0338 Non Substantive Change Request
0338 Non sub change 2017.doc
General Licensing Provisions: Biologics License Application, Changes to an Approved Application, Labeling, Revocation and Suspension, and Form FDA 356h
0338 Non Substantive Change Request
OMB: 0910-0338
Food and Drug Administration
Non-Substantive Change Request
OMB Control No. 0910-0338
Purpose: FDA is requesting a non-substantive change to Form FDA 356h, General Licensing Provisions: Biologics License Application, Changes to an Approved Application, Labeling, Revocation and Suspension, Postmarketing Studies Status Reports, Form FDA 356h , currently approved under OMB Control No. 0910-0338.
Background: Regulations in 21 CFR Parts 314 (Applications for FDA Approval to Market a
New Drug) and 601 (Biologics/Licensing) govern the submission of new drug applications and
biologics, respectively. FDA’s Center for Drug Evaluation and Research (CDER) and Center for
Biologics Evaluation and Research (CBER) review these submissions, where the agency has developed Form FDA 356h to assist respondents in this regard. The application form serves primarily as a checklist for firms to gather and submit certain information to FDA. As such, the form helps to ensure that the application is complete and contains all the necessary information, so that delays due to lack of information may be eliminated. In addition, the form provides key information to FDA for efficient handling and distribution to the appropriate staff for review. The form is available electronically and may be submitted electronically. We are currently proposing to revise Form FDA 356h to add the following:
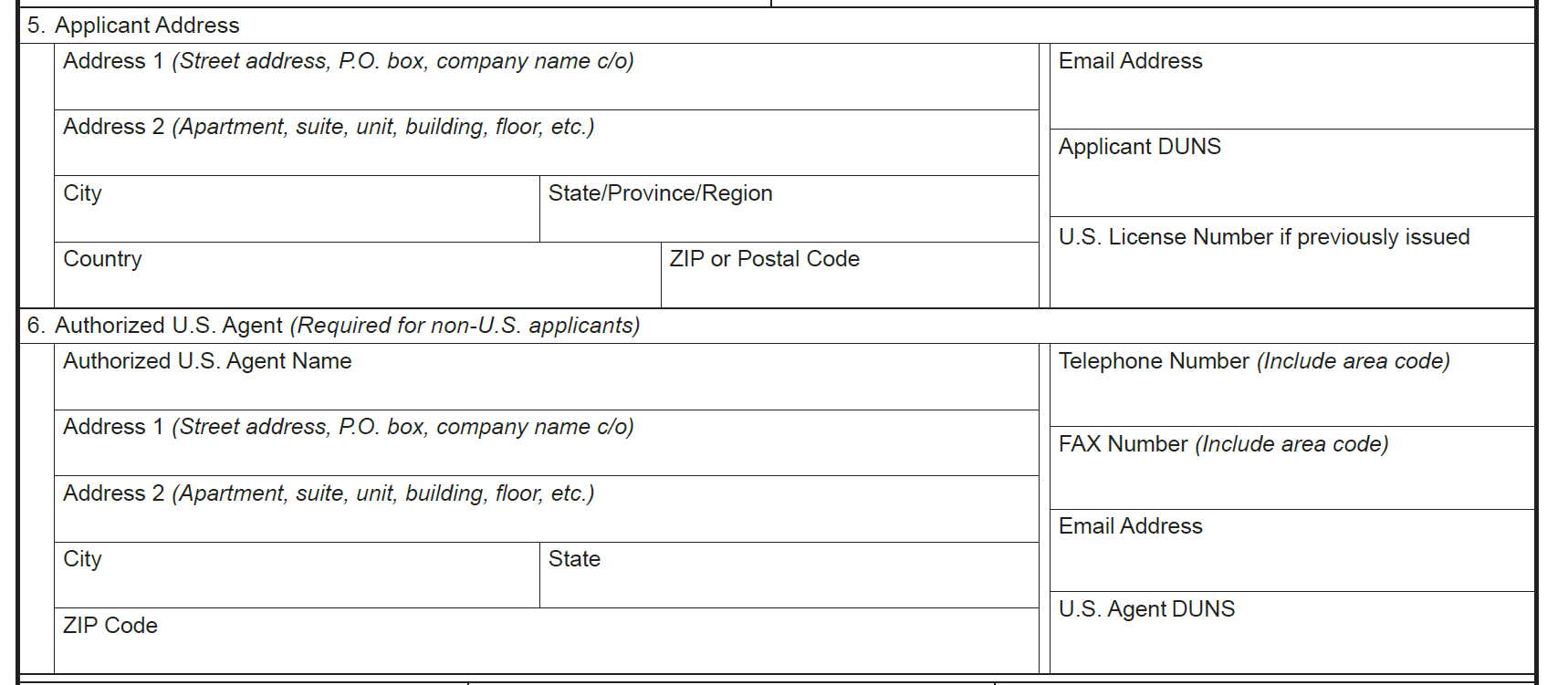
Field 5: Applicant DUNS
Field 6: US Agent DUNS
These fields would establish a reliable inventory of application sponsors for ANDA, NDA and BLA. The current form does not include a field for a unique identifier (such as DUNS) for the application sponsor. Without a unique identifier for application sponsor, it is difficult to establish a reliable inventory of application sponsors and track changes over time.


Justification: We believe these revisions will facilitate more expeditious processing as they
allow respondents to provide greater specificity regarding the submission.
We hope to release the revised form in October.
| File Type | application/msword |
| Author | Chowdhury, Ishani |
| Last Modified By | SYSTEM |
| File Modified | 2017-09-20 |
| File Created | 2017-09-20 |
© 2026 OMB.report | Privacy Policy