Part 1- Final Rule Guidance to OMB for 2017 - 10 CFR Part 30,32,and 35
Part 1 of the final guidance sent to OMB for the 2017 final Part 35 Rule .docx
10 CFR 35, Medical Use of Byproduct Material
Part 1- Final Rule Guidance to OMB for 2017 - 10 CFR Part 30,32,and 35
OMB: 3150-0010
Guidance for the Final Rule “Medical Use of Byproduct Material - Medical Event Definitions, Training and Experience, and Clarifying Amendments”
and
Comment Resolution for Proposed Guidance on the Proposed Rule “Medical Use of Byproduct Material - Medical Event Definitions, Training and Experience, and Clarifying Amendments”
Introduction
Guidance for the Final Rule “Medical Use of Byproduct Material - Medical Event Definitions, Training and Experience, and Clarifying Amendments
The guidance developed to implement the Final Rule “10 CFR Parts 30, 32 and 35 - Medical Use of Byproduct Material - Medical Event Definitions, Training and Experience, and Clarifying Amendments” is provided in three parts. The first two parts consist of revisions to the guidance already provided in the NUREG-1556, “Consolidated Guidance About Materials Licenses” series of volumes for medical uses and commercial nuclear pharmacies. These guidance documents primarily provide guidance to applicants in the completion and submission of materials license applications. They also include model procedures that an applicant may want to use as models when developing its radiation safety program, as well as tools that licensees may employ when completing the corresponding material license applications. Because the rulemaking is not limited to elements associated with obtaining a license, the third part of this guidance document includes supplemental questions and answers that will be added to the NRC’s Medical Uses Licensee Toolkit website, to assist licensees in understanding and implementing the new regulatory changes.
Part 1 consists of the “Supplemental Guidance for NUREG-1556, Volume 9, Revision 2, Consolidated Guidance About Materials Licenses: Program - Specific Guidance About Medical Use Licenses.”
Part 2 consists of the “Supplemental Guidance for NUREG-1556, Volume 13, Revision 1, Consolidated Guidance About Materials Licenses: Program-Specific Guidance About Commercial Radiopharmacy Licenses.”
For Part 1 and Part 2, the NRC extracted only those NUREG sections and appendices that it changed as part of the revised supplementary guidance documents. For each revised section and/or appendix, the NRC inserted an introductory notation describing and changes and identifying the relevant revised regulation. Red line format was used to show new text and black strikeout format was used to show eliminated text. In general, entire sections were included to put the proposed changes in proper context. If the entire appendix was not included, the NUREG-1556 page numbers were included in the introductory notation.
Part 3 includes questions and answers grouped by topic.
Comment Resolution for Proposed Guidance on the Proposed Rule “Medical Use of Byproduct Material - Medical Event Definitions, Training and Experience, and Clarifying Amendments”
There were five commenters. Three provided comments only on the proposed rule. The fourth commenter provided comments on both the proposed rule and the guidance and the Fifth only commented on the guidance. The comments on the rule were addressed in the comment resolution for the rule (XXFRNXXXX). The summary of the comments provided on the proposed guidance document and NRC’s response to those comments is provided in Part 4 of this document.
PART 1
Supplemental Guidance for NUREG-1556, Volume 9, Revision 2, Consolidated Guidance About Materials Licenses: Program - Specific Guidance About Medical Use Licenses.
[The following redline addition to the “Abbreviations” section reflects the change to 10 CFR 35.2, adding an Associate Radiation Safety Officer.]
ABBREVIATIONS
AAPM American Association of Physicists in Medicine
ACMUI Advisory Committee on the Medical Use of Isotopes
ACR American College of Radiology
ALARA as low as is reasonably achievable
ALI annual limit on intake
AMP Authorized Medical Physicist
ANP Authorized Nuclear Pharmacist
ANSI American National Standards Institute
ARSO Associate Radiation Safety Officer
AU Authorized User
bkg background
BPR Business Process Redesign
Bq becquerel
CFR Code of Federal Regulations
Ci curie
cc centimeter cubed
cm2 square centimeter
Co-57 cobalt-57
Co-60 cobalt-60
cpm counts per minute
Cs-137 cesium-137
DAC derived air concentration
DOT United States Department of Transportation
dpm disintegrations per minute
EPAct Energy Policy Act of 2005
F-18 fluorine-18
FDA United States Food and Drug Administration
GM Geiger-Mueller
GPO Government Printing Office
GSR gamma stereotactic radiosurgery
HDR high dose-rate
I-125 iodine-125
I-131 iodine-131
IN Information Notice
IP Inspection Procedure
Ir-192 iridium-192
LDR low dose-rate
mCi millicurie
ml milliliter
Mo-99 molybdenum-99
mR milliroentgen
mrem millirem
mSv millisievert
N–13 nitrogen-13
NaI(Tl) sodium iodide (thallium doped)
NARM Naturally Occurring and Accelerator-Produced Material
NCRP National Council on Radiation Protection and Measurements
NIST National Institute of Standards and Technology
NRC United States Nuclear Regulatory Commission
NVLAP National Voluntary Laboratory Accreditation Program
O-15 oxygen-15
OCFO Office of the Chief Financial Officer
OCR optical character reader
OMB Office of Management and Budget
OSL optically stimulated luminescence dosimeters
PET Positron Emission Tomography
P-32 phosphorus-32
Pd-103 palladium-103
PDR pulsed dose-rate
P&GD Policy and Guidance Directive
QA quality assurance
Ra-226 radium-226
Ru-82 rubidium-82
RG Regulatory Guide
RIS Regulatory Issue Summary
RSC Radiation Safety Committee
RSO Radiation Safety Officer
SDE shallow-dose equivalent
SI International System of Units (abbreviated SI from the French Le Système Internationale d’Unités)
Sr-82 strontium-82
Sr-85 strontium-85
Sr-90 strontium-90
SSDR Sealed Source and Device Registry
std standard
Sv Sievert
TAR Technical Assistance Request
Tc-99m technetium-99m
TEDE total effective dose equivalent
TI Transport Index
TLD thermoluminescent dosimeters
U-235 uranium-235
WD written directive
Xe-133 xenon-133
Y-90 yttrium-90
µCi microcurie
% percent
[The following redline additions to Table 1.1 reflect the changes to 10 CFR 35.2 and 35.433 adding an Associate Radiation Safety Officer and an ophthalmic physicist.]
