ZPER Sampling Protocol
Att 10 Sampling ZPER Protocol_6_20_17.docx
Zika Postpartum Emergency Response Survey (ZPER), Puerto Rico, 2017
ZPER Sampling Protocol
OMB: 0920-1199
Appendix C
Sampling
C.1 Definition
The population of interest for the Puerto Rico Pregnancy Risk Assessment Monitoring System - Zika Postpartum Emergency Response (PRAMS-ZPER) is all women who are residents of Puerto Rico and who delivered a live-born infant in Puerto Rico during the surveillance period, and their available male partners. To draw a sample from this population, a sampling frame must be identified. Since 99.17% of the births in Puerto Rico occur in the hospital setting, PRAMS-ZPER will employ hospital-based data collection. The operational target population is all resident women delivering a live birth in selected field sites (Puerto Rican hospitals) during the surveillance period, and their available male partners.
In practice, the hospital-based sampling frame is identified by the hospital's delivery log. In most hospitals the delivery log represents a complete and accurate account of all births occurring in the hospital. It will be important to insure that the delivery log in each participating hospital is reliable and kept up-to-date. If this is not the case, alternate procedures to identify women who are eligible for PRAMS-ZPER will be developed specifically for that field site.
PRAMS-ZPER data collection will take place in all hospitals in Puerto Rico with 100 or more births per year. According to 2016 birth data obtained from Demographic Registry, 34 hospitals meet this eligibility criteria. However, one qualifying facility in the Metro region will not be included as a PRAMS-ZPER field site as their delivery ward closed down during September 2016, allotting a total of 33 field sites to PRAMS-ZPER 2.0 which will represent 97.74% of all births in Puerto Rico. Data collection will take place over a 4-month period in the fall of 2017. The overall sample size for ZPER 2.0 is 3,200 women and 2,000 male partners.
Subsequently, a sub-sample of participant women will be sampled to complete the PRAMS-ZPER Telephone Follow-up Survey.
C.2 Adjustments to the Sampling Frame
C.2.a Inclusions and Exclusions. Exclusions to the PRAMS-ZPER sampling frame may be implicit or explicit. Because of the definition of the PRAMS-ZPER targeted population and the use of the hospital delivery logs as the sampling frames, certain mothers will implicitly be excluded from eligibility in the ZPER sample. An implicit exclusion is any restriction inferred by the definition of the targeted population (i.e., non-residents of Puerto Rico and women who deliver at home or at hospitals with fewer than 100 births per year) or the choice of the hospital delivery log as the PRAMS-ZPER sampling frame (i.e., stillbirths and fetal deaths). All other exclusions arise from concerns or operational difficulties in sampling certain types of births, and are termed explicit exclusions.
Stillbirths and Fetal Deaths. By definition, the targeted population of PRAMS-ZPER is limited to pregnancies resulting in a live-born infant.
Multiple Gestation Pregnancies. Mothers with a multiple gestation regardless of the order will be included in the sample if at least one infant is delivered, alive, in the sampling window. Since the survey questions are about the mother and her pregnancy and not specifically about the baby, the mother will complete one survey for this pregnancy, regardless of how many babies were delivered.
Mothers Discharged Early From the Hospital or Otherwise Missed in the Hospital. For hospital-based supplementation, mothers discharged early from the hospital or otherwise missed in the hospital are not to be excluded from the sample. Nonetheless, no additional procedures for locating and contacting them will be developed. On the other hand, mothers transferred to another hospital participating in the PRAMS-ZPER survey will not be excluded. Location and follow-up of transferred mothers will be arranged.
Deceased Infants. Mothers of deceased infants will be excluded from the sample. The Neonatal Mortality Rate for 2016 births in Puerto Rico is 5.33. Based on 2016 neonatal mortality rates in Puerto Rico, approximately 16 infant deaths will occur during the study period. This number is too small to have any impact on the sample size calculations.
Mothers with Pregnancy Complications. Mothers who are ill or suffering from complications of pregnancy and delivery will not be excluded. Contact with these women may need to be delayed until their condition has improved. However, they should be approached to complete the survey before they are discharged from the hospital.
C.3 Sampling Plan
C.3a Sampling Scheme for PRAMS-ZPER. For PRAMS-ZPER surveillance, there is a particular interest from a public health perspective in making inferences by geographic region. Some regions do not represent a large portion of a Puerto Rico's overall population. To make inferences about specific subpopulations and make comparisons among several subpopulations, women in those subpopulations (commonly called strata) will need to be oversampled.
