Request for change
0920-1102 Non Sub Change 04112018.docx
Information Collection for Tuberculosis Data from Panel Physicians
Request for change
OMB: 0920-1102
Request for Nonmaterial/Non-substantive change
Information Collection for Tuberculosis Data from Panel Physicians
OMB Control Number 0920-1102
Expiration date
02/28/2019
Program Contact
Lee Samuel
National Center for Emerging and Zoonotic Infectious Diseases
Centers for Disease Control and Prevention
1600 Clifton Road, NE
Atlanta, Georgia 30333
Phone: (404) 718-1616
Email: [email protected]
Submission Date: 4/12/2018
Circumstances of Change Request for OMB 0920-1102
The Centers for Disease Control and Prevention (CDC), Division of Global Migration and Quarantine (DGMQ) requests a nonmaterial/non-substantive of the currently approved Information Collection Request: “Information Collection for Tuberculosis Data from Panel Physicians.”
CDC is moving the collection time frame for the tuberculosis (TB) indicators data from January to March to make more efficient use of panel physician and CDC program staff time concerning the reporting and analysis of critical TB data. This has two minor impacts on the structure of the reporting form, which, in combination, are not expected to have a substantive impact on reporting burden.
DGMQ TB Indicator data provide valuable TB-related epidemiologic data from required U.S. immigration medical exams and allow CDC to monitor the effectiveness and impact of CDC’s Technical Instructions in diagnosing applicants with TB disease. These data are used to:
Improve quality assurance efforts and monitor proficiency of TB screening programs overseas
Demonstrate the impact of CDC/DGMQ TB screening programs on country-specific TB burdens
Evaluate the impact of the 2007 CDOT TB TI on the immigrant screening program
Compare TB Indicator incidence rates to WHO country-specific TB incidence rates
Detect and resolve problems at panel sites demonstrating poor performance
Provide a potentially new source of TB data to National TB Programs globally
Description of the changes
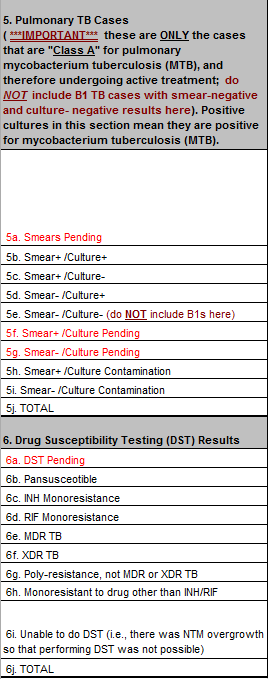
1) The additional time provides an opportunity for pending TB results to become final, negating the need for data fields requesting pending results. Therefore, CDC is removing four fields concerning pending results:
Row 5a. Smears Pending
Row 5f. Smear+ /Culture Pending
Row 5g. Smear- /Culture Pending
Row 6a. DST Pending
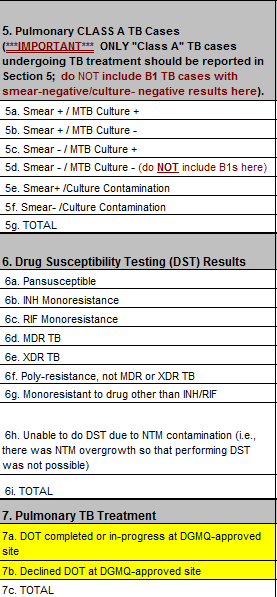
2) The additional time also provides added focus on TB outcomes, so CDC is requesting two new variables related to treatment outcomes:
Row 7a. DOT completed or in-progress at DGMQ-approved site
Row 7b. Declined DOT at DGMQ-approved site
See comparison of sections below. Red text is removed fields; yellow fields are new.
Approved form
Requested changes
Burden
The removal of the pending results fields and the addition of TB outcome fields is not expected to materially change the burden associated with submitting the TB indicators form. These data are collected as part of routine procedures during the required medical examination of applications for immigration to the United States.
The burden table below is unchanged from the approved one.
Type of Respondents |
Form Name |
Number of Respondents |
Number of Responses per Respondent |
Average Burden per Response (in hours) |
Total Burden Hours |
International Panel Physicians (All sites) |
TB Indicators Excel Spreadsheet |
353 |
1 |
7.5 |
2648 |
TOTAL |
2648 |
||||
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | JReichard |
| File Modified | 0000-00-00 |
| File Created | 2021-01-21 |
© 2026 OMB.report | Privacy Policy