Table 1.1 Sections of NUREG-1556, Volume 9, Revision 2, that Applicants for a Particular Type of Use Should Review |
||||||||
NUREG-1556 - Volume 9, Rev. 2 Section: |
Type of Use |
|||||||
100 |
200 |
300 |
400 |
500 |
600 |
1000 |
||
8.1 |
License Action Type |
● |
● |
● |
● |
● |
● |
● |
8.2 |
Applicant's Name and Mailing Address |
● |
● |
● |
● |
● |
● |
● |
8.3 |
Address(es) Where Licensed Material Will Be Used or Possessed |
● |
● |
● |
● |
● |
● |
● |
8.4 |
Person to Be Contacted about This Application |
● |
● |
● |
● |
● |
● |
● |
8.5 |
Radioactive Material |
● |
● |
● |
● |
● |
● |
● |
8.6 |
Sealed Sources and Devices (including Ra-226 Sealed Sources and Devices) |
|
|
|
● |
● |
● |
● |
8.7 |
Discrete Source of Ra-226 (other than Sealed Sources) |
● |
● |
● |
|
|
|
● |
8.8 |
Recordkeeping for Decommissioning and Financial Assurance |
● |
● |
● |
● |
● |
● |
● |
8.9 |
Purpose(s) for which Licensed Material Will Be Used |
● |
● |
● |
● |
● |
● |
● |
8.10 |
Individual(s) Responsible for Radiation Safety Program and their Training and Experience |
● |
● |
● |
● |
● |
● |
● |
8.11 |
Radiation Safety Officer (RSO) and Associate Radiation Safety Officer (ARSO) |
● |
● |
● |
● |
● |
● |
● |
8.12 |
Authorized User (AU) |
● |
● |
● |
● |
● |
● |
● |
8.13 |
Authorized Nuclear Pharmacist (ANP) |
● |
● |
● |
|
|
|
● |
8.14a |
Authorized Medical Physicist (AMP) |
|
|
|
● |
|
● |
● |
8.14b |
Ophthalmic Physicist |
|
|
|
● |
|
|
|
8.15 |
Facilities and Equipment |
● |
● |
● |
● |
● |
● |
● |
8.16 |
Facility Diagram |
● |
● |
● |
● |
● |
● |
● |
8.17 |
Radiation Monitoring Instruments |
● |
● |
● |
● |
● |
● |
● |
8.18 |
Dose Calibrator and Other Equipment Used to Measure Dosages of Unsealed Byproduct Material |
● |
● |
● |
|
|
|
● |
8.19 |
Therapy Unit - Calibration and Use |
|
|
|
● |
|
● |
● |
8.20 |
Other Equipment and Facilities |
● |
● |
● |
● |
● |
● |
● |
8.21 |
Radiation Protection Program |
● |
● |
● |
● |
● |
● |
● |
8.22 |
Safety Procedures and Instructions |
|
|
|
|
|
● |
● |
8.23 |
Occupational Dose |
● |
● |
● |
● |
● |
● |
● |
8.24 |
Area Surveys |
● |
● |
● |
● |
● |
● |
● |
8.25 |
Safe Use of Unsealed Licensed Material |
● |
● |
● |
|
|
|
● |
8.26 |
Spill/Contamination Procedures |
● |
● |
● |
● |
● |
● |
● |
8.27 |
Installation, Maintenance, Adjustment, Repair, and Inspection of Therapy Devices Containing Sealed Sources |
|
|
|
|
|
● |
● |
8.28 |
Minimization of Contamination |
● |
● |
● |
● |
● |
● |
● |
8.29 |
Waste Management |
● |
● |
● |
● |
● |
● |
● |
8.30 |
Fees |
● |
● |
● |
● |
● |
● |
● |
8.31 |
Certification |
● |
● |
● |
● |
● |
● |
● |
AA |
Authorization under 10 CFR 30.32(j) to Prepare PET Radioactive Drugs for Noncommercial Transfer |
|
|
|
|
|
|
|
PROGRAM-RELATED GUIDANCE - NO RESPONSE FROM APPLICANTS ON NRC FORM 313 |
||||||||
8.32 |
Safety Instruction for Individuals Working In or Frequenting Restricted Areas |
● |
● |
● |
● |
● |
● |
● |
8.33 |
Public Dose |
● |
● |
● |
● |
● |
● |
● |
8.34 |
Opening Packages |
● |
● |
● |
● |
● |
● |
● |
8.35 |
Procedures for Administrations When a Written Directive Is Required |
|
|
● |
● |
|
● |
● |
8.36 |
Release of Patients or Human Research Subjects |
|
|
● |
● |
|
|
● |
8.37 |
Mobile Medical Service |
● |
● |
● |
● |
● |
● |
● |
8.38 |
Audit Program |
● |
● |
● |
● |
● |
● |
● |
8.39 |
Operating and Emergency Procedures |
● |
● |
● |
● |
● |
● |
● |
8.40 |
Material Receipt and Accountability |
● |
● |
● |
● |
● |
● |
● |
8.41 |
Ordering and Receiving |
● |
● |
● |
● |
● |
● |
● |
8.42 |
Sealed Source Inventory |
● |
● |
● |
● |
● |
● |
● |
8.43 |
Records of Dosages and Use of Brachytherapy Source |
● |
● |
● |
● |
|
|
● |
8.44 |
Recordkeeping |
● |
● |
● |
● |
● |
● |
● |
8.45 |
Reporting |
● |
● |
● |
● |
● |
● |
● |
8.46 |
Leak Tests |
● |
● |
● |
● |
● |
● |
● |
8.47 |
Safety Procedures for Treatments When Patients Are Hospitalized |
|
|
● |
● |
|
● |
● |
8.48 |
Transportation |
● |
● |
● |
● |
● |
● |
● |
[The following redline additions to Section 3 reflect the changes to 10 CFR 35.2 and 35.24 adding an Associate Radiation Safety Officer.]
Part 35 |
Applicability |
100 |
✓ |
200 |
✓ |
300 |
✓ |
400 |
✓ |
500 |
✓ |
600 |
✓ |
1000 |
✓ |
3 MANAGEMENT RESPONSIBILITY
Regulations: 10 CFR 30.9, 10 CFR 35.12, 10 CFR 35.24.
The NRC endorses the philosophy that effective Radiation
Protection Program management is vital to safe operations that
comply with NRC regulatory requirements
(see 10 CFR 35.24).
“Management” refers to the chief executive officer or other individual having the authority to manage, direct, or administer the licensee’s activities or that person’s delegate or delegates (see 10 CFR 35.2). |
To ensure adequate management involvement in accordance with 10 CFR 35.12(a) and 35.24(a), a management representative (i.e., chief executive officer or delegate) must sign the submitted application acknowledging management’s commitments to and responsibility for the following:
∙ Radiation protection, security and control of radioactive materials, and compliance with regulations;
∙ Completeness and accuracy of the radiation protection records and all information provided to NRC (10 CFR 30.9);
∙ Knowledge about the contents of the license application;
∙ Compliance with current NRC and United States Department of Transportation (DOT) regulations and the licensee’s operating and emergency procedures;
∙ Provision of adequate financial and other resources (including space, equipment, personnel, time, and, if needed, contractors) to the Radiation Protection Program to ensure that patients, the public, and workers are protected from radiation hazards;
∙ Appointment of a qualified individual who has agreed in writing to work as the RSO;
∙ If applicable, appointment of one or more qualified as an ARSO;
∙ Approval of qualified individual(s) to serve as authorized medical physicists (AMPs), an ophthalmic physicist, ANPs, and AUs for licensed activities.
For information on NRC inspection, investigation, enforcement, and other compliance programs, see the following:
∙ The NRC Enforcement Policy which is included on the NRC’s Web site at http://www.nrc.gov/what-we-do/regulatory/enforcement/enforce-pol.html
∙ The NRC Inspection Manual, Chapter 2800, “Materials Inspection Program,” and
∙ Inspection Procedures:
83822 – “Radiation Protection,”
84850 – “Radioactive Waste Management - Inspection of Waste Generator Requirements of 10 CFR Part 20 and 10 CFR Part 61,”
84900 – “Low-Level Radioactive Waste Storage,”
87130 – “Nuclear Medicine Programs — Written Directive Not Required,”
87131 – “Nuclear Medicine Programs — Written Directive Required,”
87132 – “Brachytherapy Programs,”
87133 – “Medical Gamma Stereotactic Radiosurgery and Teletherapy Programs,” and
87134 – “Medical Broad-Scope Programs.”
For availability of these documents, see the Notice of Availability on the inside front cover of this report. In addition, the Inspection Manual and procedures are available at http://www.nrc.gov/materials/miau/med-use-toolkit.html.
[The following redline/strike out revisions to Section 5.1 reflect a change to 10 CFR 35.12 to require submission of only the original license application.]
5.1 PREPARING AN APPLICATION
Part 35 |
Applicability |
100 |
✓ |
200 |
✓ |
300 |
✓ |
400 |
✓ |
500 |
✓ |
600 |
✓ |
1000 |
✓ |
∙ Use the most recent guidance in preparing an application, including Appendix AA of this document, if appropriate;
∙ Complete NRC Form 313 (Appendix A), Items 1 through 4,
12, and 13, on the form itself;
∙ Complete NRC Form 313, Items 5 through 11, on supplementary pages, or use Appendix C;
∙ Complete the appropriate NRC Form 313A series of forms (Appendix B) to document training and experience, if electing to complete this optional form;
∙ Provide sufficient detail for NRC to determine that equipment, facilities, training, experience, and the Radiation Safety Program are adequate to protect health and safety and minimize danger to life and property;
∙ For each separate sheet, other than the NRC Form 313A series of forms or Appendix C, that is submitted with the application, identify and cross-reference it to the item number on the application or the topic to which it refers;
∙ Submit all documents, typed, on 8-1/2 x 11-inch paper;
∙ Avoid submitting proprietary information unless it is absolutely necessary;
∙ If submitted, proprietary information and other sensitive information must be clearly identified (see Section 5.2 below);
∙ Submit
the
an
original
signed application and
one copy;
and
∙ Retain one copy of the license application for future reference.