The main advantage of stratified sampling is that for a given overall sample size, stratifying will permit separate estimates of subgroups of interest and permit comparisons across these subgroups. The sampling plan is designed in order to allow investigators to inferences about the prevalence rates for maternal behaviors and knowledge related to Zika, and to make estimates with sufficient precision both overall in Puerto Rico and within selected strata (health regions). For PRAMS-ZPER, the 8 geographic health regions of Puerto Rico will serve as the strata. The 8 regions are Arecibo, Aguadilla, Bayamón, Caguas, Fajardo, Mayagüez, Metro, and Ponce. The sampling will be further stratified by hospital, although proportional allocation will be used (each hospital within a region will have the same sampling fraction). Unlike region, hospital is not a subgroup of analysis interest.
C.3b Determining Overall Sample Size. Required sample sizes for the questionnaire are determined in relation to the given proportion that is being estimated, at a given level of precision, and with a given level of statistical confidence. For specified levels of precision and confidence, the sample size required is at its maximum when the advance estimate (the number used in sample size calculations) of the proportion being estimated equals 0.50. PRAMS-ZPER data are used in estimates of proportions of risk factors that range from common (such as delivery paid for by Medicaid) to rare (such as a confirmed Zika diagnosis). Using 0.50 in sample size calculations leads to sufficiently large sample sizes, whatever the true population proportions are for the various risk factors.
The PRAMS-ZPER sampling plan is based upon stratified sampling by hospital within region. However, since proportional allocation by hospital is used, the formula for determining sample size for stratified sampling reduces to that used for simple random sampling. Based on the stratification measures found above, a sample size of about 400 (n = 400) is necessary in each stratum to estimate a prevalence for a dichotomous variable with a reasonable precision of 5% and a confidence level of 95%, assuming an infinitely large population size (N). The assumption of an infinitely large population will be violated in the oversampled strata. In any stratum where our desired sample size of 400 comprises more than 5% to 10% of the population, it is appropriate to apply the finite population correction (FPC). The FPC will reduce the desired sample sizes in such cases without compromising the precision of the estimates.
The formula for FPC is:
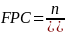
Where,
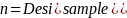
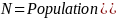
Mothers in some hospitals may be more difficult to contact than mothers in others. Thus, actual stratum sample sizes must be larger than theoretically needed to achieve a given level of statistical power. Based on the estimated stratum-specific response rates, the stratum-specific sample sizes will be inflated to ensure an adequate number of responses for analysis. Based on previous hospital-based surveillance in Puerto Rico and the US-Mexico border, an 81% response rate is assumed across all strata.
Births in Puerto Rico have been steadily declining in recent years. The most recent birth data by hospital available is for 2016. Since births have continued to decline, a sampling rate based on 2016 birth distributions would not achieve the desired sample size. Therefore, it is necessary to account for the declining birth rate. Based on estimated birth data for 2017, Table C.1 describes the drop in birth rates from 2007 to 2017. An adjustment factor of 1.054 will be used to estimate the number of 2017 births in each region.
Table C.1 |
||
Annual percent change (APC) for declining births in Puerto Rico from 2007 to 2016. |
||
Year |
Annual Births |
% Decline (APC) |
2007 |
46,750 |
|
2008 |
45,689 |
-2.27% |
2009 |
44,830 |
-1.88% |
2010 |
42,203 |
-5.86% |
2011 |
41,133 |
-2.54% |
2012 |
38,974 |
-5.25% |
2013 |
36,580 |
-6.14% |
2014 |
34,493 |
-5.71% |
2015 |
31,233 |
-9.45% |
2016 |
28,321 |
-9.32% |
2017 |
26,797 |
-5.38% |
Note. Values in italics are estimates. Data for 2016 is preliminary and was obtained from the Puerto Rico Demographic Registry on 03/06/2017. |
||
C.3c Steps for Establishing the Sample Rates
Establish the distribution of births in Puerto Rico by hospital. Obtain a list of births by hospital and identify within which health region each hospital is located. This list was provided by the Puerto Rico Health Department for 2016 births. Determine which hospitals have a sufficient number of births to support PRAMS-ZPER surveillance.
