Healthcare Professional Ihterviews: Risk Processing for Newly Promoted Prescription Drugs
Data to Support Drug Product Communications
Moderator Guide
Healthcare Professional Ihterviews: Risk Processing for Newly Promoted Prescription Drugs
OMB: 0910-0695
Moderator Guide
FDA Healthcare Professional Interviews – In-Depth Interviews
with Healthcare Professionals on Prescription Drugs
IN-DEPTH INTERVIEW DISCUSSION GUIDE
Research Objective: Conduct in-depth interviews with prescribers (general practitioners, specialists, nurse practitioners, and physician assistants) to understand how they process risk information in professional promotional materials and how it affects subsequent decision-making (Section IV), and to assess their thoughts on risk information in promotional materials more generally (Section V).
NOTES: |
Question probes are italicized. These are suggestions for the interviewer to follow and will be used or modified as deemed relevant and necessary in the natural flow of discussion. |
Moderator instructions are highlighted in yellow. |
Session Overview:
SECTION I: INTRODUCTION (5 minutes) The purpose of this section is for the moderator to explain the purpose of the research, lay down any ground rules or guidelines, and allow the participant to ask any questions. |
SECTION II: BACKGROUND INFORMATION (5 minutes) This section gathers background information, including the participant’s clinical background and exposure to and use of promotional materials for new prescription drug products. |
SECTION III: STIMULI PRESENTATION AND MEMORY TASK (15 minutes) Participants will view the promotional materials for Veridan and undergo memory tasks. |
SECTION IV: STIMULI DISCUSSION (15 minutes) The participant will view the promotional materials for Veridan again and share his or her thoughts, assessments of the risks and benefits, and subsequent prescription decision-making. |
SECTION V: GENERAL THOUGHTS ON PRESCRIBING NEW DRUGS (18 minutes) This section will include a discussion on using new prescription drugs more generally, including how the participant becomes aware of new prescription products, his or her openness to prescribing them, how he or she seeks out information, and how this information factors into his/her decision-making with regard to patient care. |
CONCLUSION (2 minutes) If time permits, the moderator will check if the research team members and observers have additional questions. If yes, the moderator will ask follow-up questions and then wrap up the discussion and ensure that the participant’s comments have been heard. |
SECTION I: INTRODUCTION (5 minutes)
[Please refer to the participant by his or her FIRST NAME only.]
My name is _________, and I’m part of an independent research company, Fors Marsh Group. This means that I’m here to listen to you and what you have to tell me, and I have no stake in how you respond.
This is a study sponsored by DHHS, the Department of Health and Human Services, to understand how healthcare professionals learn about new prescription drugs. The purpose of today’s interview is to get your thoughts and reactions about various topics we will be discussing. I do not have a medical background, so your feedback is extremely helpful.
Your thoughts are very important to us, and your time today is appreciated.
We will have about 60 minutes for our discussion.
As we begin, I want to review a few ground rules for our discussion.
Your participation is voluntary and you have the right to withdraw from the study at any time.
Most importantly, there are no “wrong” answers. We want to know your opinions and what you think about the things we will be discussing.
Just a reminder, we are not selling anything, and I do not work for the people who are sponsoring this research, so don’t hold back from giving me your honest opinions.
Your identity will not be linked to your responses. This means that no one outside of the research team will be able to link what you said back to you.
As we move through our discussions, please make sure to refrain from providing any protected health information to us. Please also refrain from providing any identifying information about your practice, such as mentioning the names of your colleagues.
I’m here with my colleague [NAME] from [COMPANY] and he/she will be [helping me to take notes/facilitating the eye-tracking portion of the interview]. This interview will also be audio recorded for data analysis and reporting, and your computer screen will be video captured as well. [If livestreaming: We are also livestreaming the interview so that other research team members can observe remotely.] We will be recording your eye movements during parts of the interview while you look at materials on the computer screen. We will give you further instructions before that section. We will not record any images of your face. Only people who are involved in the project will have access to this data. Is it ok if I start the audio recording now? [Start recording]
Do you have any questions before we begin?
Okay, great. Let’s get started.
SECTION II: BACKGROUND (5 minutes)
I’d like to start by talking a little bit about your background as a healthcare professional.
To start, could you briefly describe your clinical background and general information about your practice?
