Physician Interviews on FDA-Approved Labeling
Data to Support Drug Product Communications
Appendix E - Interview Guide
Physician Interviews on FDA-Approved Labeling
OMB: 0910-0695
Appendix E:
Interview Guide
Physician Interviews on FDA-Approved Prescribing
Information:
Research Questions and Interviewer Guide
Overview:
The interviewer will provide background information about the project and get consent to proceed (including consent to audio-record and livestream).
|
Introduction & Consent
INTERVIEWER: Hello and thank you for making time to talk with me today.
My name is [ ] and I work for RTI International, a not-for-profit research organization located in Research Triangle Park, North Carolina.
The Food and Drug Administration has contracted with RTI to conduct interviews with health care providers to better understand how people interpret information commonly used in prescription drug labels.
Informed Consent
Before we begin, I would like to review a few items:
Consent Form. I understand that you reviewed the informed consent form when you scheduled your appointment. Just a few highlights…
Participation. Your participation is voluntary and you can stop participating at any time. If at any time you are uncomfortable with any question, you can choose not to answer.
Privacy. Your name and contact information will not be given to anyone else and no one will contact you about this study after our discussion is over. During the interview, please do not tell us anything about yourself that could be used to identify you like your last name (it is okay to tell us your first name) or birthday.
Audio Recording. To make sure that we capture everything people say today, we are making an audio recording of this discussion, which will later be transcribed. We will provide the FDA with a transcript (but no audio-recording) of our discussion. Your name and any identifying information about you will not be included nor associated with the project or the report in any way.
Observation. Members of our study team, including staff from FDA, may listen and watch today’s discussion via livestream so they can hear your thoughts directly from you.
Reporting. As part of this study, we will write a report for the FDA summarizing what we learned from these interviews. We will not use your name or any identifying information in the report.
Do you have any questions before we begin?
Do I have your consent to participate? [get a verbal “yes” then continue]
Do you agree to have your interview audio-recorded and livestreamed? [get a verbal “yes” then continue; If no, remind participant that it is a condition for participation as outlined in the consent-thank them for their time and end call]
Warm-up
First, I’d like to get a better idea about the types of medical problems you typically treat and some background on your prescribing practices.
What are the most common medical problems you treat?
How many different drugs do you typically prescribe in a week?
How often do you prescribe a drug you’ve never prescribed before?
How often are the drugs newly approved vs. older drugs that you just haven’t prescribed before?
Discussion
Section 1. Resources to Find Information About Prescription Drugs
Research
Questions
Discuss
the resources physicians use to find information about prescription
drugs.
Discuss
how physicians use Prescribing Information to find information
about prescription drugs.

Next, I want to talk about resources that you use to find information about prescription drugs.
1. Under what circumstances would you look up information for a drug that you have prescribed before?
a. What about for a drug that you haven’t prescribed before? Do the circumstances differ?
2. [INTERVIEWER: ASK IF NOT COVERED BY Q1] What kind of information do you typically look up for a drug that you have prescribed before?
IF NEEDED, PROBE:
Dose?
Indication?
Contraindications?
a. What about for a drug that you haven’t prescribed before?
What resources do you use to look for information about prescription drugs?
IF NEEDED, PROBE:
App on a smart phone
Paper in drug package
Database within the EHR (e.g. Micromedex)
Health-related websites (e.g. Drugs.com)
Paper-based book/resource guide
Manufacturer’s website
a. IF NEEDED: How do you access these resources?
b. Do the resources that you use differ if it is for a drug that you have prescribed before vs. for one that you haven’t? If yes, how do they differ?
4. Do the resources that you use differ depending on the type of information you are looking for about the drug? If so, how do they differ?
5. What resources do you typically use for overdose information?
Section 2. Background on Prescribing/Labeling Information
Next, I’m going to ask you some questions about FDA-approved Prescribing Information, also known as the drug label or labeling. This is a summary of the information needed to use a prescription drug safely and effectively and is drafted by the drug’s manufacturer and approved by the FDA. Moving forward I’ll refer to the FDA-approved Prescribing Information as the “label.”
Under what circumstances do you look at the drug’s label?
Where do you look for the drug’s label? (IF NEEDED: For example, an online search, Drugs.com, a drug manufacturer’s website, FDA website, DailyMed, paper label in the drug packaging)
How do you typically find out that there has been an update to the label—for example, newly approved indications, new black box warnings or contraindications, new dosages, new risk information, new information about patients with renal impairment, or new drug interactions? (IF NEEDED: “Dear prescriber” letters from manufacturers, ads in medical journals, DTC ads, prescriber guides (e.g. Medical Letter, Monthly Prescribing Reference))
The label has three parts:
Highlights of Prescribing Information (see an example below)
Table of Contents
Full Prescribing Information (see a description of the contents of each of the sections in the Full Prescribing Information below)
Highlights of Prescribing Information (PLR-Format Labeling)