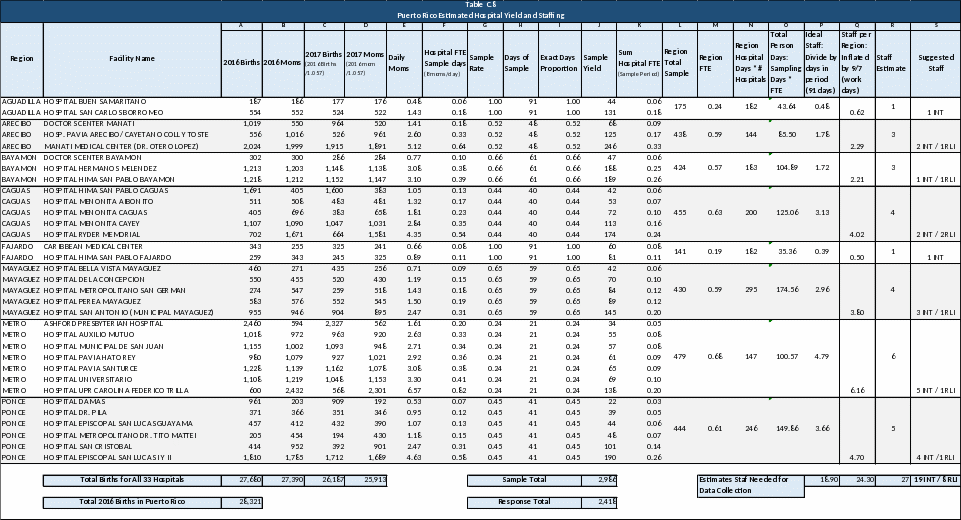
Select the hospitals where data collection will occur. Criteria for hospital selection should be defined. For ZPER, all hospitals with at least 100 births per year will be included. A total of 34 hospitals meet this eligibility criteria for PRAMS-ZPER; nonetheless, one qualifying facility in the Metro region was as their delivery ward closed down during September 2016. The adjustment leaves a total of 33 field sites for PRAMS-ZPER 2.0 which will represent 97.74% of all births in Puerto Rico. Table C.4 shows a list of the 33 selected field sites for PRAMS-ZPER 2.0 and the total number of births that occurred in that facility during 2016.
Calculate the number of eligible mothers. Mothers giving birth to double or higher plurality result in multiple births, but only one eligible mother. The multiple birth rate is 2.02% of total births. The number of distinct mothers for Puerto Rico during 2016 was determined (28,027) for each region. Thus the number of eligible mothers can be estimated as 98.96% of the total births.
Adjust for estimated declines in the birth rates from 2016 to 2017. The adjustment factor is calculated by dividing the total number of births during 2016 by the estimated births for 2017. We calculated the adjustment factor from the data in Table C.1. The adjustment factor is 1.054 for 2017 births. Divide by 1.054 to get adjusted eligible mothers for PRAMS-ZPER 2.0.
Determine the desired number of respondents in the sample. This number will be based in part upon costs and resources, but is often chosen to be 400, as an estimate of a proportion based upon 400 respondents will have a 95% confidence interval of +/- 5%.
Compute the Finite Population Correction (FPC), if applicable. See formula in section C.3b.
Estimate the completion rate of hospital-based data collection. Based on PRAMS-ZPER previous hospital-based survey implementation using the same in-hospital methodology, a total of 2,364 women completed the survey from 2,933 eligible births (80.60%). An 81% response rate is assumed across all strata. Divide the FPC Corrected Sample Size by the estimated response rate to determine the estimated sample size adjusted for non-respondents (final sample size).
Complete Table C.2 using the result of steps 3 through 7 to fill in the appropriate columns.
Carry the adjusted population size and estimated adjusted sample size from Table C.2 to Table C.3.
Compute the population size for the 3-month surveillance period. Determine the annual birth distribution by quarter, and establish the percent of births observed per quarter. Later determine the percent of births for the surveillance period to validate that there is no variation in births during the proposed sampling period. The annual births will be divided into quarters, thereby in order to calculate the population size for the 3-month surveillance period the annual population size must be divided by 4 (number of quarters in a year). Puerto Rico’s births are divided equitably throughout all quarters (25% of births per quarter).