Applications must be signed by the applicant’s or licensee’s management as required by 10 CFR 35.12(a); see Section 8.31, “Certification.” |
[The following redline additions to Section 8 reflect changes to 10 CFR 35.2 and 35.433 adding an Associate Radiation Safety Officer and an ophthalmic physicist.]
8 CONTENTS OF AN APPLICATION
This section explains, item by item, the information that medical use applicants must provide on NRC Form 313 (see Appendix A) and should provide on the appropriate NRC Form 313A series of forms if electing to use this optional form (see Appendices B and D). If an application contains security-related sensitive information (see Section 5.2), the cover letter should state that the “attached documents contain security-related sensitive information.” If a cover letter is not used, NRC Form 313 should include this statement. The information needed to complete Items 5 through 11 on Form 313 describes the applicant’s proposed medical use Radiation Safety Program. To assist the applicant in submitting complete information on these items, the applicable regulations are referenced in the discussion of each item. Appendix AA explains additional information the applicant must provide on NRC Form 313 when requesting authorization under 10 CFR 30.32(j) for preparing PET radioactive drugs for noncommercial distribution to medical use licensees within its consortium.
Table 1 in Appendix C is provided to help applicants determine which procedures must be developed, implemented, and maintained for the type of medical use requested. Several appendices in this report present sample procedures that applicants may use in developing their procedures. Suggested responses for each block on the NRC Form 313 appear under “Response from Applicant” in this guide.
If a particular part of a section does not apply, simply note “N/A” for “not applicable.” If a particular section applies, but a procedure does not have to be developed, simply note “N” for “no response required.” N/A, N, or short sentence responses to Items 5 through 11 should run consecutively on one or more sheets separate from responses provided on NRC Form 313. Lengthy responses should be appended as attachments.
As indicated on NRC Form 313 (see Appendix A), responses to Items 5 through 11 should be submitted on separate sheets of paper. Applicants may use the appropriate NRC Form 313A series of forms (see Appendix B) to document training and experience for new AUs, medical physicists, ophthalmic physicists, nuclear pharmacists, ARSOs and RSOs. The NRC Form 313A series of forms may also be used by experienced individuals seeking additional authorizations. Applicants may use Appendix C to assist with completion of the application. |
[The following redline/strikeout revisions to Item 5 reflect changes to 10 CFR 35.65 to prohibit bundling of single sources or use of calibration, transmission, or reference sources for medical use under the provisions of 10 CFR 35.65; and to clarify that certain calibration, transmission, or reference sources may not have to be listed on the license when used under the provisions of 10 CFR 35.500.]
8.5 ITEM 5: RADIOACTIVE MATERIAL
Part 35 |
Applicability |
100 |
✓ |
200 |
✓ |
300 |
✓ |
400 |
✓ |
500 |
✓ |
600 |
✓ |
1000 |
✓ |
10 CFR 35.100, 10 CFR 35.200, 10 CFR 35.300, 10 CFR 35.400,
10 CFR 35.500, 10 CFR 35.600, 10 CFR 35.1000.
Criteria: Byproduct material for medical use in 10 CFR Part 35
is divided into seven types of use (10 CFR 35.100, 35.200, 35.300,
35.400, 35.500, 35.600, and 35.1000).
Discussion: The applicant should indicate the byproduct material requested. The amount and type of information necessary will vary according to the type of use and material requested.
Under Section 651c of the EPAct, the NRC now has regulatory authority over accelerator-produced byproduct material as well as discrete sources of Ra-226. Although sealed Ra-226 sources (e.g., Ra-226 needles) were once used for manual brachytherapy and are no longer believed to be used for medical uses, the medical use of discrete sources of Ra-226 is included in this guidance because its use for this purpose is not prohibited. The guidance also distinguishes between discrete sources of Ra-226 and sealed sources of Ra-226 because not all discrete sources are sealed sources.
The medical uses of the other new byproduct materials are essentially the same as the uses of the previously defined byproduct materials. However, some of the radionuclides now included in the expanded definition of byproduct material have significantly shorter half-lives and higher energy levels (e.g., PET radionuclides) that may result in delivery of the unsealed material by direct transfer tube from the accelerator production facility to the 35.100 and 35.200 medical use areas. This may result in higher potential radiation doses to workers and the public if additional handling and shielding precautions are not implemented, and licensees should consider this in evaluating their equipment, facilities, and programs.
35.100 and 35.200 Use: For 10 CFR 35.100 and 35.200 medical uses, the chemical/physical form may be “Any” unsealed byproduct material permitted by 10 CFR 35.100 or 35.200, as appropriate. For 10 CFR 35.100 and 35.200 medical uses, the total amount requested may be “As Needed.”
The following format may be used:
Byproduct Material |
Chemical/Physical Form |
Maximum Amount |
Any byproduct material permitted by 10 CFR 35.100 |
Any |
As needed |
Any byproduct material permitted by 10 CFR 35.200 |
Any |
As needed1 |
35.300 Use: For 10 CFR 35.300 use, the chemical/physical form may be “Any” unsealed byproduct material permitted by 10 CFR 35.300. The total amount requested must be specified. The following format may be used:
Byproduct Material |
Chemical/Physical Form |
Maximum Amount |
Any byproduct material permitted by 10 CFR 35.300 |
Any |
300 millicuries |
35.400,
35.500, 35.600, and 35.1000 Use:
For 10 CFR 35.400, 35.500,
35.600, and 35.1000 use, the radionuclide, the chemical/physical form
(e.g., sealed source or device identified by manufacturer and model
number), the total amount in becquerels (Bq), microcuries (µCi),
millicuries (mCi), or curies (Ci), and the maximum number of sources
or activity possessed at any one time must be specified. For
10 CFR 35.500 use, the sealed source information described above does
not need to be provided for calibration, transmission, or reference
sources used for this medical use if the individual activity or a
bundled activity is not greater than the maximum activity of any
single source authorized in 10 CFR 35.65. The sealed source and
device information described above must be provided for all other
sources and devices used under 35.500.