Full Prescribing Information Sections of PLR-Formatted Labeling1
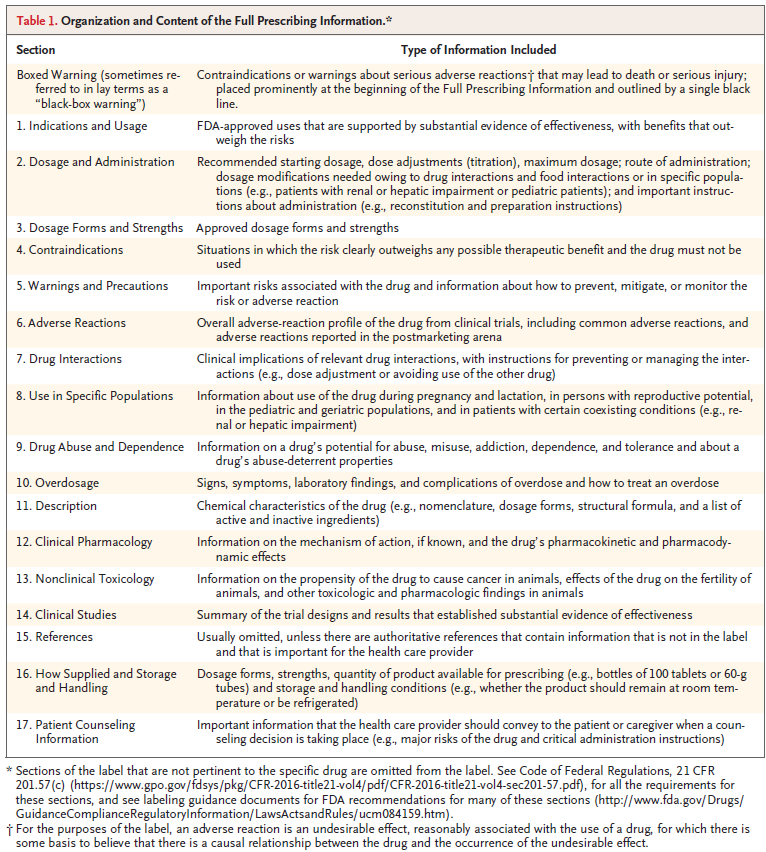
4. What sections or parts of the label do you find most useful in your practice? Why?
5. What sections or parts of the label do not provide enough information?
a. What information should be added?
6. What sections or parts of the label includes too much information?
a. What information should be deleted?
7. Are there sections or parts of the label that are confusing? If so, what should be done to make them clearer?
Section 3. Interpretation of Language in the Prescribing Information
Research Questions
How do healthcare providers interpret the following terminology in
the Prescribing Information: “contraindications”, “not
recommended”, or “avoid use”?
How do healthcare providers interpret language in the INDICATIONS
AND USAGE section of fixed-combination drugs that describes an
indication for only one of the two components?
What are the merits and disadvantages of including the age groups
in the INDICATIONS AND USAGE section for diseases that do not occur
or rarely occur in pediatric patients?
How do healthcare providers interpret adverse reaction data from
actively controlled trials in the ADVERSE REACTIONS section?

