ASC OMB Draft B_2-5-2018
ASC OMB Draft B_2-5-2018.docx
Ambulatory Surgery Center Survey on Patient Safety Culture Database
OMB: 0935-0242
SUPPORTING STATEMENT
Part B
Agency for Healthcare Research and Quality’s (AHRQ)
Ambulatory Surgical Center Survey on Patient Safety
Comparative Database
February 5, 2018
Agency of Healthcare Research and Quality (AHRQ)
Supporting Statement B
ASC SOPS Database
1. Description of Respondent Universe
The AHRQ Surveys on Patient Safety Culture™ (SOPS™) Ambulatory Surgical Center (ASC) Database will serve as a central U.S. repository for data on the survey; AHRQ will house the largest database of the survey’s results. However, the database will be comprised of data that are voluntarily submitted by ASCs that have administered the survey and, therefore, would not be a statistically representative sample of all U.S. ASCs.
AHRQ developed and pilot tested the Ambulatory Surgical Center Survey on Patient Safety Culture (ASC SOPS) with OMB approval (OMB No. 0935-0216; approved 10/31/2013). AHRQ piloted the ASC SOPS in early 2014. Participants included 1,800 staff members from 59 ASCs—or approximately one percent of the total number of ASCs at that time.i AHRQ made the survey publicly available along with a Survey User’s Guide, the pilot study results,ii and related toolkit materials on the AHRQ Ambulatory Surgery Center Survey on Patient Safety Culture Web site in April 2015. Results from the 59 ASCs that participated in the pilot test are available at: https://www.ahrq.gov/sites/default/files/wysiwyg/professionals/quality-patient-safety/patientsafetyculture/asc/resources/asc_pilotstudy.pdf.
The Centers for Medicare and Medicaid Services (CMS) defines ASCs as distinct entities that operate exclusively to provide surgical services to patients who do not require hospitalization and are not expected to need to stay in a surgical facility longer than 24 hours.iii iv The number of Medicare-certified ASCs was estimated to be 5,475 in 2015.v
MedPAC reports that the most frequently provided ASC services are cataract surgeries (16.9%), upper gastrointestinal endoscopies (8.1%), and colonoscopies (5.8%). ASCs that provided these services were included in the pilot study and are expected to be among the types of ASCs that would submit to the ASC SOPS Database. Tables 1 and 2 show a comparison of national data on ASC affiliation and specialty type compared to those who voluntarily participated in the ASC SOPS pilot study.
Table 1. National and Pilot Study ASC Affiliation
ASC Affiliation |
U.S. ASCs |
Pilot Study ASCs |
|||
|
Number |
Percent |
Number |
Percent |
|
Hospital-Affiliated |
1,880* |
63.2%* |
15 |
25% |
|
Freestanding |
1,096* |
36.8%* |
44 |
75% |
|
Subtotal |
2,976* |
100% of 16 states* |
54.4% of 2015 ASC population** |
|
|
Unknown |
2,499 |
45.6%** |
|
|
|
Total |
5,475** |
100%** |
59 |
100% |
|
*Based on data available through the HCUP Central Distributor, 2007. It includes only ASCs in the 16 states that participate in HCUP.vi
Based on MedPAC Report to Congress, May 2017.vii
Table 2. National and Pilot Study Specialty Type
ASC Specialty Type |
U.S. ASCs* |
Pilot Study ASCs |
||
|
Number |
Percent |
Number |
Percent |
Multispecialty |
1,802 |
39% |
41 |
69% |
Single-specialty (e.g., ophthalmology, dermatology, pain, gastroenterology) |
2,878 |
61% |
18 |
31% |
Total |
4,680 |
100% |
59 |
100% |
* “Single-specialty ASCs” are defined as those with more than 67 percent of their Medicare claims in one clinical specialty. “Multispecialty ASCs” are defined as those with more than 67 percent of their Medicare claims in more than one clinical specialty. ASCs included in this analysis are limited to those in the 50 states and the District of Columbia with a paid Medicare claim in 2015. Source: MedPAC analysis of Medicare carrier file claims, 2015.viii
2. Information Collection Procedures
Information collection for the AHRQ ASC SOPS Database will occur in a periodic data collection cycle beginning in fall 2019. Information collection procedures for submitting and processing data are shown in Figure 1.
