OMB approval memo
0695 OMB Approval Memo IMPROVE.doc
Data to Support Drug Product Communications
OMB approval memo
OMB: 0910-0695
FDA DOCUMENTATION FOR THE GENERIC CLEARANCE,
“TESTING
COMMUNICATIONS ON DRUGS PRODUCTS”
(0910-0695)

.
TITLE OF INFORMATION COLLECTION: Identifying Messages to Promote Value and Education (IMPROVE) of Generic Prescribing (Pilot Testing)
DESCRIPTION OF THIS SPECIFIC COLLECTION
Statement of need:
The Food and Drug Administration (FDA), Center for Drug Evaluation and Research (CDER), Office of Generic Drugs (OGD) is seeking OMB approval under the generic clearance 0910-0695 to conduct pilot testing of messages for the project “Identifying Messages to Promote Value and Education (IMPROVE) of Generic Prescribing.”
Based on the supporting statement for generic clearance 0910-0695,1 the purpose of information collection under this generic clearance is “to identify strengths and weaknesses of current services and make improvements in service delivery based on feedback.” One of the services that FDA offers is the delivery of quality and affordable generic drugs. For certain classes of drugs, the brand drug is preferred, despite the cost-savings conferred through use of the generic alternative. The specific collection described in this memo aims to assess the need for communications directed at healthcare providers on the use of generic drugs for certain drug classes, to enhance FDA’s delivery of generic drugs. Exhibit 1 illustrates the full set of research phases for this project. Phases 1 and 2 have been previously approved. Exhibit 1 illustrates the full set of research phases for this project. Please note that this information collection request concerns only Phase 3.
Exhibit 1. Overview of Research Phases
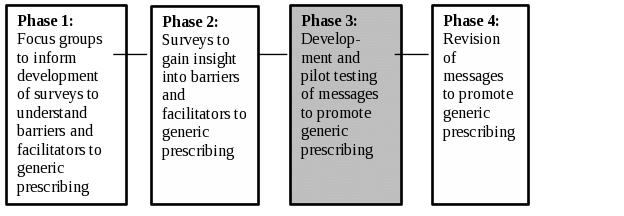
Congress passed the Generic Drug User Fee Amendments (GDUFA, Title III of the Food and Drug Administration Safety and Innovation Act (Public Law 112-144)) in July 2012. Under GDUFA, FDA obtains industry and public input to create regulatory science initiatives regarding research on generic drugs that advance public health.2 Once marketed, certain generic drugs are often not preferred over brand drugs3 4 5 6 even though generic drugs generally cost less than brand drugs.7 After identifying the barriers to prescribing generics, there is a need to develop and pilot test of messages to promote generic prescribing catered around these barriers. To address this regulatory science need regarding generic drugs, the FDA entered into a cooperative agreement with the University of Chicago (UChicago) (Grant number 1U01FD005485-01).
As prescribers of brand or generic drugs, healthcare providers can influence consumer use of generic drugs. Many healthcare providers have been slow to adopt generic drugs despite data suggesting clinical equivalence between generic and brand drugs.
In primary care practice, both clinicians and patients’ express concerns regarding use of certain generic drug classes such as antidepressants, oral contraceptives, and cholesterol lowering drugs. Through a cooperative agreement with UChicago, development and pilot testing of messages to promote generic prescribing will be deployed.
Intended use of information:
Development and pilot testing of messages to promote generic prescribing will be used to evaluate and improve the effectiveness of messages.
Information collected in the Phase 3 pilot testing will be used to inform the improvement of educational messages regarding generic drugs (Phase 4). By understanding the effectiveness of messaging, messages to promote generic prescribing can be improved.
Description of respondents:
Development and pilot testing of messages to promote generic prescribing will be disseminated through the American College of Physicians (ACP) and the American Association of Nurse Practitioners (AANP).
ACP is the largest medical-specialty organization and the second largest physician group in the United States. General internist primary care physicians typically provide comprehensive longitudinal care and coordinate complex treatment for adults. The Internal Medicine Insider Research Panel is a community of approximately 1100 U.S. ACP members and 300 non-members who participate in research surveys distributed by the ACP Research Center.
AANP is a union of the American Academy of Nurse Practitioners and the American College of Nurse Practitioners. It is currently the largest full-service national professional membership organization for nurse practitioners (NPs) of all specialties. AANP maintains the only national NP database of all practicing NPs. NPs are authorized to prescribe medications in all fifty states and Washington, D.C.
The AANP Network for Research (AANPNR) is a practice-based research network open to all members and allows them to participate in research surveys distributed by the AANP. The AANPNR consists of approximately 1400 NPs from a variety of practice settings.