Establish the sampling fraction. The sampling fraction is the proportion of the sample that will be included in the sample. The sampling fraction is calculate by dividing the desired sample size (ZPER Estimated Adjusted Sample Size) by the population size (ZPER Estimated Population Size during the 3 Month Period). See formula for sampling fraction below:
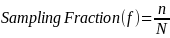
Where,

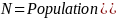
Calculate the Operational Sample Size. The operational sample size is the expected sample size based on the number of births actually occurring in each region. The operational sample size is calculated by multiplying the Estimated Sample Size (ZPER Estimated Adjusted Sample Size) times the sampling fraction (f). Note that in two regions, Fajardo and Aguadilla, the calculated operational sample size is higher than the Estimated Population Size for the Sampling Period (ZPER Estimated Population Size during 3 Month Study Period). Meaning all women having a live birth during the surveillance period will be included.
Determine the sampling fraction in days. The three month sampling period (September 2017 to November 2017) has a total of 91 days. From the sampling fraction, determine the number of days over the 3 month (91 day) surveillance period during which sampling will be conducted.
Using the number of days during which sampling will be conducted, determine the intervals for sampling. Using the example of 2 out of every 15 days, the two days should be randomly chosen at one hospital. For nearby hospitals, the sampling days can be shifted by one or two days to distribute the workload. Care should be taken to vary the days of the week that sampling occurs throughout the surveillance period. Specific sampling schedules for each field site will be provided by CDC.
Region |
Total Number of Live Births |
Live Births in Eligible Hospitals |
Total ZPER Eligible Population of Mothers (adjusting for non-residents and Multiple Births) in Eligible Hospitals |
ZPER Population Size Adjusted for Projected 2017 decline in births |
Estimated Unadjusted Sample Size |
FPC Corrected Sample Size |
Estimated Sample Size for Non-Respondents (81% based on ZPER 1.0) |
AGUADILLA |
741 |
741 |
738 |
700 |
400 |
254 |
314 |
ARECIBO |
3,599 |
3,599 |
3,561 |
3,379 |
400 |
358 |
441 |
BAYAMON |
2,733 |
2,733 |
2,713 |
2,574 |
400 |
346 |
427 |
CAGUAS |
4,416 |
4,416 |
4,362 |
4,139 |
400 |
365 |
450 |
FAJARDO |
602 |
602 |
595 |
565 |
400 |
234 |
289 |
MAYAGUEZ |
2,822 |
2,822 |
2,792 |
2,649 |
400 |
347 |
429 |
METRO |
8,549 |
8,549 |
8,390 |
7,960 |
400 |
381 |
470 |
PONCE |
4,218 |
4,218 |
4,170 |
3,956 |
400 |
363 |
448 |
OTHER |
641 |
|
|
|
|
|
|
TOTAL |
28,321 |
27,680 |
27,321 |
25,922 |
3,200 |
2,649 |
3,270 |
Table C.3 |
||||||
PRAMS-ZPER 2.0 Sampling Fractions and Estimated Sample Sizes |
||||||
Region |
ZPER Population Size Adjusted for Projected 2017 decline in births |
ZPER Estimated Population size During 3 Month Study Period |
ZPER Estimated Adjusted Sample Size |
|
Operational Sample Size |
f in days (number of days to sample out of the 91 days) |
Aguadilla |
700 |
175 |
314 |
1.00 |
175 |
91 |
Arecibo |
3,379 |
845 |
442 |
0.52 |
442 |
48 |
Bayamón |
2,574 |
644 |
427 |
0.66 |
427 |
60 |
Caguas |
4,139 |
1,035 |
450 |
0.44 |
450 |
40 |
Fajardo |
565 |
141 |
289 |
1.00 |
141 |
91 |
Mayagüez |
2,649 |
662 |
429 |
0.65 |
429 |
60 |
Metro |
7,960 |
1,990 |
470 |
0.24 |
470 |
22 |
Ponce |
3,956 |
989 |
448 |
0.45 |
448 |
41 |
Total |
25,922 |
6,481 |
3,269 |
|
2,982 |
|
Source: Demographic Registry 2016 preliminary data. |