Sealed sources of Ra-226 may be used for 10 CFR 35.400, 35.500, and
35.1000 uses. Unsealed Ra-226 can only be used for medical use under
35.1000. Applicants should include all possible new sources they
might use, in order to minimize the need for license amendments if
they change model or vendor. The following format may be used:
Byproduct Material |
Chemical/Physical Form |
Maximum Amount |
I-125 (specific radiation therapy system liquid brachytherapy source, 35.1000 use) |
Liquid source (Manufacturer Name, Model #DEF) |
2 curies total |
Ra-226 |
Sealed source or device (Manufacturer Name, Model #HIJ) |
Not to exceed 50 millicuries per source and 250 millicuries total |
Cesium 137 (i.e., specific brachytherapy radionuclide, 35.400 use) |
Sealed source or device (Manufacturer Name, Model #MNO) |
2 curies total |
Pd-103 (i.e., specific manual brachytherapy source, 35.400 use) |
Sealed source or device (Manufacturer Name, Model #QRS) |
Not to exceed 0.5 millicuries per source and 3 curies total |
Gadolinium 153 (i.e., specific diagnostic sealed-source radionuclide, 35.500 use) |
Sealed source or device (Manufacturer Name, Model #TUV) |
Not to exceed 500 millicuries per source and 1 curie total |
Germanium 68 (transmission sources bundled to exceed 30 millicuries, in PET scanners, 35.500 use) |
Sealed source or device (Manufacturer Name, Model #CTR) |
Not to exceed 30 millicuries per source and 1 curie total |
Cobalt 60 (i.e., specific teletherapy sealed-source radionuclide, 35.600 use) |
Sealed source or device (Manufacturer Name, Model #XYZ) |
Not to exceed 9,000 curies per source and 18,000 curies total |
Iridium 192 (i.e., specific afterloader sealed-source radionuclide, 35.600 use) |
Sealed source or device (Manufacturer Name, Model #XYZ) |
Not to exceed 10 curies per source and 20 curies total |
Cobalt 60 (i.e., specific gamma stereotactic radiosurgery sealed- source radionuclide, 35.600 use) |
Sealed source or device (Manufacturer Name, Model #XYZ) |
Not to exceed 36 curies per source and 6,600 curies total |
For sealed sources used in devices, an applicant may wish to request a possession limit adequate to allow for the possession of a spare source, to accommodate the total quantity of material in the licensee’s possession during replacement of the source in the device. The maximum activity for a single source or source loading may not exceed the activity specified by the manufacturer for the specific device and source combination as stated in the Sealed Source and Device Registry (SSDR) certificate. However, an applicant may request a maximum activity for the source in the shipping container that exceeds the maximum activity allowed in the device. To request this authorization, applicants should provide certification that the source transport container is approved for the requested activity. A source that is received with a higher activity than permitted in the device must be allowed to decay to or below the licensed activity limit prior to installation in the device.
Calibration, Transmission, and Reference Sources: For all calibration, transmission, and reference sources, including those with Ra-226, covered under 10 CFR 35.65, the specific sources do not need to be listed on the license as long as the licensee is authorized pursuant to 10 CFR 35.11 for the medical use of byproduct material. 10 CFR 35.65 only permits use of the calibration, transmission, and reference sources for intentional internal or external administration of byproduct material, or the radiation from byproduct material, to patients or human research subjects (i.e. medical use) in accordance with the requirements in 10 CFR 35.500, in which case they may be used without listing them on the license for medical use. Calibration, transmission, and reference sealed sources with an individual activity or a bundled activity greater than the maximum activity of any single source authorized in 10 CFR 35.65 must be listed on the license.
Shielding Material/Depleted Uranium: Some high-activity radionuclide generators used to produce byproduct materials for 10 CFR 35.200 and 35.300 uses (e.g., Tc-99m generators) may include depleted uranium (i.e., uranium depleted in uranium-235 (U-235)) as shielding material. If a generator has depleted uranium shielding, an applicant should request authorization to possess depleted uranium as shielding material. Applicants receiving large therapy sources and devices also should determine if depleted uranium is used to shield the therapy sources and devices. This includes identifying depleted uranium used as shielding in linear accelerators because, even though NRC does not regulate the accelerator, it does regulate the depleted uranium in the accelerator. If applicable, the applicant should request authorization to possess depleted uranium (i.e., uranium depleted in U-235) in quantities sufficient to include shielding material in both the device(s) and source containers used for source exchange and shielding for other devices. The applicant should review the manufacturer’s specifications for each device specified in the license request to determine: (1) if depleted uranium is used to shield the source(s) within the device; and (2) the total quantity of depleted uranium present in the device (in kilograms). The applicant should also consult the manufacturer’s specifications or the source supplier to determine if depleted uranium is contained in shielding source containers used during source exchange, as well as the total quantity of depleted uranium in such containers (in kilograms). The following format may be used:
Byproduct Material |
Chemical/Physical Form |
Maximum Amount |
Depleted Uranium |
Metal |
999 kilograms |
Other Material: The applicant should make a separate entry for other required items (e.g., Ra-226 not previously described, more byproduct material for in vitro testing than is allowed under 10 CFR 31.11, survey meter calibration source, dosimetry system constancy check source, material for in vitro, animal, or human research studies). The following format may be used:
Byproduct Material |
Chemical/Physical Form |
Maximum Amount |
Any byproduct material permitted by 10 CFR 31.11 |
Prepackaged kits |
50 millicuries |
Ra-226 |
unsealed |
1 millicurie |
Sources that are authorized by 10 CFR 35.65, “Authorization for calibration, transmission, and reference sources,” should not be listed.
Applicants should number each line entry consecutively, following the 10 CFR Part 35 material.
Blood Irradiators: If the use of a device to irradiate blood is anticipated, the applicant should review NUREG-1556, Volume 5, “Consolidated Guidance About Materials Licenses: Program-Specific Guidance About Self-Shielded Irradiator Licenses.”
Production of Radionuclides by Accelerators: If the applicant will use an accelerator to produce radionuclides, a separate license application will be needed for the production of the radionuclides. The applicant should review NUREG-1556, Volume 21, “Consolidated Guidance About Materials Licenses: Program-Specific Guidance about Possession Licenses for Production of Radioactive Materials Using an Accelerator.”
Production of PET Radioactive Drugs for Noncommercial Distribution to Medical Use Licensees Within a Consortium: If the applicant will use PET radionuclides to produce PET radioactive drugs for its own medical use and noncommercial distribution to other members of its consortium, the applicant, to satisfy 10 CFR 30.33(a)(1), should identify the PET radionuclides, the proposed use of the material, and the maximum activity. The applicant should also review Appendix AA.
The following format may be used for unsealed PET radionuclides used to produce PET radioactive drugs for noncommercial transfer to other members within the consortium.
Byproduct Material |
Chemical/Physical Form |
Maximum Amount |
PET Radionuclides for noncommercial distribution |
Any |
____ curies |
When applying for this authorization, the applicant should also consider applying for authorization to take back potentially contaminated transport shields from other consortium members. Each consortium member should dispose of unused dosages and used syringes and vials at its own facility.
When determining both individual radionuclide and total quantities, all materials to be possessed at any one time under the license should be included (i.e., materials received awaiting use (new teletherapy or brachytherapy sources for exchange), materials in use or possessed, material used for shielding, and materials classified as waste awaiting disposal or held for decay-in-storage).
Response from Applicant: The applicant should submit the information as described above. Certain information about quantities of radioactive materials is no longer released to the public and needs to be marked “security-related information – withhold under 10 CFR 2.390.” Therefore, when responding to this section, follow the guidance in Section 5.2 to determine if the response includes security-related sensitive information and needs to be marked accordingly. Applicants requesting authorization for the medical use of a discrete source of Ra-226 (which includes a sealed source of Ra-226) or other NARM sources or devices containing NARM sources that do not have the information described above (e.g., manufacturer and model number from an SSDR certificate), or the information required in 10 CFR 30.32(g)(3), should consult the appropriate NRC Regional Office to discuss the contents of their application.
[The following redline additions to Section 8.6 reflect changes to 10 CFR 35.65 to prohibit bundling of single sources or use of calibration, transmission, or reference sources for medical use under the provisions of 10 CFR 35.65, and to clarify that when used in accordance with the requirements in 35.500 the sources need not be listed on the license.]
Part 35 |
Applicability |
100 |
|
200 |
|
300 |
|
400 |
✓ |
500 |
✓ |
600 |
✓ |
1000 |
✓ |
DEVICES (including Ra-226 sealed
sources and devices)
Regulations: 10 CFR 30.32(g), 10 CFR 30.33(a)(2),
10 CFR 32.210, 10 CFR 35.65.
Criteria: In accordance with 10 CFR 30.32(g), applicants must
provide the manufacturer’s name and model number for each
requested sealed source and device (except for calibration,
transmission, and reference sources authorized by 10 CFR 35.65, and certain NARM sources for which this information is not available). 10 CFR 35.65(c) provides the exception for calibration, transmission, or reference sources and, as indicated in the regulation, applies to either medical uses under 10 CFR 35.500 or non-medical uses under 10 CFR 35.65. (Note: If the single or bundled activity of calibration, transmission, and reference sources exceeds the single source activity limit in 10 CFR 35.65, then the exception in 10 CFR 35.65(c) does not apply and under the requirements of 10 CFR 30.32(g) the manufacturer’s name and model number for each requested sealed source and device must be provided.) Licensees will be authorized to possess and use only those sealed sources and devices specifically approved or registered by NRC, an Agreement State or a non-Agreement State, or certain sources when information required in 10 CFR 30.32(g)(3) is provided.