How long have you been practicing medicine?
What are some typical patient issues you treat?
How frequently do you come across promotional materials about prescription drugs?
[If needed:] Examples of these materials would be advertisements in a medical journal, a mailer, an email, or a brochure or pamphlet.
How do you typically use or interact with these materials?
[If the participant uses them] How much time do you typically spend looking over these materials?
SECTION III: STIMULI PRESENTATION AND MEMORY TASK (15 minutes)
I am going to show you prescription drug promotional materials on the screen. Please keep in mind this drug may or may not be applicable to your current practice or the patients you see; we are using these promotional materials to facilitate discussion and gain a better understanding of how promotional items like these are typically used.
While you are viewing the page, we will be using an eye tracker [Point to the eye tracker below the screen] to record where you are looking on the screen.
[If the participant asks questions about the eye tracker, use this language, otherwise, skip to next paragraph.] The eye tracker uses infrared light to illuminate the eye and very small cameras to record the location of your eyes. Only the location of your eyes is recorded by the eye tracker.
We’ll first calibrate the eye tracker. I’d like you to get in a comfortable position for you to view the screen. [Adjust the participant’s positioning as necessary.] You’re going to see a red dot appear on the screen. I’d like you follow the red dot with your eyes while keeping your head still. [Run calibration].
Ok, we are all set with the calibration. While you are viewing the pages, you don’t need to sit as still as a statue, but if you move your head too much, the eye tracker may lose the location of your eyes. If that occurs, I may ask you to adjust how you’re sitting. [Typically, we have to ask participants to sit back in the chair more.]
Now, I’d like you to review the promotional material that will be presented on your screen. You will have up to two minutes to review as you typically would in order to evaluate the prescription drug being described. There are two pages and you can scroll using your mouse to see the second page. Let me know when you have finished reviewing. During your review of the materials, you and I will not be interacting because I want you to continue looking at the materials as you review. If any questions come to mind as you review, we can address those after your review is complete.
[Allow the participant to read for 2 minutes (hard stop)
Advance the screen once the participant has finished reviewing, if he/she is done before the 2 minutes have passed. If the participant asks about whether Veridan is real, disclose that the drug is fictional, but tell the participant that he/she should treat it as though it is a real drug.]
For the rest of the session today we will not be eye tracking.
Next, I’d like you to read the instructions and complete the tasks that will be presented on the screen. You will be completing these tasks on your own. Please let me know if you have any questions before advancing past the instructions page. [Start the memory task in Decipher.]

SECTION IV: sTIMULI DISCUSSION (15 minutes)

What is your initial overall impression of this promotional piece?
While answering the previous questions in the memory task, would you say you specifically remember seeing the information in the promotional materials?
Did you have to guess any of your answers?
[If yes] Please walk me through how you arrived at your guess(es).
Now I am going to bring the promotional piece back for you to look at as I ask you some follow up questions. [Display stimuli to participant again.]
Can you walk me through your process of evaluating this promotional material in general? Feel free to look through the materials again if there is anything specific you would like to refer to. [Allow the participant to walk through his/her process; use probes below as needed to ensure coverage.]
What sort of information were you looking for?
What specific information in the Veridan materials was most important to you?
What, if any, sections were unclear or confusing to you when going through the materials?
When reviewing this Veridan piece, how much time did you spend looking specifically at the risk information?
What sorts of red flags did you look for?
How much time do you typically spend looking at the risk information when you receive promotional materials like this?
What are your thoughts on how the risk information was presented?
What, if any, new thoughts or questions come to mind about the risk-benefit ratio of Veridan after viewing this information?
During initial review of the material, did you notice the disclosure information on the screen about how Veridan compares to Lyrica? [If needed for clarification, show stimulus and point out the disclosure to the participant.]
[If yes] Did you take time to read it?
i. [If yes] What were your initial thoughts about it?
ii. [If no] Why not?
[If no] What are your initial thoughts about the disclosure information now that you are reading it over?
i. Do you typically look at similar disclosure information on drug promotional materials?
Does Veridan remind you of any other drugs?
[If yes] Which ones?
How similar do you think Veridan is to other drugs that treat diabetic peripheral neuropathy?
How similar do you think the risk factors for Veridan are?
Which drug would you say has greater risks, if any?