Now I’m going to show you some specific examples of language from the label. After each set, I’ll ask you some follow-up questions.
EXAMPLE SCREEN 1
 Please
read the following three examples on the screen:
Please
read the following three examples on the screen:
|
Questions for Example Screen 1
1. Looking at these three statements, are the messages the same or different? Please explain.
2. Would you consider using DRUG-X in a patient with severe renal impairment based on Statement 1? Statement 2? Statement 3? Why or why not?
EXAMPLE SCREEN 2
Please read the following two examples of language in the INDICATIONS AND USAGE section of the label. Note that the examples are identical, but Example #2 has one additional sentence at the end that is highlighted.
1 INDICATIONS AND USAGE DRUG X, a combination of drug1 and drug2, is indicated to improve glycemic control in adults with type 2 diabetes mellitus (T2DM) when treatment with both drug1 and drug2 is appropriate. Drug1 is also indicated to reduce the risk of cardiovascular death in adults with T2DM and established cardiovascular disease. Example #2: 1 INDICATIONS AND USAGE DRUG X, a combination of drug1 and drug2, is indicated to improve glycemic control in adults with T2DM when treatment with both drug1 and drug2 is appropriate. Drug1 is also indicated to reduce the risk of cardiovascular death in adults with T2DM and established cardiovascular disease. However, the effectiveness of DRUG X on reducing the risk of cardiovascular death in adults with T2DM and cardiovascular disease has not been established. |
Questions for Example Screen 2
For Example 1, would you expect DRUG X to reduce the risk of cardiovascular death in adults with type 2 diabetes mellitus and established cardiovascular disease? Why or why not?
What about for Example 2 (if answer is different ask why?)
For Example 1, would you prescribe DRUG X to reduce cardiovascular death in your diabetes patients? Why or why not?
What about for Example 2 (if answer is different ask why?)
EXAMPLE SCREEN 3
FDA generally recommends including the age group in the INDICATIONS AND USAGE section.
Please take a moment and read the examples I’m showing on the screen.

Questions for Example Screen 3
If a drug is indicated for adults, do you think it needs to explicitly state so in the INDICATIONS AND USAGE section – as in Example 1? Why or why not?
If the INDICATIONS AND USAGE section did not specifically include “adults” for diseases that typically do not occur or rarely occur in pediatric patients (as in Example 2) how would you interpret this information?
[IF NOT CLEAR FROM Q1 and Q2]. Looking at both examples (Example 1 specifically referencing adults and Example 2 not), which one do you think is best? Why do you say that?
EXAMPLE SCREEN 4
Please review the example on the screen of adverse reactions data from a trial comparing DRUG X to DRUG Y. DRUG X is the subject drug and DRUG Y is the comparator drug.

DRUG X LABELING Table 1: Most Common Adverse Reactions in the Plaque Psoriasis Studies
|
Questions for Example Screen 4
How would you interpret the liver enzyme elevations in patients treated with DRUG X and DRUG Y?
[INTERVIEWER NOTE: liver enzyme elevations refer to the last two rows/options: Increased ALT, Increased AST]
If the following footnote was added to the table would this change your interpretation of the elevated liver enzymes: “Because the study was not designed to evaluate the relative safety between DRUG-X and DRUG Y, comparison of adverse reactions data between the DRUG-X and DRUG Y groups may be misleading.” Please explain.
Section 4. Presenting Risk Information in the Prescribing Information
Research
Questions
Is
there a preferred method for listing adverse reactions (e.g., by
body system or decreasing frequency) in the ADVERSE REACTIONS
section?
Is
there an optimal way to organize the following information in the
WARNINGS AND PRECAUTIONS section: description of the clinically
significant adverse reaction, risk factors, and steps to take to
prevent or manage the adverse reactions?