Under the EPAct, the NRC was given regulatory authority over additional byproduct material including accelerator-produced radionuclides and discrete sources of Ra-226. See 10 CFR 30.4 for a complete definition of byproduct material.
Applicants and licensees should determine whether they possess, or will possess, sealed sources or devices containing this new byproduct material for uses under 10 CFR 35.400, 10 CFR 35.500, 10 CFR 35.600, or 10 CFR 35.1000, as well as check, calibration, transmission, and references sources that are not included in 10 CFR 35.65.
Applicants will need to request authorization for possession of these sealed source(s) or device(s). It should also be noted that NRC’s regulatory authority includes the new byproduct material produced prior to August 8, 2005. As a result, neither the NRC, an Agreement State, nor a non-Agreement State, may have performed a safety evaluation of the sealed source or device and it may not have an Sealed Source and Device Registry (SSDR) certificate. Information that must be submitted for all sources is described in 10 CFR 30.32(g).
Discussion: The NRC or an Agreement State performs a safety evaluation of sealed sources and devices before authorizing a manufacturer to distribute the sources or devices to specific licensees. The safety evaluation is documented in an SSDR certificate. Some non-Agreement States may also have performed similar safety evaluations for sealed sources and devices containing NARM, and these safety evaluations may be documented in SSDR certificates.
Applicants must provide the manufacturer’s name and model number for each requested sealed source and device so that NRC can verify whether they have been evaluated in an SSDR certificate or specifically approved on a license. Applicants should include all possible new sources they might use, in order to minimize the need for license amendments if they change model or vendor.
If such a review has not been conducted for the specific source/device model(s), licensees should request a copy of the latest version of NUREG-1556, Volume 3, Revision 1, “Consolidated Guidance about Materials Licenses: Applications for Sealed Source and Device Evaluation and Registration,” from an NRC Regional Office and submit the information requested therein to NRC for review.
If the sealed source or device that has not been reviewed contains NARM material and was produced before the effective date of the rule, November 30, 2007, the information required by 10 CFR 32.210 may not be available. If this is the case, the applicant must provide the information required in 10 CFR 30.32(g)(3).
An applicant may consult with the proposed supplier or manufacturer to ensure that requested sources and devices are compatible with each other and that they conform to the SSDR designations registered with NRC or an Agreement State. Licensees may not make any changes to the sealed source, device, or source-device combination that would alter the description or specifications from those indicated in the respective SSDR certificates without obtaining NRC’s prior permission in a license amendment. Licensees providing information in accordance with the provisions of 10 CFR 30.32(g) may not make changes to the sealed sources, device, or source-device combination that would alter the description provided to NRC without obtaining NRC’s prior permission in a license amendment. To ensure that sealed sources and devices are used in ways that comply with the SSDR certificates, applicants may want to review or discuss them with the manufacturer.
Response from Applicant: If the possession of a sealed source(s) or device(s) is requested, the applicant shall submit the information described above.
Reference: See the Notice of Availability on the inside front cover of this report to obtain a copy of NUREG-1556, Volume 3, Revision 1, “Consolidated Guidance About Materials Licenses: Applications for Sealed Source and Device Evaluation and Registration,” and NUREG-1556, Volume 11, “Consolidated Guidance About Materials Licenses: Program-Specific Guidance About Licenses of Broad Scope.”
Note: To obtain copies of the SSDR certificate, applicants should contact the manufacturer/distributor of the device or the appropriate NRC Regional Office (see Figure 2.1 for addresses and telephone numbers).
[The following redline/strikeout revisions to Section 8.9 reflect changes to: 10 CFR 35.65 to clarify when used under 35.500 calibration, transmission, or references sources may not have to be listed on the license; changes to 10 CFR 35.400, 35.500, and 35.600 requiring sources be used in accordance with the radiation safety conditions and limitations described in the Sealed Source Device Registration not “as approved” in the Sealed Source Device Registration; and changes in 10 CFR 35.12 describing information needed for 10 CFR 35.1000 medical uses.]
8.9 ITEM 6: PURPOSE(S) FOR WHICH
LICENSED MATERIAL WILL BE USED
Part 35 |
Applicability |
100 |
✓ |
200 |
✓ |
300 |
✓ |
400 |
✓ |
500 |
✓ |
600 |
✓ |
1000 |
✓ |
Regulations: 10 CFR 30.32(j), 10 CFR 30.33(a)(1), 10 CFR 35.100, 10 CFR 35.200, 10 CFR 35.300, 10 CFR 35.400, 10 CFR 35.500, 10 CFR 35.600, 10 CFR 35.1000.
Criteria: In 10 CFR Part 35, byproduct material
for medical use is divided into seven types of use as follows:
10 CFR 35.100 |
Use of unsealed byproduct material for uptake, dilution, and excretion studies for which a written directive is not required |
10 CFR 35.200 |
Use of unsealed byproduct material for imaging and localization studies for which a written directive is not required |
10 CFR 35.300 |
Use of unsealed byproduct material for which a written directive is required |
10 CFR 35.400 |
Use of sources for manual brachytherapy |
10 CFR 35.500 |
Use of sealed sources for diagnosis |
10 CFR 35.600 |
Use of a sealed source(s) in a device for therapy-teletherapy unit |
Use of a sealed source(s) in a device for therapy-remote afterloader unit |
|
Use of a sealed source(s) in a device for therapy-gamma stereotactic radiosurgery unit |
|
10 CFR 35.1000 |
Other medical uses of byproduct material or radiation from byproduct material |
Under 10 CFR 30.32(j), medical use licensees within a consortium are authorized to produce PET radioactive drugs for noncommercial distribution to medical use licensees within the consortium. Appendix AA provides additional information on this 10 CFR Part 30 use.
Discussion:
10 CFR 35.100, 35.200, and 35.300 Use: For 10 CFR 35.100, 35.200, and 35.300 use, the applicant should define the purpose of use by stating the applicable section of 10 CFR Part 35 (e.g., 10 CFR 35.100) and the description of the applicable modality (e.g., any uptake, dilution, and excretion procedure for which a written directive is not required).
The use of unsealed byproduct material in therapy (10 CFR 35.300) involves administering a byproduct material, either orally or by injection, to treat or palliate a particular disease. The most common form of use of unsealed byproduct material for therapy is the treatment of hyperthyroidism with iodine-131 (I-131) sodium iodide. Other therapeutic procedures include, for example, ablation of thyroid cancer metastasis, treatment of malignant effusions, treatment of polycythemia vera and leukemia, palliation of bone pain in cancer patients, and radiation synovectomy for rheumatoid arthritis patients. References to particular diagnostic or treatment modalities in this section are intended to be examples and are not intended to imply that licensees are limited to these uses.
If an applicant’s request is limited to I-131 under 10 CFR 35.300, the license will be limited to that radionuclide.
35.400 Use: The applicant should define the purpose of use by stating that the applicable section of 10 CFR Part 35 is 10 CFR 35.400. If a source is to be used in a device, applicants may need to define the purpose of use by including the manufacturer’s name and model number of the device. The licensee should relate the sealed sources, including sealed sources of Ra-226, listed in Item 5 to the devices described in this item.
In manual brachytherapy, several types of treatments are available. These may include, for example:
∙ Interstitial Treatment of Cancer.
∙ Eye Plaque Implants. This is considered interstitial, not topical, treatment.
∙ Intracavitary Treatment of Cancer. For purposes of NRC’s sealed source and device evaluation on radiation safety issues, intraluminal use is considered analogous to intracavitary use.
∙ Topical (Surface) Applications.
35.500
Use:
For 10 CFR 35.500 use, the applicant should define the
purpose of use by stating that the applicable section of 10 CFR 35
is 10 CFR 35.500 and including the manufacturer’s
name(s) and model number(s) of devices containing sealed sources
(where applicable).