||||||
Table C.4 |
||
PRAMS-ZPER 2.0 Selected Field Sites, Hospitals |
||
Region |
Field Site Name (Hospital Name) |
Total Births During 2016 |
AGUADILLA |
HOSPITAL BUEN SAMARITANO |
187 |
AGUADILLA |
HOSPITAL SAN CARLOS BORROMEO |
554 |
ARECIBO |
DOCTORS CENTER MANATI |
1,019 |
ARECIBO |
HOSP. PAVIA ARECIBO/ CAYETANO COLL Y TOSTE |
556 |
ARECIBO |
MANATI MEDICAL CENTER (DR. OTERO LOPEZ) |
2,024 |
BAYAMON |
DOCTORS CENTER BAYAMON |
302 |
BAYAMON |
HOSPITAL HERMANOS MELENDEZ |
1,213 |
BAYAMON |
HOSPITAL HIMA SAN PABLO BAYAMON |
1,218 |
CAGUAS |
HOSPITAL HIMA SAN PABLO CAGUAS |
1,691 |
CAGUAS |
HOSPITAL MENONITA AIBONITO |
511 |
CAGUAS |
HOSPITAL MENONITA CAGUAS |
405 |
CAGUAS |
HOSPITAL MENONITA CAYEY |
1,107 |
CAGUAS |
HOSPITAL RYDER MEMORIAL |
702 |
FAJARDO |
CARIBBEAN MEDICAL CENTER |
343 |
FAJARDO |
HOSPITAL HIMA SAN PABLO FAJARDO |
259 |
MAYAGUEZ |
HOSPITAL BELLA VISTA MAYAGUEZ |
460 |
MAYAGUEZ |
HOSPITAL DE LA CONCEPCION |
550 |
MAYAGUEZ |
HOSPITAL METROPOLITANO SAN GERMAN |
274 |
MAYAGUEZ |
HOSPITAL PEREA MAYAGUEZ |
583 |
MAYAGUEZ |
HOSPITAL SAN ANTONIO (MUNICIPAL MAYAGUEZ) |
955 |
METRO |
ASHFORD PRESBYTERIAN HOSPITAL |
2,460 |
METRO |
HOSPITAL AUXILIO MUTUO |
1,018 |
METRO |
HOSPITAL MUNICIPAL DE SAN JUAN |
1,155 |
METRO |
HOSPITAL PAVIA HATO REY |
980 |
METRO |
HOSPITAL PAVIA SANTURCE |
1,228 |
METRO |
HOSPITAL UNIVERSITARIO |
1,108 |
METRO |
HOSPITAL UPR CAROLINA FEDERICO TRILLA |
600 |
PONCE |
HOSPITAL DAMAS |
961 |
PONCE |
HOSPITAL DR. PILA |
371 |
PONCE |
HOSPITAL EPISCOPAL SAN LUCAS GUAYAMA |
457 |
PONCE |
HOSPITAL METROPOLITANO DR. TITO MATTEI |
205 |
PONCE |
HOSPITAL SAN CRISTOBAL |
414 |
PONCE |
HOSPITAL EPISCOPAL SAN LUCAS I Y II |
1,810 |
TOTAL BIRTHS IN ZPER SELECTED FACILITIES |
27,680 |
|
Source: Demographic Registry 2016 preliminary data. |
||
C.4 Selection of Sample
Proportional sampling within each stratum is used for drawing the sampling schedule based on the time of birth. The time and date of birth is written on the hospital delivery log. All births for a particular region that fall within the pre-established sampling time intervals for that region are selected for the study provided they do not satisfy an exclusion criterion. Where possible, sampling intervals will consist of complete days (midnight to midnight) for ease of selection. However, there may be some regions where the sampling interval will include partial days. The sampling schedule is designed to be balanced by weeks in the surveillance period and days within each week.
Based on the desired sample size and the number of live births occurring at each hospital for each region, sampling fractions can be computed. The length of the sampling interval will vary by region and is determined from the sampling fractions. For a multiple birth, the mother is selected only once. The selection procedures must satisfy the probability requirements of the sample. The sample is chosen so that, within each region, each record has an equal probability of being selected. Based on these probabilities, weights can be determined for island-wide estimates.
Because of shorter hospital delivery stays and earlier discharges of mothers, the hospital delivery log must be frequently monitored during defined sampling intervals. No more than 24 hours should lapse between the beginning of the sampling interval and when the delivery log is checked. Similarly no more than 24 hours should lapse between checks of the delivery log within a sampling interval. For regions with 100% sampling, birth logs should be checked a minimum of every other day.