EXAMPLE SCREEN 5
Now I’d like to ask you some questions about drug risk information included in the label. The ADVERSE REACTIONS section contains a listing of the adverse reactions associated with the drug or related drugs. Adverse reactions can be listed in the ADVERSE REACTIONS section in different ways.
Note the two examples contain the identical adverse reactions but they are presented in two different ways:
In Table 5a, adverse reactions are ordered by body system and then by decreasing frequency (example #1)
In Table 5b, adverse reactions are ordered by decreasing frequency (example #2)
 Table
5a: Most Common Adverse Reactions in Patients
Table
5a: Most Common Adverse Reactions in Patients
with Hypertension
(Studies 1 and 2)
|
DRUG-X (n=1129) |
Placebo (n=350) |
|
||
Gastrointestinal |
||
Abdominal pain |
12% |
8% |
Dyspepsia |
0% |
17% |
Respiratory |
||
Upper respiratory tract infection |
32% |
25% |
Sinusitis |
14% |
8% |
Pharyngitis |
12% |
8% |
Coughing |
12% |
8% |
Skin and appendages disorders |
||
Rash |
10% |
5% |
Pruritus |
7% |
2% |
Body as a whole-general disorders |
||
Fatigue |
9% |
7% |
Resistance mechanism disorders |
||
Fever |
7% |
4% |
Moniliasis |
5% |
3% |
Central and peripheral nervous system disorders |
||
Headache |
18% |
14% |
Urinary system disorders |
||
Urinary tract infection |
8% |
6% |
Cardiovascular disorders, general |
||
Hypertension |
7% |
5% |
 Table
5b: Most Common Adverse Reactions in Patients
Table
5b: Most Common Adverse Reactions in Patients
with Hypertension
(Studies 1 and 2)
|
DRUG-X |
Placebo |
Upper respiratory tract infection |
32% |
25% |
Headache |
18% |
14% |
Sinusitis |
14% |
8% |
Abdominal pain |
12% |
8% |
Pharyngitis |
12% |
8% |
Coughing |
12% |
8% |
Rash |
10% |
5% |
Dyspepsia |
10% |
7% |
Fatigue |
9% |
7% |
Urinary tract infection |
8% |
6% |
Pruritus |
7% |
2% |
Fever |
7% |
4% |
Hypertension |
7% |
5% |
Moniliasis |
5% |
3% |
Questions for Example Screen 5
Looking at how the adverse reactions are presented in the ADVERSE REACTIONS section of the label in Table 5a and 5b, which version do you prefer and why?
EXAMPLE SCREEN 6
Information about clinically significant adverse reactions and risks can be presented in different ways in the WARNINGS AND PRECAUTIONS section. Please review the two examples on the screen.
Example 6a orders information in the following way: (1) description of the clinically significant adverse reaction, (2) risk factors, and (3) steps to take to prevent or manage the adverse reactions.
 Example
6a.
Example
6a.
5 WARNINGS AND PRECAUTIONS
5.1 Increase in Blood Pressure
In Studies 1 and 2, DRUG-X increased systolic blood pressure (BP) during six months of treatment by an average of 5.9 mmHg (ambulatory blood pressure monitoring) and by an average of 3.8 mmHg (blood pressure cuff measurements) from baseline [see Adverse Reactions (6.1)]. In the six-month study, 8% of DRUG-X treated patients were started on antihypertensive medications or required intensification of their antihypertensive medication regimen.
These BP increases can increase the risk of myocardial infarction and stroke with a greater risk in those with established cardiovascular disease or risk factors for cardiovascular disease.
Before initiating DRUG-X, consider the patient’s baseline cardiovascular risk and ensure blood pressure is adequately controlled. Check BP approximately 3 weeks after initiating DRUG-Xor increasing the dose and periodically thereafter. Re-evaluate whether the benefits of continued treatment with DRUG-X outweigh its risks in patients who develop cardiovascular risk factors or cardiovascular disease.
Example 6b orders information in the following way: (1) steps to take to prevent or manage the adverse reactions (2) description of the clinically significant adverse reaction, (3) risk factors. (Section 1 and 2 are reversed in Example 6b)

Example 6b.
5 WARNINGS AND PRECAUTIONS
5.1 Increase in Blood Pressure
Before initiating DRUG-X, consider the patient’s baseline cardiovascular risk and ensure blood pressure is adequately controlled. Check BP approximately 3 weeks after initiating DRUG-X or increasing the dose and periodically thereafter. Re-evaluate whether the benefits of continued treatment with DRUG-X outweigh its risks in patients who develop cardiovascular risk factors or cardiovascular disease.
In Studies 1 and 2, DRUG-X increased systolic blood pressure (BP) during six months of treatment by an average of 5.9 mmHg (ambulatory blood pressure monitoring) and by an average of 3.8 mmHg (blood pressure cuff measurements) from baseline [see Adverse Reactions (6.1)]. In the six-month study, 8% of DRUG-X treated patients were started on antihypertensive medications or required intensification of their antihypertensive medication regimen.
These BP increases can increase the risk of myocardial infarction and stroke with a greater risk in those with established cardiovascular disease or risk factors for cardiovascular disease.
Questions for Example Screen 6
Looking at the information in the WARNINGS AND PRECAUTIONS section in Examples 6a and 6b, which format do you prefer and why?