In accordance with 35.65(c), calibration, reference and transmission
sources used under the provisions of 35.500 do not have to be listed
on a specific medical use license; therefore in this case the
manufacturer and model numbers do not have to be provided. The
licensee should correlate the sealed sources, including sealed
sources of Ra-226, listed in Item 5 with the devices described in
this item. Typically,
a
A
licensee
should use the sealed sources according to the manufacturer’s
radiation safety and handling instructions and must use the sources
in
accordance with the radiation safety conditions and limitations
described
as
approved
in the SSDR.
35.600 Use: For 10 CFR 35.600 use, the applicant should define the purpose of use by stating the applicable section of 10 CFR Part 35.600 (e.g., teletherapy, remote afterloading, GSR) and including the manufacturer’s name(s) and model number(s) of the device(s) containing a sealed source(s) (e.g., for use in a [Manufacturer’s Name and Unit Type, Model xxxx] radiation therapy unit for the treatment of humans). The applicant should correlate the sealed source(s) listed in Item 5 with the device described in this item. If applicable, the applicant should state that depleted uranium is used as shielding for the device and specify that authorization is being requested for an additional source to be stored in its shipping container, incident to source replacement.
35.1000 Use: Applicants must apply for authorization to use byproduct material, or radiation therefrom, in medical applications under 10 CFR 35.1000 when the type of use is not covered under 10 CFR 35.100-35.600. This includes the medical use of unsealed Ra-226 or of Ra-226 sealed sources for uses other than those described by 10 CFR 35.400 or 35.500.
When applying for use under the provisions of 10 CFR 35.1000, applicants should:
Describe the purpose of use and submit the information required under Section 35.12(b) through (d).
,R
review regulatory requirements in other Subparts of 10 CFR Part 35, and use them as a guideon howto determine what should be included in an application that is required in Section 35.12.Address any aspects of the medical use of the material applicable to radiation safety but not addressed in, or different from those in, subparts A through C, L and M of 10 CFR Part 35.
Identify and commit to follow the applicable radiation safety program requirements in subparts D through H of 10 CFR Part 35.
Provide information, if needed, for additional radiation safety precautions and instructions, to describe methodologies for measurement of dosages or doses to be administered to patients or human research subjects, and describe any calibration, maintenance, and repair of instruments and equipment necessary for radiation safety.
If applying to use a 10 CFR 35.1000 medical use for which NRC has already developed guidance, review the applicable licensing guidance posted on NRC’s Medical Uses Licensee Toolkit website (http://www.nrc.gov/materials/miau/med-use-toolkit.html).
It is anticipated that many of the uses of byproduct material under the provisions of Section 35.1000 may involve research or product development; thus, applicants should ensure review and compliance with 10 CFR 35.6, “Provisions for the protection of human research subjects,” and 10 CFR 35.7, “FDA, other Federal, and State requirements.” Use of byproduct material in a source or device after approval by the U.S. Food and Drug Administration (FDA) (e.g., under an IDE (investigational device exemption) or an IND (investigational new drug exemption)), does not relieve individuals of the responsibility to obtain a license to use the byproduct material in medicine under the provisions of 10 CFR Part 35.
If the source for the type of use sought under 10 CFR 35.1000 is a sealed source, including sealed sources of Ra-226, Section 8.6 of this guide describes the information that must be provided at the time of application. Broad-scope licensees are exempted under 35.15(a) from requirements of 35.12(d) (which relates to the need to put into an application certain information about the radiation safety aspects of medical use under Section 35.1000). However, broad-scope licensees should ensure that the quantity needed for the proposed use is authorized on their license or apply for an increase if not. Applicants should refer to IN 99-024, “Broad-Scope Licensees’ Responsibilities for Reviewing and Approving Unregistered Sealed Sources and Devices” for more information on sealed sources.
Applicants for uses under Section 35.1000 should consult with the appropriate NRC Regional Office to discuss the contents of their application.
Nonmedical Uses: Applicants may also describe nonmedical uses (e.g., survey meter calibrations with NIST-traceable brachytherapy sources) and reference the applicable radioactive material provided in response to Item 5. This would include the nonmedical use of discrete sources of Ra-226.
Authorization under 10 CFR 30.32(j) to produce PET radioactive drugs for noncommercial transfer to licensees in its consortium for medical use is another nonmedical use. Applicants intending to produce PET radioactive drugs under this provision should include this use under this section, list the applicable radioactive materials under Item 5, and review Appendix AA for additional information.
Radionuclide Production by an Accelerator: Production of radionuclides for both medical and nonmedical uses is beyond the scope of this guidance and a medical use license. See NUREG-1556, Volume 21, “Consolidated Guidance About Materials Licenses: Program-Specific Guidance about Possession Licenses for Production of Radioactive Materials Using an Accelerator.”
Response from Applicant: The applicant must submit information regarding the purpose for which the licensed material will be used. The applicant should consider including the information described above, as applicable to the type of use(s) proposed.
When responding to this section, follow the guidance in Section 5.2 to determine if the response includes security-related sensitive information and needs to be marked accordingly.
[The following redline/strikeout revisions to Section 8.10 reflect changes to 10 CFR 35.57 grandfathering individuals that were board certified by boards listed in NRC regulations on or prior to October 24, 2005, and changes to 10 CFR 35.24 permitting licensees to name one or more Associate Radiation Safety Officers.]
8.10 ITEM 7: INDIVIDUAL(S) RESPONSIBLE FOR RADIATION SAFETY PROGRAMS AND THEIR TRAINING AND EXPERIENCE
Part 35 |
Applicability |
100 |
✓ |
200 |
✓ |
300 |
✓ |
400 |
✓ |
500 |
✓ |
600 |
✓ |
1000 |
✓ |
Regulations: 10 CFR 30.33(a)(3), 10 CFR 30.34(j), 10 CFR 33.13, 10 CFR 35.24, 10 CFR 35.50, 10 CFR 35.51, 10 CFR 35.55, 10 CFR 35.57, 10 CFR 35.59, 10 CFR 35.190, 10 CFR 35.290, 10 CFR 35.390, 10 CFR 35.392, 10 CFR 35.394, 10 CFR 35.396, 10 CFR 35.433(a)(2),10 CFR 35.490, 10 CFR 35.491, 10 CFR 35.590, 10 CFR 35.690.
Criteria: The RSO, ARSOs, AUs, AMPs, ophthalmic physicists, and ANPs must have adequate training and experience.
Discussion: “Authorized user (AU)” is not defined for nonmedical use, but for purposes of this discussion, the term AU will be used to also mean individuals who are authorized for such nonmedical uses. The requirements in 10 CFR 35.24 describe the authority and responsibilities for the Radiation Protection Program, including those of the licensee’s management and the RSO appointed by licensee management. Other personnel who have a role in the Radiation Protection Program are ARSOs, AUs, AMPs, ophthalmic physicists, ANPs, and members of the Radiation Safety Committee (RSC) (if the licensee is required to establish an RSC). The [date of the final rule] rule added two new individuals, the ARSO and the ophthalmic physicist, described in sections 8.11 and 8.14b, respectively.
In
10 CFR 30.33(a)(3), the NRC requires that an applicant be
qualified by training and experience to use licensed materials for
the purposes requested in such a manner as to protect health and
minimize danger to life or property. Subparts B, D, E, F, G, and H
of 10 CFR Part 35 give specific criteria for acceptable
training and experience for AUs for medical use, ANPs, the RSO,
ARSOs,
and
AMPs,
and ophthalmic physicists;
AUs for nonmedical uses must meet the criteria in 10 CFR 30.33(a)(3).
A résumé or a curriculum vitae is likely to be insufficient because such documents usually do not supply all the information needed to evaluate an individual’s training and experience for NRC purposes. Applicants should ensure that they submit the specific training information required by NRC regulations in 10 CFR Part 35. The NRC Form 313A series of forms provides a convenient format for submitting the information required in 10 CFR Part 35, Subparts B, D, E, F, G, and H. For nonmedical use AUs, the information provided should focus on educational training and radiation safety training and experience specific to the radionuclides and uses requested.
