Studies to Enhance FDA Communications Addressing Biosimilar Drug Products: Focus Groups with Patients
Data to Support Drug Product Communications
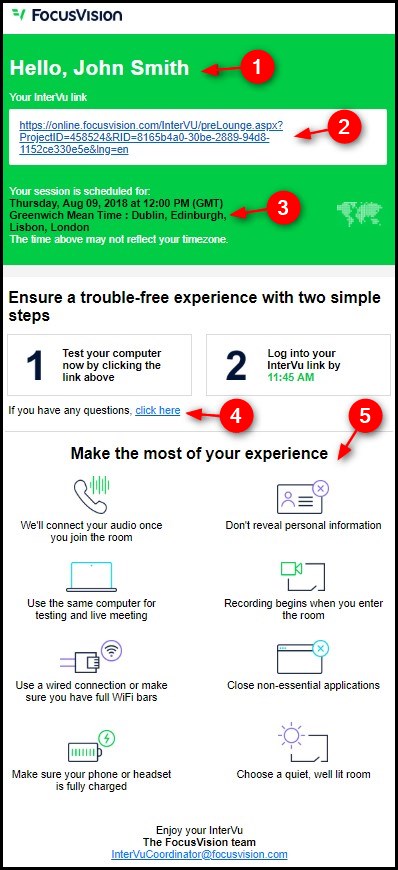
InterVu Respondent Invitation Template
Studies to Enhance FDA Communications Addressing Biosimilar Drug Products: Focus Groups with Patients
OMB: 0910-0695
OMB Control No. 0910-0695
Expiration Date 02/28/2021
InterVu RESPONDENT INVITATION TEMPLATE
1. Greeting: The respondent's name will automatically replace the listed example name "John Smith". The normal format of the name is forename + initial of surname (e.g., "Mike C"), as this helps to maintain anonymity whilst reassuring respondents that the invite was intentionally and correctly sent to them.
2. InterVu Link: The InterVu link is uniquely generated for each respondent. Respondents can use this link to test their system (as well as webcam where applicable) and to access the live meeting once it has started.
3. Meeting Time: The meeting date and time, as previously confirmed by the respondent. All times will be listed in respondents' personal time zones, if known.
4. Contact Us: If respondents need help or have any questions regarding their scheduled meeting, they can contact FocusVision directly via this link.
5. Notes: The icons / notes in this section are designed to help answer the most common questions, and to curtail the most commonly encountered issues.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Elise Bui |
| File Modified | 0000-00-00 |
| File Created | 2021-01-16 |
© 2026 OMB.report | Privacy Policy