The ACP and AANP serve as collaborators in this study under the 1U01FD005485-01 collective agreement. The well-established networks and infrastructures of the ACP and AANP guarantee the success of this study by ensuring feasibility and a representative recruitment pool that can be surveyed with minimal logistical burden.
Date(s) to be Conducted:
Messaging and pilot testing will be launched and conducted electronically in April-June 2018.
How the Information is being collected:
Target Participants
Pilot testing of messages to promote generic prescribing will utilize ACP and AANP’s preexisting research panel of participants detailed above. Both panels select participants using stratified random sampling. Participants fill out profiles to confirm their eligibility and ensure the panels are geographically and demographically representative of the organization’s membership. These profiles include information including member ID numbers, names, gender, member class, primary specialty, practice site, and percentage of time spent in patient care. These profiles are updated by the organizations regularly. Non-members are recruited to the panel using a similar profile database to be representative of the larger provider population.
Using their respective database of information on panel participants, ACP and AANP will work with UChicago to identify eligibility for pilot testing of messages to promote generic prescribing to ensure participants have adequate experience with patients and the messaging subject matter. The eligibility screening process is conducted using a preexisting database and bears no burden on participants.
Pilot Module
Eligible participants will receive an email inviting them to complete a pilot module consisting of a video (Appendix A: Script), a PowerPoint presentation (Appendix B: Presentation), and a post-test (Appendix C: Post Test) via a URL embedded in the email. The module will be hosted via a UChicago CME channel. The video portion of the pilot module is designed to represent a typical scenario of a clinician prescribing an oral contraceptive. Following the video, a short PowerPoint is presented to provide further education regarding generic prescribing. Finally, a post-test will be taken to access the effectiveness of the video and PowerPoint on presenting messaging to promote generic prescribing.
Recruitment Procedures
All email communication regarding the pilot module will be from the respective organizations’ CEO or Director of the Research Center. Emails will contain the ACP/AANP logo and will be formatted to match standard messaging practices of the respective organization.
One week after the initial invitation email, up to 10 email reminders will be sent at intervals of 4-21 days to participants who have not yet completed the post-test portion of the pilot module. The post-test will be closed and all contact regarding the pilot module will end when an approximate response rate of 50% is reached or when all planned contact has been completed.
Emails to participants will follow the standard format and structure used by the ACP and AANP (Appendix D: ACP/AANP Example Emails). Emails will include basic information about the messaging topic, expected length of time to complete the module (video, PowerPoint, and post-test), privacy information, and consent process. As with other AANP and ACP panel pilot tests, participants’ participation and submission of the post-test is considered implied consent. Participation is voluntary and participants may choose to not participate at any time.
Recruitment Size
Given the eligibility criteria for pilot testing, the target sample sizes for the ACP and AANP pilot tests are 850 participants and 900 participants, respectively, with an approximate goal response rate of 50% based on past surveys administered by these organizations. Participants will access the pilot module via a URL provided in the recruitment emails. The email will include an introductory section (Appendix D) including the statement, “submission of the post-test is considered implied consent.”
Confidentiality of Respondents:
UChicago and FDA will comply with safeguards for ensuring participant information is kept private to the extent permitted by law. All data will be collected with an assurance that your identity and information will remain private to the extent permitted by law. The pilot module introduction will contain a statement that participant responses will be kept private to the extent permitted by law.
Personally identifiable information, including membership ID numbers, names, and emails, are used by ACP and AANP in order to contact participants and re-contact non-respondents for this survey. All identifiable data is password-protected and stored on a secured platform according to each organization’s respective data security and confidentiality protocols. Only ACP/AANP research staff working on this project will have access to identifiable data. Once survey responses are collected by ACP/AANP, deidentified data sets are sent to UChicago for combined analysis.
All results from the study will be reported only in the aggregate. Data will be kept for six years after the close of the study, per UChicago Institutional Review Board (IRB) policy. At the end of this period, written documentation will be destroyed according to proper University of Chicago protocols and channels regulated by the IRB and Federal Policy for the Protection of Human Subjects. Any digital data will be destroyed using commercial software applications designed to remove all data from the storage device. Digital data will be password protected and accessed using devices maintained on secure servers with regular and secured back-up.
Amount and justification for any proposed incentive
Compensation for pilot test participants is proposed in accordance with the ACP and the AANP standard practices regarding any research activities involving their research networks. To maintain equipoise with other ACP and AANP studies, physician and nurse practitioner survey respondents are proposed to receive a $10 Amazon gift card.
The incentive is not a reward or salary. Rather, it is a stimulus to ensure adequate participation, high-quality data, and that participants are reasonably representative of the ACP and AANP membership.
Questions of a Sensitive Nature
There will be no questions of a sensitive nature asked of participants.
Description of Statistical Methods
Kirkpatrick Model: Our evaluation plan will use the Kirkpatrick model, which specifies four levels of educational outcomes for a specific training program for health professionals. The model acknowledges that while the goal of training is to impact society, measuring the impact of any educational program targeting health professionals is increasingly difficult as you move up the pyramid. Using this framework, we will assess if the pilot module translates into behavior change. While the Kirkpatrick model includes patient outcomes as the pyramid apex, we will primarily focus on the first three levels, but will also brainstorm how to collect data on impact to society. We will plan to test the pilot module with NPs and PCPs who participated in survey from Phase 2.
Satisfaction: Learner satisfaction will be assessed using Likert-type satisfaction questions. (Very Unsatisfied to Very Satisfied) in addition to open-ended response items for constructive feedback on how to make the educational program better for widespread dissemination. Here are examples: “I found this module useful”, “as a result of this module, I have less distrust of generic OCPs”, “as a result of this module, I will discuss generic OCPs with my patients”, and “as a result of this module, I will prescribe generic OCPs.”
Knowledge: Knowledge will be surveyed using a pre- and post- knowledge test regarding generic drugs. The test will be developed based on the knowledge gaps identified in focus groups and literature review. Item writing theory will be utilized to ensure test questions are clear and written to assess the specified learning objectives and evaluate the outcomes of interest.
Behavior: Participant behavior will be measured first using commitment to change questions immediately after the pilot module (i.e. “how likely are you to order generic antidepressants as a result of this intervention”). In addition, we will select the vignettes from the initial survey with baseline low levels of willingness to prescribe generic drug to determine if the willingness to prescribe generic drug has changed. Here are example questions: “Which of the following is TRUE about generic OCPs?” “Which is NOT considered a barrier when prescribing generic OCPs?” “When generic drugs are therapeutically equivalent, it means that which of the following are required to be the same?”
BURDEN HOUR COMPUTATION (Number of responses (X) estimated response or participation time in minutes (/60) = annual burden hours):
The pilot module takes approximately 15 minutes to complete.
Type/Category of Respondent |
No. of Respondents |
Participation Time (minutes) |
Burden (hours) |
General internist primary care physicians (ACP) |
425 |
15 |
106.25 |
Nurse practitioners (AANP) |
425 |
15 |
106.25 |
Total |
850 |
30 |
212.50 |
REQUESTED APPROVAL DATE: Approval is requested no later than March 2018.
NAME OF PRA ANALYST & PROGRAM CONTACT:
Ila S. Mizrachi
Paperwork Reduction Act Staff
301.796.7726
Oluwamurewa Oguntimein MHS, CHES
LCDR-USPHS
Social Scientist, Division of Therapeutic Performance
Office of Research Standards
301.796.4869
FDA CENTER: Center for Drug Research and Evaluation (FDA/CDER)
? Scher, S. (2013) The Branded Advantage. Ophthamol Mgmt. July: p18
http://www.ophthalmologymanagement.com/printarticle.aspx?articleID=108618
? Alloway, RR, Isaacs R, Lake K, Hoyer P, First R, Helderman H, Bunnapradist S, Leichtman A, Bennett MW, Tejani A, Takemoto SK. (2003) Report of the American Society of Transplantation Conference on Immunosuppressive Drugs and the Use of Generic Immunosuppressants. A J Transpl 3: 1211.
? Liow K, Barkley GL, Pollard JR, Harden CL, Bazil CW. (2007) Position statement on the coverage of anticonvulsant drugs for the treatment of epilepsy. Neurology 68: 1249.
? American Thyroid Association, The Endocrine Society, and American Association of Clinical Endocrinologists. (2004) Joint Statement on the U.S. Food and Drug Administration’s Decision Regarding Bioequivalence of Levothyroxine Sodium. Thyroid 14: 486.
? IMS Institute for Healthcare Informatics. (2013) Avoidable Costs in U.S. Healthcare: The $200 Billion Opportunity from Using Medicines More Responsibly. http://www.imshealth.com/deployedfiles/imshealth/Global/Content/Corporate/IMS Institute/RUOM- 2013/IHII_Responsible_Use_Medicines_2013.pdf
| File Type | application/msword |
| File Title | OMBMemoMERCPtP |
| Subject | MERC OMB MEP |
| Author | Hillabrant |
| Last Modified By | SYSTEM |
| File Modified | 2018-02-23 |
| File Created | 2018-02-23 |
© 2026 OMB.report | Privacy Policy