Licensees are responsible for their Radiation Protection Programs; it is essential that strong management control and oversight exist to ensure that licensed activities are conducted properly. The licensee’s management must appoint an RSO, who agrees in writing to be responsible for implementing the Radiation Protection Program, and must provide the RSO sufficient authority, organizational freedom, time, resources, and management prerogative to communicate with personnel and direct personnel regarding NRC regulations and license provisions, including: identifying radiation safety problems; initiating, recommending, or providing corrective actions; stopping unsafe operations; and verifying the implementation of corrective actions. Nevertheless, the licensee retains the ultimate responsibility for the conduct of licensed activities.
The licensee’s management can name only one RSO, who is the individual who remains responsible for implementing the entire radiation protection program. Although not required, the licensee’s management may appoint one or more ARSOs to support the RSO. The ARSO is delegated radiation safety duties and tasks for the types of uses for which he or she is listed on the license. These duties and task are also commensurate with his or her training and experience.
Licensees that are authorized for two or more different types of uses of byproduct material under Subparts E, F, and H, or two or more types of units under Subpart H are required under 10 CFR 35.24(f) to establish an RSC to oversee all uses of byproduct material permitted by the license. Membership in the committee must include an AU for each type of use permitted by the license, the RSO, a representative of the nursing service, and a representative of management who is neither an AU nor the RSO. The committee may include other members the licensee considers appropriate.
Licensees may contract for medical use services, including those involving patient services. However, the licensee should not assume that, by hiring a contractor to provide certain services, it has satisfied all regulatory requirements or that it has transferred responsibility for the licensed program to the contractor. Licensee management should ensure that adequate mechanisms for oversight are in place to determine that the Radiation Protection Program, including the training of contractor staff, is effectively implemented by the appropriate individuals.
Training
for an experienced RSO, teletherapy or medical physicist, AU or
nuclear pharmacist; recentness of training.
Under 10 CFR 35.57(a)(1) and
a(2),
experienced individuals, who may
be candidates to
serve as a
RSO, AMP, or ANP, before
[INSERT
DATE 180 DAYS AFTER PUBLICATION IN THE FEDERAL REGISTER]
are not required to meet the requirements of Sections 35.50, 35.51,
or 35.55, respectively (are “grandfathered”) for
the same materials and authorizations. Experienced RSOs and AMPs
after
[INSERT
DATE 180 DAYS AFTER PUBLICATION IN THE FEDERAL REGISTER]
must meet the requirements in Sections 35.51(d) and 35.51(c) for new
uses and authorizations. Under 10 CFR 35.57 (a)(2) and
(a)(3), experienced individuals, who may be candidates to serve as
RSO, AMP, or ANP, are not required to meet the requirements of
Sections 35.50, 35.51, or 35.55, respectively (are “grandfathered”),
for those materials and uses that these individuals performed on or
before October 24, 2005. (e.g.
the individual is named on an NRC or Agreement State license).
Under 10 CFR 35.57(b)(1) and
(b)(2)AUs
are also
not required to meet the requirements in Subparts D-H of
10 CFR Part 35 for
the same materials and uses performed before [INSERT
DATE 180 DAYS AFTER PUBLICATION IN THE FEDERAL REGISTER].
Under
10 CFR 35.57(b)(2), individuals not listed as an AU on
medical licenses or permits are not required to meet the requirements
in Subparts D-H of 10 CFR Part 35
for
the same materials and uses performed on October 24, 2005.
Subsequent
to the EPAct, RSOs, medical physicists, nuclear pharmacists,
physicians, podiatrists, and dentists that only used
accelerator-produced radioactive material, discrete sources of
Ra-226, or both, are also grandfathered, under NRC regulations in
10 CFR 35.57(a)(43)
and (b)(3), for medical uses or the practice of nuclear pharmacy when
using materials for the same uses performed before or under NRC’s
waiver issued August 31, 2005. The requirements in 10 CFR 35.59
(that the training and experience specified in 10 CFR 35,
Subparts B, D, E, F, G, and H, must have been obtained within 7 years
preceding the date of application or the individual must have related
continuing education and experience) do not apply to those
individuals “grandfathered” under the regulations
implementing the EPAct. Also, 10 CFR 35.57 provides that
nuclear pharmacists, medical physicists, physicians, dentists, and
podiatrists that meet the criteria in 10 CFR 35.57(a)(43)
and (b)(3) qualify as ANPs, AMPs, and AUs for those materials and
uses performed before or under NRC’s waiver of August 31, 2005.
Resolution to a petition for rulemaking by American Association of Physicists in Medicine allows recognition of certifications issued by boards previously listed in 10 CFR Part 35, Subpart J, which under September 16, 2004, rulemaking expired on October 24, 2005. This recognition permits experienced board certified individuals who were not recognized as authorized individuals on licensees or permits to be “grandfathered” for modalities they practiced on or before October 24, 2005. The recognized certification boards are named in 10 CFR 35.57.
Response from Applicant: Refer to the subsequent sections specific to the individuals described above.
[The following redline/strikeout revisions to Section 8.11 reflect changes to 10 CFR 35.57 grandfathering individuals that were board certified by boards listed in NRC regulations on or before October 24, 2005; changes to 10 CFR 35.24 permitting licensees to name one or more Associate Radiation Safety Officers; and changes to 10 CFR 35.50 training and experience pathways.]
8.11 ITEM 7: RADIATION SAFETY OFFICER
Part 35 |
Applicability |
100 |
✓ |
200 |
✓ |
300 |
✓ |
400 |
✓ |
500 |
✓ |
600 |
✓ |
1000 |
✓ |
Regulations: 10 CFR 30.33(a)(3), 10 CFR 35.2, 10 CFR 35.14, 10 CFR 35.24, 10 CFR 35.50, 10 CFR 35.57, 10 CFR 35.59, 10 CFR 35.2024.
Criteria: The RSOs and ARSOs must have adequate training and experience. The training and experience requirements for the RSO and ARSOs are described in 10 CFR 35.50 and allow for the following training pathways:
∙ Certification
as provided in 10 CFR 35.50(a) by a specialty board whose
certification process has been recognized by the NRC or an Agreement
State,
plus a written attestation signed by a preceptor RSO as provided in
35.50(d)
and completion
of
training as specified in 35.50(de);
or
∙ Completion
of classroom and laboratory training (200 hours) and 1 year of
full-time radiation safety experience as described in
10 CFR 35.50(b)(1) plus a written attestation signed by a
preceptor RSO or
ARSO
as provided in 10 CFR 35.50 (bd)(2)
and training as specified in 35.50(de);
or
∙ Certification
as provided in 10 CFR 35.50(c)(1) as a medical physicist
under 35.51(a), plus
a written attestation signed by a preceptor RSO as provided in
35.50(d)
demonstrating
that the proposed RSO or ARSO is qualified by experience with the
radiation safety aspects of similar types of use of byproduct
material for which the applicant seeks approval of an individual to
serve as RSO or ARSO,
and has completed training as specified in 35.50(de);
or
∙ Identification
as provided in 10 CFR 35.50(c)(2)on the
licensee’sa
Commission or Agreement State license,
a permit issued by a
Commission master
material license, a permit issued by a Commission or Agreement State
licensee of broad scope, or a permit issued by a Commission master
material license broad scope permittee
as an AU, AMP, or ANP with experience in the radiation safety aspects
of similar types of use
of
byproduct material use for which the licensee
seeks the approval of
the individual as
the has
RSO responsibilities
or
ARSO,
with
a written attestation signed by a preceptor RSO as provided in 10
CFR35.50(d)and
completion of
training as specified in 35.50(ed).
∙ Completion of training and experience required to be named as an AU and meet the requirements in 35.50(d) when simultaneously applying to be the AU and RSO on a new medical license as permitted by 10 CFR 35.50(c)(3).