C.5 Paternal In-hospital Sampling
A convenience sample will be done for paternal surveying as we are unaware of the population of fathers that will be available during the interviewers visit during maternal in-hospital surveying. Convenience sampling is a non-probability sampling, meaning that the sample is not randomized, and fathers will be selected based on their presence in the hospital during the time the surveyor is contacting mothers or subsequent time prior to the mother being discharged. Only fathers of qualifying mothers will be approached. Additional procedures can be developed to address fathers of qualifying infants that are not present during the interviewer’s initial visit.
The sample size for paternal surveying is expected to be 40-60% of the sample size determined for mothers. The sample size determined for PRAMS-ZPER 2.0 is 2,975, meaning father sampling will be between 1,193 and 1,789. Table C.5 shows the sample distribution by region and the daily estimates to achieve proposed goal.
Sample size for fathers will be dependent on encountering the father in the hospital during the period in which the mother is eligible to be interviewed. Completion of paternal survey does not replace the need to promote postpartum women’s participation. In the event that women decide to be non-participants, available fathers may still be considered for participation. Paternal in-hospital data collection modes will include paper/pen, tablet, or laptop.
Table C.5 |
||||||
ZPER Estimated Sample Sizes for Paternal Survey and Daily Estimates. |
||||||
Region |
PRAMS-ZPER Operational Sample Size (from Table C.3) |
Paternal Estimated Sample Size (40%) |
Paternal Estimated Sample Size (60%) |
f in days (from Table C.3 ) |
Daily Estimate of Paternal Survey based in daily f (40%) |
Daily Estimate of Paternal Survey based in daily f (60%) |
Aguadilla |
175 |
70 |
105 |
91 |
1 |
1 |
Arecibo |
442 |
177 |
265 |
48 |
4 |
6 |
Bayamón |
427 |
171 |
256 |
60 |
3 |
4 |
Caguas |
450 |
180 |
270 |
40 |
5 |
7 |
Fajardo |
141 |
56 |
85 |
91 |
1 |
1 |
Mayagüez |
429 |
172 |
257 |
60 |
3 |
4 |
Metro |
470 |
188 |
282 |
22 |
9 |
13 |
Ponce |
448 |
179 |
269 |
41 |
4 |
7 |
Total |
2,982 |
1,193 |
1,789 |
|
|
|
C.6 Educational Intervention Sampling
The PRAMS-ZPER Educational Intervention (EI) will be provided to all eligible women and available partners, regardless of their participation.
C.7 Telephone Follow-Up Survey Sampling
C.7a Definition. The population of interest for the Puerto Rico PRAMS Zika Postpartum Emergency Response (PRAMS-ZPER) Telephone Follow-up survey is Puerto Rican residents who delivered a live-born infant in Puerto Rico and participated in the PRAMS-ZPER hospital-based survey in the fall of 2017.
To draw a sample from this population, a sampling frame must be identified. The original PRAMS-ZPER study used a hospital-based sampling frame identified by the hospital's delivery log in all hospitals in Puerto Rico with 100 or more births per year. Table C.4 shows all eligible field sites for PRAMS-ZPER 2.0 data collection.
The PRAMS-ZPER Telephone Follow-up Survey (TS) will target a subset of the in-hospital study participants. The Puerto Rico Department of Health will match information from PRAMS-ZPER respondents with the birth certificate record of their baby. The list of original study participants who can be matched to a birth certificate record will serve as the sampling frame. The sampling frame is estimated to include women who gave birth between September 2017 and November 2017, this is the estimated time period for births eligible for the PRAMS-ZPER In-hospital survey. Potentially, women who give birth in December 2017 may be included if sampling adjustments are required during in-hospital data collection. Since the ZPER follow-up survey will be focused on postpartum behaviors, recall should not be a concern. Thus, there is no need to impose age limits on the infants included in the study. Among ZPER participants, a proportional sample by region will be drawn.
C.7b Inclusions and Exclusions. Exclusions to the PRAMS-ZPER Telephone Follow-up Survey sampling frame may be implicit or explicit. Because of the definition of the PRAMS-ZPER targeted population and the need to match PRAMS-ZPER data with birth certificate data to identify birth to PRAMS-ZPER participants, certain mothers will implicitly be excluded from eligibility in the PRAMS-ZPER follow-up sample. An implicit exclusion is any restriction inferred by the definition of the targeted population (i.e., women who did not participate in PRAMS-ZPER or PRAMS-ZPER respondents who gave birth outside the stated time period) or the choice of the list of PRAMS-ZPER respondents matched to a birth certificate record as the PRAMS-ZPER follow-up sampling frame (i.e., records that could not be matched to a birth certificate). All other exclusions arise from concerns or operational difficulties in sampling certain types of births, and are termed explicit exclusions.