Figure 1. ASC SOPS Comparative Database Data Submission
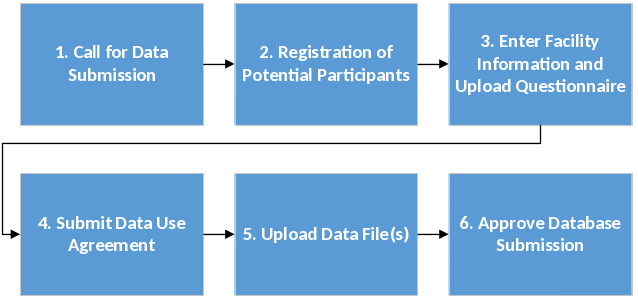
Step 1: Call for Data Submission. Announcements about the opening of data submission go out through various publicity sources. AHRQ’s patient safety and electronic newsletters target approximately 50,000 subscribers. In addition, the AHRQ Surveys on Patient Safety Culture listserv targets approximately 34,000 subscribers. An example of email announcements calling for data submission is shown in Attachment D, Email # 1 and # 3. Through these efforts, U.S. ASCs will be made aware of and invited to submit their survey data to the database.
As the administrator of the database and under contract with AHRQ, Westat provides free technical assistance to submitting ASCs through a dedicated email address ([email protected]) and toll-free phone number (1-888-324-9790).
Step 2: Registration for Potential Participants. A secure data submission Web site allows interested parties, such as ASCs, to register and submit data. The interested party’s point-of-contact (POC), often the manager of the ASC, will complete a number of data submission steps and forms. First, the online one-page Eligibility and Registration Form will take approximately 3 minutes to complete (see Attachment A). After registering, if registrants are deemed eligible to submit data, an automated email is sent to authenticate the account and update the user password (see Attachment D, Email #2).
Once users are registered and have a password, they can enter the main page menu of the Web site. Information about eligibility requirements, data use agreements, and data file specifications regarding how to prepare their data for inclusion in the SOPS database is posted and can be reviewed.
Step 3: Enter Facility Information and Upload Questionnaire. At this step, users provide information about their ASCs, such as point of contact, method of survey administration, overall response rate, and other characteristics that we will use to analyze collected data (e.g., ownership, specialty type, and size ) (see Attachment B). They also upload their survey questionnaire that they administered to enable us to determine whether any changes were made to the survey.
Step 4: Submit Data Use Agreement (DUA). To protect the privacy of all participating ASCs, a duly authorized representative from the ASC must sign a data use agreement (DUA) (see Attachment C). The DUA language was reviewed and approved by AHRQ’s general counsel and asserts that the ASC’s data will be handled in a secure manner using necessary administrative, technical, and physical safeguards to limit access to the data and maintain its confidentiality. In addition, the DUA explains that the data are used for the purposes of the database, that only aggregated results will be reported, and that the ASC will not identified by name. Data are not included in the database without this signed DUA. Users can fax, mail, or upload a copy of the signed agreement.
Step 5: Upload Data File(s). At this step, users are asked to upload their individual-level survey data for each ASC. Data submitted through the secure data submission Web site are encrypted to ensure secure, confidential transmission of the survey data. Data are accepted in Microsoft Excel® format since this is the format preferred by ASCs. Users must upload one data file per ASC. The data file specifications (see Attachment E) are provided to data submitters to ensure that users submit standardized and consistent data in the way variables are named, coded, and formatted.
Once a data file is uploaded, a separate load program developed in Visual Basic (VB) reads the submitted files and loads them into the SQL database that stores the data. A data quality report is then produced and made available to the participant. This report displays item frequencies and flags out-of-range values and incorrectly reverse-coded items. If there are no problems with the data, an acknowledgement of data upload and accepted will be granted during the user session. If data are improperly coded, the user is informed that the data file failed during the user session by having a message post on the screen. Users are expected to fix any errors and resubmit their data file(s) for processing. Once there are no problems, the user is informed of the acceptance of data during the user session with an online message of acceptance.
Step 6: Approve Data Submission. Once all of the information required for submission has been submitted and approved, an email is sent to the ASC POC indicating that their data have received final acceptance.
3. Methods to Maximize Response Rates
AHRQ makes a number of toolkit materials available to assist ASCs with the SOPS surveys. ASC SOPS has a Survey User’s Guide, which provides instructions and tips about survey administration on the following topics: planning; selecting a sample; determining a data collection method; data collection procedure—including a section on conducting Web surveys; and analyzing data and producing reports (at https://www.ahrq.gov/sops/quality-patient-safety/patientsafetyculture/asc/index.html). The Survey User’s Guide also gives ASCs tips about how to increase response rates through publicity efforts, top management support, use of incentives, and following all steps of proper data collection protocols. Of the ASCs that voluntarily participated in the 2014 pilot study, the average response rate was 77% across the 59 ASCs.