Research Questions
Should
the labeling include explicit instructions that a drug can be taken
with or without food?
For
dosage modifications due to drug interactions, are healthcare
providers more likely to look under the Highlights “Dosage
and Administration” heading or the Highlights “Drug
Interactions” heading?
For
dosage recommendations in specific populations (e.g., patients with
renal impairment), are healthcare providers more likely to look
under the Highlights “Dosage and Administration”
heading or the Highlights “Use in Specific Populations”
heading?
Section 5. Dosage Information in the Prescribing Information.
Should the labeling include explicit instructions that a drug can be taken with or without food?
For dosage modifications due to drug interactions, are healthcare providers more likely to look under the Highlights “Dosage and Administration” heading or the Highlights “Drug Interactions” heading?
For dosage recommendations in specific populations (e.g., patients with renal impairment), are healthcare providers more likely to look under the Highlights “Dosage and Administration” heading or the Highlights “Use in Specific Populations” heading?
Next, I have a few questions about the best way to include dosage information in the label.
EXAMPLE SCREEN 7
Some labels include specific instructions in the DOSAGE AND ADMINISTRATION section about whether the drug should be administered with or without food. Please take a minute and review the examples on the screen.

Example 7a.
2 DOSAGE AND ADMINISTRATION
“Take DRUG-X orally once daily with food.”
Example 7b.
2 DOSAGE AND ADMINISTRATION
“Take DRUG-X orally once daily with or without food”.
Example 7c.
Other labels do not include specific instructions about whether the drug should be administered with or without food, for example:
2 DOSAGE AND ADMINISTRATION
“Take DRUG-X orally once daily.”
Questions about Example Screen 7
1. If the DOSAGE AND ADMINISTRATION section said “Take Drug-X orally once daily” – Example 7c (and did not include “with or without food”) how would you interpret this information? [IF NEEDED: Would you think it needed to be taken either with food or without food, or would you think it didn’t matter if not specifically stated?)
2. If a drug can be taken with or without food, do you think it needs to explicitly state so in the DOSAGE AND ADMINISTRATION section – as in example 7b? Why or why not?
EXAMPLE SCREEN 8
 The
following questions refer specifically to the dosage and
administration information in the Highlights of Prescribing
Information. Please take a minute to review the example on the
screen.
The
following questions refer specifically to the dosage and
administration information in the Highlights of Prescribing
Information. Please take a minute to review the example on the
screen.
Highlights of Prescribing Information (PLR-Format Labeling)

Questions about Example Screen 8
Looking at this example, where specifically would you look to find information about how to modify a dosage because of drug interactions? [IF NEEDED: Under the “Dosage and Administration” heading or under the “Drug Interactions” heading?]
Is this (ANSWER) the best place for it, or should it be included somewhere else? IF SOMEWHERE ELSE, where? [INTERVIEWER NOTE: If participant says that it should be in multiple places, remind them that the Highlights section is meant to be a concise summary, so redundancy is avoided]
Where would you look for information about how to modify a dosage for use in a specific population (e.g., patients with renal impairment)? [IF NEEDED: Under the “Dosage and Administration” heading or “Use in Specific Populations” heading?]
Is this (ANSWER) the best place for it, or should it be included somewhere else? IF SOMEWHERE ELSE, where? [INTERVIEWER NOTE: If participant says that it should be in multiple places, remind them that the Highlights section is meant to be a concise summary, so redundancy is avoided]
Section 6. Drug Interactions
Research Questions
Placement of drug interaction information in the prescribing
information.
Optimal way to convey information on interacting drugs that involve
specific metabolic pathways (e.g., CYP450) and/or transporter
systems (e.g., P-gp) in the DRUG INTERACTIONS section.

