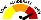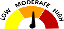Manipulation 5: Diabetes (All Conditions)
M5_DSC Diabetes_All conditions_final.docx
Data to Support Drug Product Communications as Used by the FDA
Manipulation 5: Diabetes (All Conditions)
OMB: 0910-0695

Text
FDA Drug Safety Communication
FDA approves label changes for diabetes DRUG D due to risk of serious psychiatric and nerve side effects
RISK RATING: High
Text |
Legend |
Low |
The severity of the adverse event or risk is low or mild. Only some users will experience the adverse event described. |
Moderate |
The severity of the adverse event or risk is moderate. Only some users will experience the adverse event described. |
High |
The adverse event or risk is serious and potentially life-threatening or deadly. Only some users will experience the adverse event described. |
Safety Announcement
[7-29-2013] The U.S. Food and Drug Administration (FDA) has strengthened and updated warnings regarding nerve and psychiatric side effects associated with DRUG D, used to treat diabetes. A boxed warning, the most serious kind of warning about these potential problems, has been added to the drug label. We have revised the patient Medication Guide dispensed with each prescription and wallet card to include this information and the possibility that the side effects may persist or become permanent.
Patients, caregivers, and health care professionals should watch for nerve and psychiatric side effects when using DRUG D. The nerve side effects can include dizziness, loss of balance, or ringing in the ears. These can occur at any time during drug use, and can last for months to years after the drug is stopped or can be permanent. The psychiatric side effects can include feeling anxious, mistrustful, depressed, or having hallucinations. Patients who develop nerve or psychiatric symptoms should contact their prescribing health care professional. Do not stop taking DRUG D before discussing symptoms with your health care professional.
We will continue to evaluate the safety of DRUG D and will communicate with the public again when additional information becomes available.

FACE
FDA Drug Safety Communication
FDA approves label changes for diabetes DRUG D due to risk of serious psychiatric and nerve side effects
RISK RATING:
Face |
Legend |
|
The severity of the adverse event or risk is low or mild. Only some users will experience the adverse event described. |
|
The severity of the adverse event or risk is moderate. Only some users will experience the adverse event described. |
|
The adverse event or risk is serious and potentially life-threatening or deadly. Only some users will experience the adverse event described. |
Safety Announcement
[7-29-2013] The U.S. Food and Drug Administration (FDA) has strengthened and updated warnings regarding nerve and psychiatric side effects associated with DRUG D, used to treat diabetes. A boxed warning, the most serious kind of warning about these potential problems, has been added to the drug label. We have revised the patient Medication Guide dispensed with each prescription and wallet card to include this information and the possibility that the side effects may persist or become permanent.
Patients, caregivers, and health care professionals should watch for nerve and psychiatric side effects when using DRUG D. The nerve side effects can include dizziness, loss of balance, or ringing in the ears. These can occur at any time during drug use, and can last for months to years after the drug is stopped or can be permanent. The psychiatric side effects can include feeling anxious, mistrustful, depressed, or having hallucinations. Patients who develop nerve or psychiatric symptoms should contact their prescribing health care professional. Do not stop taking DRUG D before discussing symptoms with your health care professional.
We will continue to evaluate the safety of DRUG D and will communicate with the public again when additional information becomes available.

COLOR
FDA Drug Safety Communication
FDA approves label changes for diabetes DRUG D due to risk of serious psychiatric and nerve side effects
|
Color |
Legend |
|
The severity of the adverse event or risk is low or mild. Only some users will experience the adverse event described. |
|
The severity of the adverse event or risk is moderate. Only some users will experience the adverse event described. |
|
The adverse event or risk is serious and potentially life-threatening or deadly. Only some users will experience the adverse event described. |
Safety Announcement
[7-29-2013] The U.S. Food and Drug Administration (FDA) has strengthened and updated warnings regarding nerve and psychiatric side effects associated with DRUG D, used to treat diabetes. A boxed warning, the most serious kind of warning about these potential problems, has been added to the drug label. We have revised the patient Medication Guide dispensed with each prescription and wallet card to include this information and the possibility that the side effects may persist or become permanent.
Patients, caregivers, and health care professionals should watch for nerve and psychiatric side effects when using DRUG D. The nerve side effects can include dizziness, loss of balance, or ringing in the ears. These can occur at any time during drug use, and can last for months to years after the drug is stopped or can be permanent. The psychiatric side effects can include feeling anxious, mistrustful, depressed, or having hallucinations. Patients who develop nerve or psychiatric symptoms should contact their prescribing health care professional. Do not stop taking DRUG D before discussing symptoms with your health care professional.
We will continue to evaluate the safety of DRUG D and will communicate with the public again when additional information becomes available.

NUMBER
FDA Drug Safety Communication
FDA approves label changes for diabetes DRUG D due to risk of serious psychiatric and nerve side effects
RISK RATING: 3
# |
Legend |
1 |
The severity of the adverse event or risk is low or mild. Only some users will experience the adverse event described. |
2 |
The severity of the adverse event or risk is moderate. Only some users will experience the adverse event described. |
3 |
The adverse event or risk is serious and potentially life-threatening or deadly. Only some users will experience the adverse event described. |
Safety Announcement
[7-29-2013] The U.S. Food and Drug Administration (FDA) has strengthened and updated warnings regarding nerve and psychiatric side effects associated with DRUG D, used to treat diabetes. A boxed warning, the most serious kind of warning about these potential problems, has been added to the drug label. We have revised the patient Medication Guide dispensed with each prescription and wallet card to include this information and the possibility that the side effects may persist or become permanent.
Patients, caregivers, and health care professionals should watch for nerve and psychiatric side effects when using DRUG D. The nerve side effects can include dizziness, loss of balance, or ringing in the ears. These can occur at any time during drug use, and can last for months to years after the drug is stopped or can be permanent. The psychiatric side effects can include feeling anxious, mistrustful, depressed, or having hallucinations. Patients who develop nerve or psychiatric symptoms should contact their prescribing health care professional. Do not stop taking DRUG D before discussing symptoms with your health care professional.
We will continue to evaluate the safety of DRUG D and will communicate with the public again when additional information becomes available.

METER
FDA Drug Safety Communication
FDA approves label changes for diabetes DRUG D due to risk of serious psychiatric and nerve side effects
RISK RATING:
Meter |
Legend |
|
The severity of the adverse event or risk is low or mild. Only some users will experience the adverse event described. |
|
The severity of the adverse event or risk is moderate. Only some users will experience the adverse event described. |
|
The adverse event or risk is serious and potentially life-threatening or deadly. Only some users will experience the adverse event described. |
Safety Announcement
[7-29-2013] The U.S. Food and Drug Administration (FDA) has strengthened and updated warnings regarding nerve and psychiatric side effects associated with DRUG D, used to treat diabetes. A boxed warning, the most serious kind of warning about these potential problems, has been added to the drug label. We have revised the patient Medication Guide dispensed with each prescription and wallet card to include this information and the possibility that the side effects may persist or become permanent.
Patients, caregivers, and health care professionals should watch for nerve and psychiatric side effects when using DRUG D. The nerve side effects can include dizziness, loss of balance, or ringing in the ears. These can occur at any time during drug use, and can last for months to years after the drug is stopped or can be permanent. The psychiatric side effects can include feeling anxious, mistrustful, depressed, or having hallucinations. Patients who develop nerve or psychiatric symptoms should contact their prescribing health care professional. Do not stop taking DRUG D before discussing symptoms with your health care professional.
We will continue to evaluate the safety of DRUG D and will communicate with the public again when additional information becomes available.

SYMBOL
FDA Drug Safety Communication
FDA approves label changes for diabetes DRUG D due to risk of serious psychiatric and nerve side effects
RISK RATING:
Symbol |
Legend |
|
The severity of the adverse event or risk is low or mild. Only some users will experience the adverse event described. |
|
The severity of the adverse event or risk is moderate. Only some users will experience the adverse event described. |
|
The adverse event or risk is serious and potentially life-threatening or deadly. Only some users will experience the adverse event described. |
Safety Announcement
[7-29-2013] The U.S. Food and Drug Administration (FDA) has strengthened and updated warnings regarding nerve and psychiatric side effects associated with DRUG D, used to treat diabetes. A boxed warning, the most serious kind of warning about these potential problems, has been added to the drug label. We have revised the patient Medication Guide dispensed with each prescription and wallet card to include this information and the possibility that the side effects may persist or become permanent.
Patients, caregivers, and health care professionals should watch for nerve and psychiatric side effects when using DRUG D. The nerve side effects can include dizziness, loss of balance, or ringing in the ears. These can occur at any time during drug use, and can last for months to years after the drug is stopped or can be permanent. The psychiatric side effects can include feeling anxious, mistrustful, depressed, or having hallucinations. Patients who develop nerve or psychiatric symptoms should contact their prescribing health care professional. Do not stop taking DRUG D before discussing symptoms with your health care professional.
We will continue to evaluate the safety of DRUG D and will communicate with the public again when additional information becomes available.

CONTROL
FDA Drug Safety Communication
FDA approves label changes for diabetes DRUG D due to risk of serious psychiatric and nerve side effects
Safety Announcement
[7-29-2013] The U.S. Food and Drug Administration (FDA) has strengthened and updated warnings regarding nerve and psychiatric side effects associated with DRUG D, used to treat diabetes. A boxed warning, the most serious kind of warning about these potential problems, has been added to the drug label. We have revised the patient Medication Guide dispensed with each prescription and wallet card to include this information and the possibility that the side effects may persist or become permanent.
Patients, caregivers, and health care professionals should watch for nerve and psychiatric side effects when using DRUG D. The nerve side effects can include dizziness, loss of balance, or ringing in the ears. These can occur at any time during drug use, and can last for months to years after the drug is stopped or can be permanent. The psychiatric side effects can include feeling anxious, mistrustful, depressed, or having hallucinations. Patients who develop nerve or psychiatric symptoms should contact their prescribing health care professional. Do not stop taking DRUG D before discussing symptoms with your health care professional.
We will continue to evaluate the safety of DRUG D and will communicate with the public again when additional information becomes available.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Winthrop, Sally |
| File Modified | 0000-00-00 |
| File Created | 2021-01-21 |
© 2026 OMB.report | Privacy Policy