Deceased infants. Mothers of infants who have died will be included in the sample if they completed the PRAMS-ZPER survey.
Multiple Gestation Pregnancies. Mothers with a multiple gestation regardless of the order will be included in the sample. In PRAMS-ZPER, the mother was asked to respond to the infant questions for just one of her randomly selected infants. The same randomly selected infant will be eligible for the PRAMS-ZPER follow-up survey. All other infants from the pregnancy will be excluded.
Mothers with Zika-affected or special needs infants. Mothers whose infants have microcephaly or other birth defects or special health conditions will not be excluded. If they are contacted, the interviewer should be aware of extra sensitivity of questions related to the baby’s health.
Zika Positive Sampling. Due to the need of extensive evaluation and follow-up for women receiving Zika-related post-natal services, 100% of women who are identified as having Zika virus infection during their pregnancy will be included in the PRAMS-ZPER TS.
C.7c Sampling Plan. For the PRAMS-ZPER follow-up study, there is a particular interest from a public health perspective in making island-wide inferences. The PRAMS-ZPER In-Hospital sampling design was a stratified by region. Thus smaller regions are overrepresented in the PRAMS-ZPER sample. Since region-specific estimates are not needed for the PRAMS-ZPER Telephone Follow-up Survey, proportional sampling by region is recommended.
The main advantage of proportional sampling is that regions will be sampled in accordance with their contribution to overall births on the island, yet the sample size is more manageable than it would be if stratified sampling by region were used. The sampling plan is designed so that island-wide inferences about prevalence rates for maternal behaviors and knowledge of Zika can be estimated with sufficient precision. For PRAMS-ZPER, 8 geographic regions will be sampled proportionally: Arecibo, Aguadilla, Bayamón, Caguas, Fajardo, Mayagüez, Metro, and Ponce.
C.7d Determining Overall Sample Size. Required sample sizes for the TS are determined in relation to the given proportion that is being estimated, at a given level of precision, and with a given level of statistical confidence. For specified levels of precision and confidence, the sample size required is at its maximum when the advance estimate (the number used in sample size calculations) of the proportion being estimated equals 0.50. PRAMS-ZPER data are used in estimates of proportions of risk factors that range from common (such as delivery paid for by Medicaid) to rare (such as a confirmed Zika diagnosis). Using 0.50 in sample size calculations leads to sufficiently large sample sizes, whatever the true population proportions are for the various risk factors.
The PRAMS-ZPER TS sampling plan is based upon proportional sampling by region. However, since proportional allocation by region is used, the formula for determining sample size reduces to that used for simple random sampling. We will establish the necessary sample size in order to estimate a prevalence for a dichotomous variable with a reasonable precision of 3% and a confidence level of 95%, assuming an infinitely large population size (N). The assumption of an infinitely large population will be violated since our population size estimated to be 6,833 based on 2016 birth data and adjustment for estimated decline (see Table C.1). If the estimated sample size comprises more than 5% to 10% of the population, it is appropriate to apply the finite population correction (FPC). The FPC will reduce the desired sample sizes in such cases without compromising the precision of the estimates. The formula for FPC can be seen in section C.3b.
Since all sampled mothers will not participate, sample sizes must be larger than theoretically needed to achieve a given level of statistical power. Based on the estimated response rate for the telephone follow-up, the sample sizes will be inflated to ensure an adequate number of responses for analysis. Based on previous surveys implemented survey, ESMIPR (Maternal-Infant Study of Puerto Rico, English for Estudio Materno-Infantil de Puerto Rico) that followed a similar methodology to that implemented by PRAMS-ZPER (in-hospital contact and a follow-up survey), a 60% response rate to the follow-up survey is assumed.
C.7e Steps for Establishing Sample Rates. (Will be completed once PRAMS-ZPER HS data collection is completed)
Identify eligible mothers. Determine the overall resident live-births in Puerto Rico that occurred throughout the sampling months (estimated to be September to November 2017). Additionally, determine the number of mothers who gave birth on PRAMS-ZPER sampling days. Determine the number of women who had a live-birth during the sampling period and completed the survey.