What additional information would you want to see about Veridan in order to make a prescribing decision?
Where else would you go for more information?
What factor(s) would go into your decision to prescribe or not prescribe Veridan to a patient?
What are the most important considerations?
How would you go about assessing Veridan’s potential benefits and risks for an individual patient?
What role, if any, would the risk information play in your decision?
To what extent would you use your prior knowledge about other similar drugs to assess the risk information of Veridan?
How would you explain the contents of the Veridan material to a patient?
Imagine you were going to prescribe Veridan to a patient—how would you explain the risks and benefits of Veridan to them?
[Remove the stimulus from the screen.]
SECTION V: general thoughts on PRESCRIBING NEW DRUGS (18 minutes)
Thank you very much for your thoughts on the promotional materials for Veridan. Now, I would like to ask you some more general questions about when and how you make decisions with regard to prescribing new drugs. You can still think about Veridan but also consider other medications you are familiar with.
How do you typically become aware of new prescription drugs?
How open are you to prescribing new drugs in general?
What positives and negatives do you perceive when it comes to prescribing new drugs?
[If expressing doubt, skepticism, or hesitation, etc.] Where does your hesitation toward new drugs come from?
In what situations would you consider prescribing new prescription drugs?
If you want to find more information about a drug, how would you go about doing so?
How often do you use information in promotional materials to decide whether to prescribe new drugs?
How important is this information compared to other sources?
What would you consider the most useful and least useful pieces of information contained in prescription promotional materials?
[If needed, ask the participant to elaborate on reasons for their response.]
[If not already mentioned] How useful or important is the risk information?
[If appropriate, probe on the trustworthiness of the information.]
If you had limited time to learn about a new prescription drug, what types of information would take priority?
How, if at all, does your decision-making process differ when it comes to deciding whether to prescribe new drugs compared to ones you are more familiar with?
How do you counsel patients with regard to medications?
Are there any particular steps you take in order to mitigate risks?
I know we have already discussed a number of factors that influence your prescription decisions for new drugs. Are there any others that we have not yet talked about?
Does time play a role? That is, if you are under time pressure, does it affect your prescription decision-making?
How do patient factors play a role?
How does cost or insurance play a role, if any?
How do pharmaceutical representatives play a role, if any?
How does a drug’s class affect your decision-making about a new drug?
When deciding whether to prescribe a new drug, to what extent would you use your prior knowledge of the drug class it belongs to?
How much do you perceive drugs that share a class to be similar or different from each other?
_____________________________________________________________________________
S ection
Vi: Closing
(2 minutes)
ection
Vi: Closing
(2 minutes)
[If time permits—please check with the research team/observers for follow-up questions.]
Thank you, this has been helpful. I am going to check quickly with my colleagues to see if there are any follow-up questions for you in the time we have remaining.
[Discuss with observers over online chat or phone (for pilot study), or confer with research team in person, as appropriate.]
Moderator asks follow-up questions (if applicable).
[Thank participant.]
The promotional materials that you saw today for Veridan were created for the purposes of research and do not advertise a real prescription drug. Thank you very much for participating in this interview. I appreciate your time and feedback. Is there anything that you would like to share that you didn’t have the chance to share yet?
MEMORY TASK
Task Introduction:
While the eye-tracking measures will be used to assess attention to design elements and written information within the stimulus, memory task measures will be used to assess information processing, encoding, and retrieval of the information. Considering eye-tracking and memory task measures in combination will provide additional insight into the cognitive processing of healthcare professionals as they review new promotional material for medications. A four-part task is utilized for the memory task that includes indirect and explicit recall measures.
First, a distraction task will be administered to reduce the possibility of the participant recalling information from working memory for the explicit and indirect memory tasks. A study by Liu and Fu (2007)1 found that there is a significant effect in the availability of long-term memory after a distraction task. The distraction task improves attention due to the required processing while also priming the participant for recall. After this one-minute task, the participant is presented with five explicit recall questions in relation to the drug promotional document. The main areas of focus include the risk information, prescribing considerations, and cost savings highlighted in the drug promotion.