Drug interaction information may be included in several sections of the label.
Questions
Where would you look for information on the “results of positive and pertinent negative results from specific clinical pharmacology studies, population analyses, or other modeling and simulation approaches that evaluate drug interactions?”
[SHOW ANSWER CATEGORIES ON SCREEN: Under “Dosage and Administration” section, “Drug Interactions” section or “Clinical Pharmacology” section?]
Is this (ANSWER) the best place for it, or should it be included somewhere else? IF SOMEWHERE ELSE, where?
Where would you look for information on the “Specific dosage modifications of the drug due to drug interactions?”
[SHOW ANSWER CATEGORIES ON SCREEN: Under “Dosage and Administration” section, “Drug Interactions” section or “Clinical Pharmacology” section?]
Is this (ANSWER) the best place for it, or should it be included somewhere else? IF SOMEWHERE ELSE, where?
Where would you look for information on the “recommendations to prevent and manage clinically significant drug interactions?”
[SHOW ANSWER CATEGORIES ON SCREEN: Under “Dosage and Administration” section, “Drug Interactions” section or “Clinical Pharmacology” section?]
Is this (ANSWER) the best place for it, or should it be included somewhere else? IF SOMEWHERE ELSE, where?
EXAMPLE SCREEN 9
The DRUG INTERACTIONS section of the label should include clinically significant known and potential drug interactions (DI), mechanisms of the DI, clinical implications of the DI, and practical instructions to prevent and manage the DI.
Please take a look at the following examples showing different ways of incorporating this information in the label. Note that the examples are identical except for the additional highlighted sentence at the end of each.
Example 9a: Include Only the Category of Interacting Drugs without
Examples
7 DRUG INTERACTIONS
Avoid use with strong CYP3A inhibitors. Concomitant use of DRUG X with strong CYP3A inhibitors increases drugoxide concentrations [see Clinical Pharmacology (12.3)] which may increase the risk of DRUG X adverse reactions.
Example 9b: Include a Few Representative Examples From FDA Sources
7 DRUG INTERACTIONS
Avoid use with strong CYP3A inhibitors. Concomitant use of DRUG X with strong CYP3A inhibitors increases drugoxide concentrations [see Clinical Pharmacology (12.3)] which may increase the risk of DRUG X adverse reactions. Examples of strong CYP3A inhibitors include but are not limited to ketoconazole, clarithromycin, and ritonavir.
Example 9c: Include an Entire List of Representative Examples from FDA Sources
7 DRUG INTERACTIONS
Avoid use with strong CYP3A inhibitors: Concomitant use of DRUG X with strong CYP3A inhibitors increases drugoxide concentrations [see Clinical Pharmacology (12.3)] which may increase the risk of DRUG X adverse reactions. Examples of strong CYP3A inhibitors include but are not limited to clarithromycin, cobicistat, conivaptan, elvitegravir and ritonavir, grapefruit juice, idelalisib, indinavir and ritonavir, itraconazole, ketoconazole, lopinavir and ritonavir, nefazodone, nelfinavir, paritaprevir and ritonavir and ombitasvir and dasabuvir, posaconazole, ritonavir, saquinavir and ritonavir, tipranavir and ritonavir, and voriconazole.
Example 9d: Include a Hyperlink to an FDA Drug Interactions Webpage
7 DRUG INTERACTIONS
Avoid use with strong CYP3A inhibitors. Concomitant use of DRUG X with strong CYP3A inhibitors increases drugoxide concentrations [see Clinical Pharmacology (12.3)] which may increase the risk of DRUG X adverse reactions. See www.fda.gov/DrugInteractions for examples of strong CYP3A inhibitors.
Questions for Example Screen 9
Which format do you like best and why?

Research Question
Would healthcare providers prefer that the Overdosage
section of the Prescribing Information not include details about
overdosage management and instead refer healthcare providers to the
Poison Control phone number, or would healthcare providers prefer
all the details related to overdosage management information
included in the Overdosage section?
Section 7. Overdosage Information in the Prescribing
Information
Would healthcare providers prefer that the Overdosage section of the Prescribing Information not include details about overdosage management and instead refer healthcare providers to the Poison Control phone number, or would healthcare providers prefer all the details related to overdosage management information included in the Overdosage section?
Finally, I’d like to ask you a question about information related to overdose.
Thinking about your use of the drug label, do you think that the OVERDOSAGE section of the label should include all details of the overdosage management information, or do you think that section should include a summary of the overdosage management information and provide the Poison Control Number phone number? Why do you say that?
Conclusion
These are all of my questions. Is there anything else you would like to share before we wrap up?
Thank you for your time. You will receive a check for [$200 for PCP/$300 for specialists] in the mail in approximately 4-6 weeks.
1 Gassman, A. L, Nguyen, C. P., and Joffe, H. V. (2017). FDA Regulation of Prescription Drugs. The New England Journal of Medicine, 376: 674-82. doi: 10.1056/NEJMra1602972
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Sullivan, Helen W (CDER) |
| File Modified | 0000-00-00 |
| File Created | 2021-01-15 |
© 2026 OMB.report | Privacy Policy
 Example
#1:
Example
#1: