0014 Non Substantive Change Request
0014 Non sub change 2017.doc
Investigational New Drug Regulations
0014 Non Substantive Change Request
OMB: 0910-0014
Food and Drug Administration
Investigational New Drug Regulations
(OMB Control Number 0910-0014)
CHANGE REQUEST (83-C)
FDA is proposing a non-substantive change to Form FDA 1571: Investigational New Drug Application (IND). We are currently proposing to review the form to include the following:
Field 6B:

Reason:
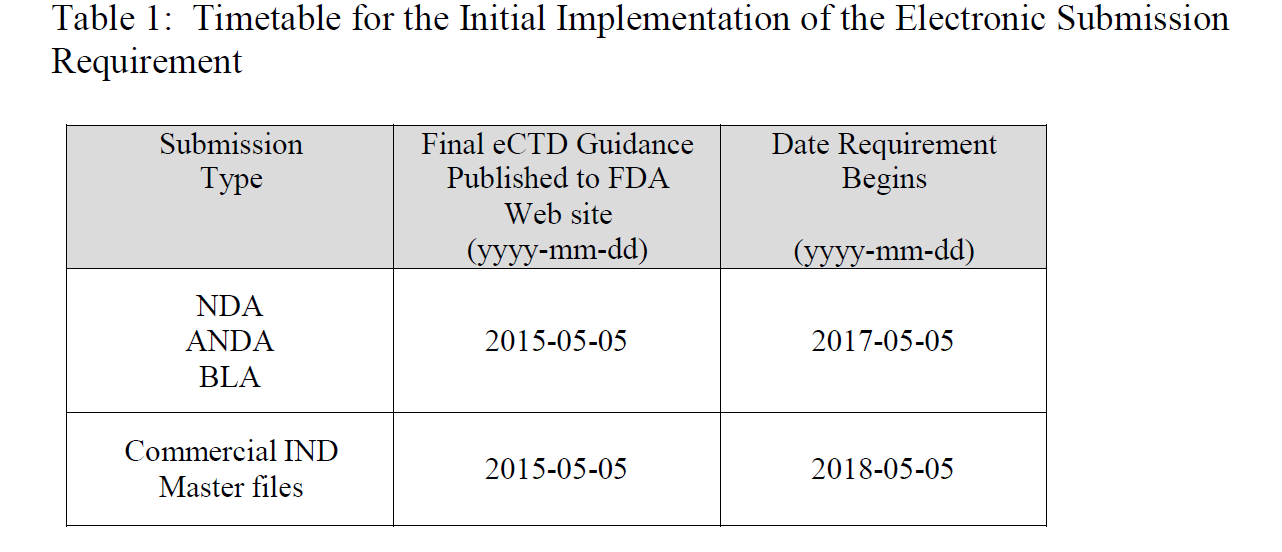
Effective May 5, 2018, commercial INDs will be required in electronic (eCTD) format. In preparation for this change, FDA believes an identifier is needed on the collection instrument to ensure efficient and appropriate processing of the submission (i.e., differentiate between a commercial and research IND).
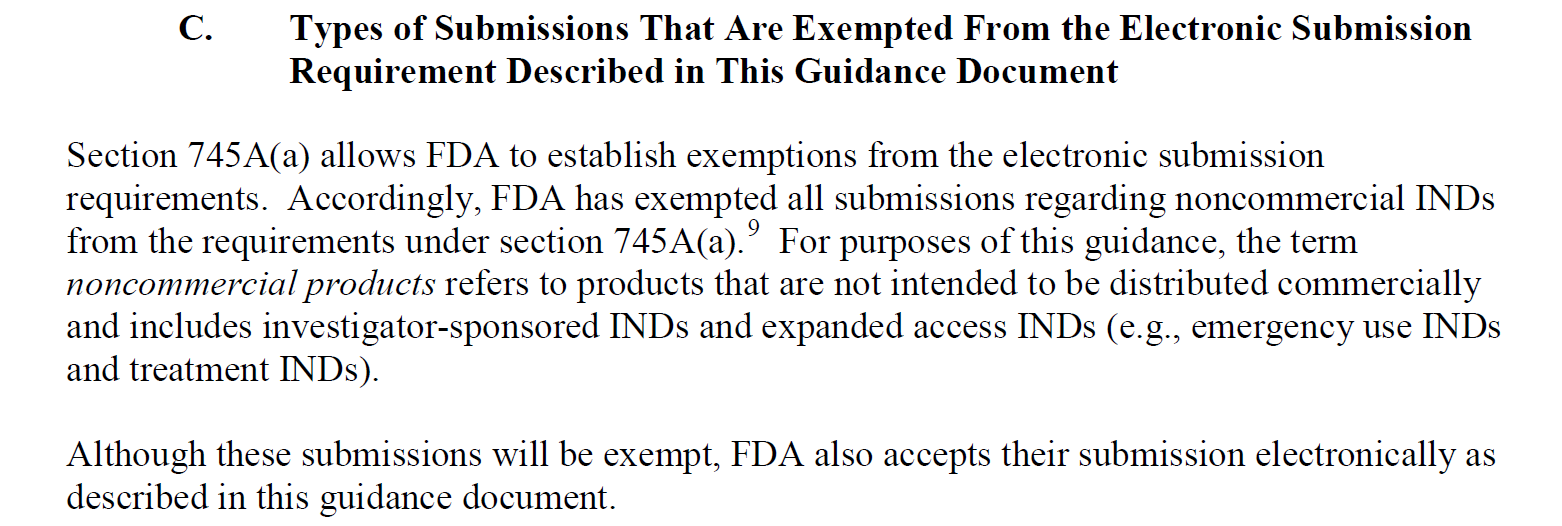
Page 4 of Sections B (Timetable for Implementation of Electronic Submission Requirements) and C (Types of Submissions That Are Exempted From the Electronic Submission Requirement Described in This Guidance Document) of “Providing Regulatory Submissions in Electronic Format — Certain Human Pharmaceutical Product Applications and Related Submissions Using the eCTD Specifications Guidance for Industry”, a specific guidance under 745a, provides both a timetable for electronic submissions by category.
We hope to release the revised form in October.
| File Type | application/msword |
| Author | Chowdhury, Ishani |
| Last Modified By | SYSTEM |
| File Modified | 2017-09-21 |
| File Created | 2017-09-21 |
© 2026 OMB.report | Privacy Policy