Link mothers with birth certificates. PRAMS-ZPER participants must be matched to their infant’s birth certificate in order to collect current contact information. The Puerto Rico Department of Health is performing the linkage based on 5 fields, mother’s name, mother’s date of birth, baby’s date of birth, hospital of delivery, and method of delivery. Based on PRAMS-ZPER 1.0 activities, there is a 99.7% matching rate. The PRAMS-ZPER participants who are successfully matched to their infant’s birth certificate record form the sampling frame for the PRAMS-ZPER Telephone Follow-Up survey.
Determine the desired number of respondents in the sample. This number is based upon the desired precision level for study estimates. For the follow-up, a precision level of +/- 3% is desired. The estimated proportion of respondents will have a 95% confidence interval of +/- 3%.
Compute the Finite Population Correction (FPC), if applicable. The formula for FPC can be seen in section C.3b.
Estimate the participation rate. Based on previous hospital-based surveillance in Puerto Rico that included a telephone follow-up component, a 60% response rate is expected.
Compute final adjusted sample size. Divide the FPC corrected sample size by the estimated response rate (0.60) to determine the final sample size.
Identify all births to Zika-positive women, based on their self-reported information on the hospital survey, and include them in the sample. Based on the distribution of all births during the PRAMS-ZPER study period by region, proportionally allocate the remaining births among the 8 regions.
Complete Table C.6 using the result of steps 3 through 7 to fill in the appropriate columns.
Carry the adjusted population size and estimated adjusted sample size from Table C.6 to Table C.7.
Divide the final adjusted sample size by the number of PRAMS-ZPER participants to compute the sampling fraction for each region.
From the sampling fraction, determine the operational sample size. The operational sample size is calculated by multiplying the Estimated Sample Size times the sampling fraction (f). if the obtained operational sample is less than the Estimated adjusted sample size, use the estimated adjusted sample size value as the operational sample size. Note that in the Metro region, all PRAMS-ZPER participants linked with a birth certificate will be included.
Table C.6 |
|||||||
Calculation of PRAMS-ZPER Telephone Follow-up Survey Sample Size |
|||||||
Region |
All Live Births* |
Proportion of Births (%) |
PRAMS-ZPER Participants |
Adjustments for Unmatched Records |
Estimated Unadjusted Sample Size for PRAMS-ZPER Follow-up Study |
FPC Adjusted Sample Size# |
Estimated Sample Size Adjusted for Nonresponse |
Aguadilla |
|
|
Sample
Table |
|
|
|
|
Arecibo |
|
|
|
|
|
|
|
Bayamón |
|
|
|
|
|
|
|
Caguas |
|
|
|
|
|
|
|
Fajardo |
|
|
|
|
|
|
|
Mayagüez |
|
|
|
|
|
|
|
Metro |
|
|
|
|
|
|
|
Ponce |
|
|
|
|
|
|
|
Total |
|
|
|
|
|
|
|
*Numbers adjusted for multiple births and non-residents of Puerto Rico. |
|||||||
Table C.7 |
||||
PRAMS-ZPER Sampling Fractions and Estimated Sample Sizes |
||||
Region |
PRAMS-ZPER
Participants Adjusted for Matching (from
Table C.6)
|
Estimated
Adjusted Sample Size (from
Table C.6)
|
|
Operational Sample Size
=
|
Aguadilla |
|
|
|
|
Arecibo |
|
|
|
|
Bayamón |
|
|
|
|
Caguas |
|
|
|
|
Fajardo |
|
|
|
|
Mayagüez |
|
|
|
|
Metro |
|
|
|
|
Ponce |
|
|
|
Sample
Table |
Total |
|
|
|
|
Both Table C.6 and Table C.7 will be completed once PRAMS-ZPER HS data collection is completed.
C.7f Selection of Sample. Proportional sampling is used for drawing the sample. First all births to Zika-positive mothers will be identified and included in the sample. Then all remaining matched birth certificates will be first separated by region. Within each region the birth certificates will be sorted by hospital and date of birth within hospital. The sampling rates for each region will be applied to randomly select the birth certificates for the follow-up. For the Metro region, all matched births will be included in the sample. For the other regions, the sample is chosen so that, within each region, each record has an equal probability of being selected. Based on these probabilities, weights can be determined for island-wide estimates.
ZPER
2.0 Sampling | Page
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | LDF8 |
| File Modified | 0000-00-00 |
| File Created | 2021-01-21 |
© 2026 OMB.report | Privacy Policy