Next, the indirect memory task aims to identify recognition of risk information and key branding elements (as a comparison) in the promotional document. Inspiration for the indirect memory task came from a study by Wedel and Peters (2000),2 whose goal was to test the effectiveness of advertisements in leaving traces of brands in perceptual memory. They compared the findings of the indirect memory task against the number of eye-gaze fixations on the brand elements. They found that the number of fixations directly correlated with faster recognition of information. Similarly, the indirect memory task in this study is intended to determine the efficacy of retrieving key information of the drug from memory.
Lastly, an explicit ranking task is administered to compare the findings from the memory tasks and to decide whether information ranked to be important for decisions by medical practitioners is perceived to be important in practice.
Task Overview:
Part 1 – Distraction Task (1 minute):
Off-load short-term memory by asking participants to complete a task that is not directly related to the stimuli.
We will assess the value of the distraction task during the pilot and determine whether it should be included in the full study.
Part 2 – Explicit Recall (45 seconds per question; 3 minutes and 45 seconds in total):
Assess recall of Veridan risk information, prescribing considerations, cost savings, and other factors important in decision-making.
Five questions are proposed.
Part 3 – Indirect Memory Task (30 seconds per question; 2 minutes and 30 seconds in total)
Assess recognition of risk information and key branding elements (as a comparison) from the promotional document.
Each question will consist of a pixelated/blurred image along with five options. Participants will be instructed to identify the correct answer from the options presented.
Response time and accuracy will be measured.
Five questions are proposed.
Part 4 – Explicit Ranking (3 minutes):
Identify the decision-making process and information hierarchy when health professionals are considering a new drug.
The task will provide insight into the information hierarchy that is desired by the medical practitioners. Information that is thought to be important for decisions by medical practitioners could possibly be deemed less important in practice.
No ties will be allowed in the ranking task in order to reduce ambiguity in scoring the responses. Furthermore, each item must be assigned a rank.
Part 1 – Distraction Task:
Q1. Drawing from your experiences, please list the 10 medications that are most frequently prescribed to your patients.
[Stop after 1 minute]
Part 2 – Explicit Recall Task:
Q1. The promoted medication is available only by prescription for what type of condition?
[Text field]
Q2. Based on the document for the promoted medication, how much money does the promoted medication save per year compared to Lyrica?
[Text field]
Q3. Name one serious adverse reaction to the promoted medication at treatment initiation.
[Text field]
Q4. In the promotional document, the promoted medication is compared to Lyrica. However, the promoted medication may or may not be ________________ as Lyrica.
[Text field]
Q5. What is the risk associated with prescribing the promoted drug to women who are nursing?
[Text field]
Part 3 – Indirect Memory Task:
Q1: Drug manufacturer
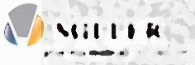
A) Muller Pharmaceuticals
B) Miller Pharmaceuticals
C) Multer Pharmaceuticals
D) Mulder Pharmaceuticals
E) Miltek Pharmaceuticals
Q2: Brand name

A) Vexirtan
B) Viritan
C) Veridan
D) Veritun
E) Vieritun
Q3: Generic name of drug

A) Verdexetine
B) Veridicine
C) Veridiotene
D) Veridantonine
E) Veridinine
Q4: Contraindicated conditions

A) Asthma
B) Tuberculosis
C) Bronchitis
D) Hypoglycemia
E) Hypertension
Q5: Common Adverse Reactions

A) Drowsiness
B) Itchiness
C) Irritation
D) Dizziness
E) Fever
Part 4 – Explicit Ranking Task:
Drawing from your experience, knowledge, and daily needs, which of the following information do you deem most important to be included in a drug’s promotional material? Rank specific needs from 1 to 10 with 1 being the most important.
Comparison to competitor drug |
Contraindications |
Cost savings |
Prescribing considerations |
Risks at treatment initiation |
Adverse reactions |
Conditions requiring adjustments or special tests |
Drug manufacturer contact information |
Indication |
Patient counseling information |
1 Liu, Y., & Fu, X. (2007). How Does Distraction Task Influence the Interaction of Working Memory and Long-Term Memory? (pp. 366–374).
2 Wedel, M., & Pieters, R. (2000). Eye Fixations on Advertisements and Memory for Brands: A Model and Findings. Marketing Science, 19, 297–312.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Betts, Kevin |
| File Modified | 0000-00-00 |
| File Created | 2021-01-16 |
© 2026 OMB.report | Privacy Policy