The SOPS User Network promotes the database to encourage data submission in a number of ways:
AHRQ and AHRQ SOPS email listservs;
Organizational partners and stakeholders that have national reach to ASCs;
Users that have contacted the SOPS technical assistance helpline about the ASC survey;
Other outlets such as national Webcasts and conferences.
As noted earlier in this document under Information Collection Procedures, Step 1 – Call for Data Submission, announcements about the opening of data submission go out through various publicity sources as a way to boost ASC participation in the database. AHRQ’s electronic newsletter targets approximately 50,000 subscribers. In addition, the AHRQ Surveys on Patient Safety Culture listserv targets approximately 34,000 subscribers. AHRQ, through its contractor Westat, provides free technical assistance to users through a dedicated email box and toll-free phone number. In addition, reminders are sent to database registrants to remind them of the deadline for data submission.
4. Tests of Procedures
Input and Feedback for the Development of the SOPS Database Submission System. Because the Surveys on Patient Safety Culture are public-use instruments, the SOPS program has generally modeled its data submission processes after those utilized by the Consumer Assessment of Healthcare Providers and Systems (CAHPS) Database that has been in operation since 1998. SOPS staff consulted with CAHPS Database staff and programmers to determine best practices for data submission. This information, as well as feedback obtained during the provision of technical assistance each year the database has been running, has been used to improve the SOPS online data submission system and process over time.
5. Statistical Consultants
Joann Sorra, PhD
Westat
1600 Research Blvd.
Rockville, MD 20850
Naomi Yount, PhD
Westat
1600 Research Blvd.
Rockville, MD 20850
i MedPAC Report to the Congress, 2017: Medicare payment policy. Washington, DC. 2017. Available at http://medpac.gov/docs/default-source/reports/mar17_entirereport.pdf. Last accessed 11/20/2017.
ii Sorra J, Smith S, Franklin M, et al. Results from the 2014 AHRQ Ambulatory Surgery Center Survey on Patient Safety Culture Pilot Study. (Prepared by Westat, Rockville, MD, under Contract No. HHSA290201000025I.) Rockville, MD: Agency for Healthcare Research and Quality; April 2015. AHRQ Publication No. 15-0019-1-EF. See https://www.ahrq.gov/sites/default/files/wysiwyg/professionals/quality-patient-safety/patientsafetyculture/asc/resources/asc_pilotstudy.pdf. Last accessed 11/20/2017.
iii See 42 C.F.R. §416.2. See https://www.gpo.gov/fdsys/granule/CFR-2010-title42-vol3/CFR-2010-title42-vol3-sec416-2. Last accessed 11/20/2017.
iv Frequently Asked Questions: Surveys on Patient Safety Culture. Content last reviewed April 2015. Agency for Healthcare Research and Quality, Rockville, MD. http://www.ahrq.gov/professionals/quality-patient-safety/patientsafetyculture/pscfaq.html. Last accessed 11/20/2017.
v MedPAC Report to Congress, March 2017. http://www.medpac.gov/docs/default-source/reports/mar17_medpac_ch5.pdf?sfvrsn=0. Last accessed 11/20/2017.
vi Senathirajah M, Steiner C. Evaluation of the State Ambulatory Surgery Databases, Available through the HCUP Central Distributor, 2007. HCUP Methods Series Report # 2010-04. Online October 6, 2010. U.S. Agency for Healthcare Research and Quality. Available at https://hcup-us.ahrq.gov/reports/methods/2010_04.pdf. See “Table 2: Number of hospital-based and non-hospital based facilities by state available through the HCUP Central Distributor, 2007 SASD-CD” (p. 9). Last accessed 11/20/2017.
vii MedPAC Report to Congress, 2017. Chapter 5: Ambulatory Surgical Center Services. March 2017. http://www.medpac.gov/docs/default-source/reports/mar17_medpac_ch5.pdf?sfvrsn=0. Last accessed 11/20/2017.
viii Report to the Congress: Medicare Payment Policy, March 2017. Chapter 5: Ambulatory Surgical Center Services. Available at http://www.medpac.gov/docs/default-source/reports/mar17_medpac_ch5.pdf?sfvrsn=0 Last accessed 11/20/2017.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Sari Siegel |
| File Modified | 0000-00-00 |
| File Created | 2021-01-20 |
© 2026 OMB.report | Privacy Policy