The
licensee must also establish, in writing, the authority, duties, and
responsibilities of the RSO as required by 10 CFR 35.24(eb).
Discussion:
Radiation Safety Officer
The RSO is responsible for day-to-day oversight of the Radiation Protection Program. In accordance with 10 CFR 35.24, the licensee must provide the RSO sufficient authority, organizational freedom, time, and resources to perform his or her duties. Additionally, the RSO must have a sufficient commitment from management to fulfill the duties and responsibilities specified in 10 CFR 35.24 to ensure that radioactive materials are used in a safe manner. The NRC requires the name of the RSO on the license, and an agreement in writing from the RSO, to ensure that licensee management has identified a responsible, qualified person and that the named individual knows of his or her designation and assumes the responsibilities of an RSO.
Usually,
the RSO is a full-time employee of the licensed facility. The NRC
has authorized individuals who are not employed by the licensee, such
as a consultant, to fill the role of RSO
or to provide support to the facility RSO.
In order to fulfill the duties and responsibilities, the RSO should
be on site periodically to conduct meaningful, person-to-person
interactions with licensee staff, commensurate with the scope of
licensed activities, to satisfy the requirements of 10 CFR 35.24.
Appendix I contains a model RSO Delegation of Authority. Appendix B
contains NRC Form NRC 313A (RSO), “Radiation
Safety Officer or Associate Radiation Safety Officer Training,
Experience and Preceptor Attestation [10 CFR 35.57, 35.50]Medical
Use Training and Experience and Preceptor Attestation [35.50],”
which can be used to document the RSO’s training and
experience.
RSO Responsibilities: Some of the typical duties and responsibilities of RSOs include ensuring the following:
∙ Unsafe activities involving licensed materials are stopped;
∙ Radiation exposures are ALARA;
∙ Material accountability and disposal;
∙ Interaction with NRC;
∙ Timely and accurate reporting and maintenance of appropriate records;
∙ Annual program audits;
∙ Proper use and routine maintenance;
∙ Personnel
training;
and
∙ Investigation
of incidents involving byproduct material (e.g., medical events).;
and
∙ Assigning specific duties and tasks to an ARSO, restricted to the types of use for which the ARSO is listed on the license.
Appendix I contains a detailed list of typical duties and responsibilities of the RSO.
In
implementing the EPAct, the NRC “grandfathered” RSOs that
performed as RSOs for medical uses of only accelerator-produced
radioactive material, discrete sources of Ra-226, or both. These
individuals do not have to meet the requirements in either
10 CFR 35.59 or 10 CFR 35.50 when
performing the same uses;
however, the applicant must document that the individual meets the
criteria in 10 CFR 35.57 (a)(34).
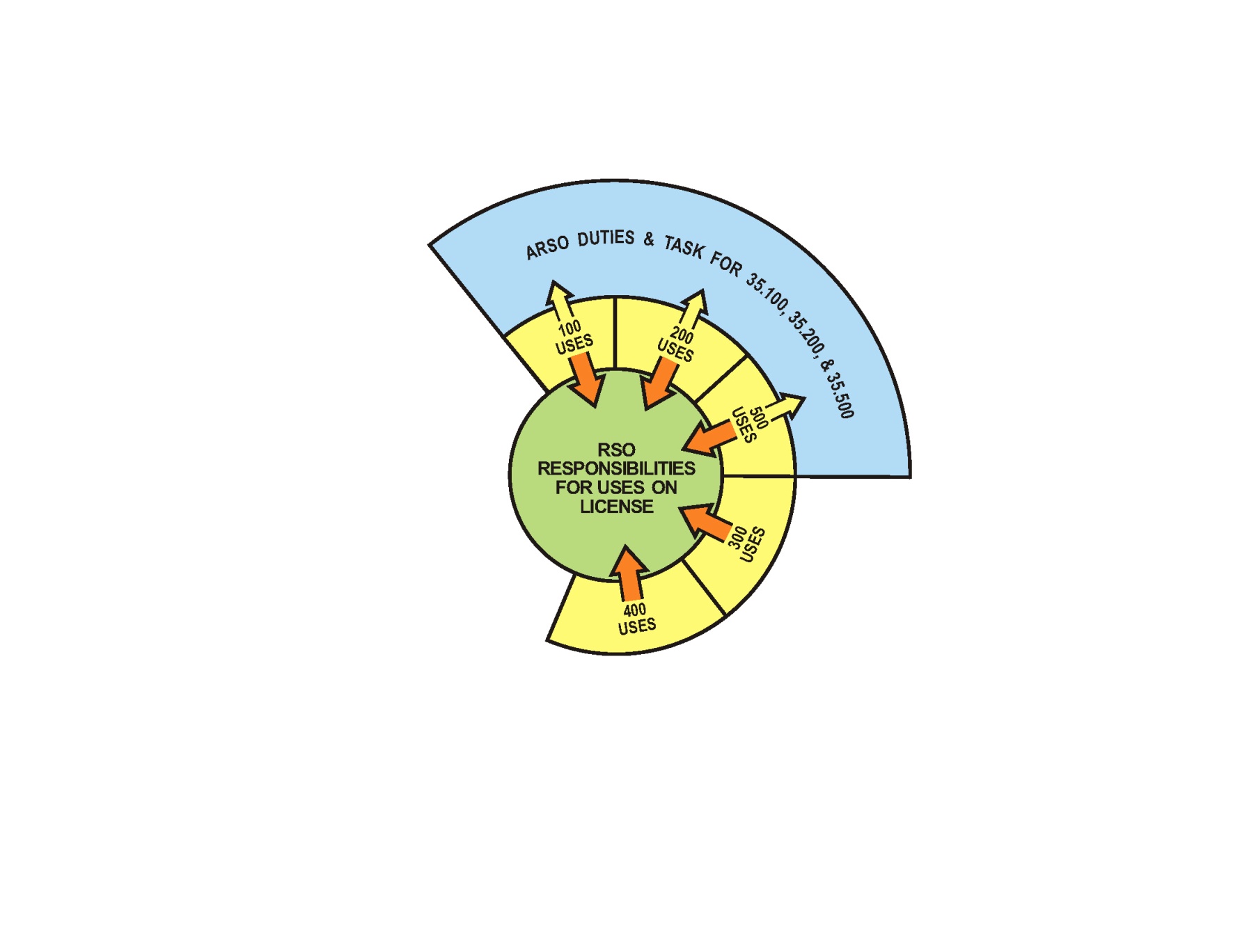
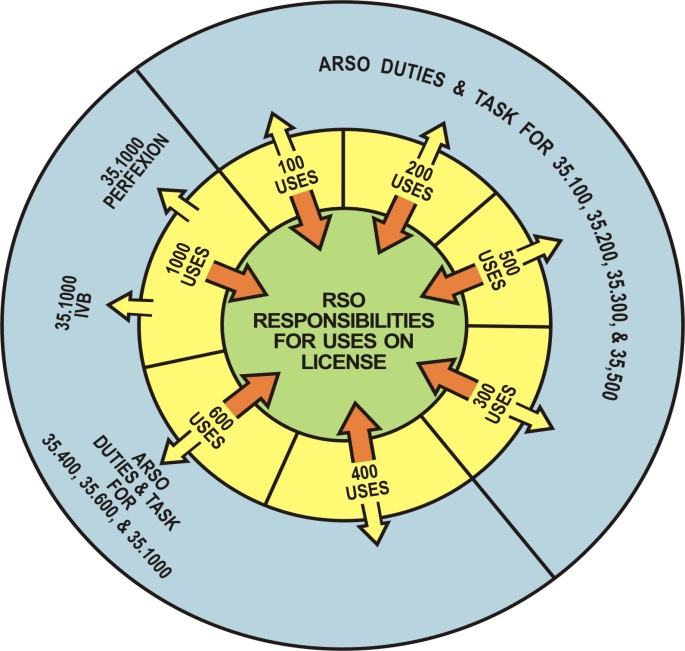
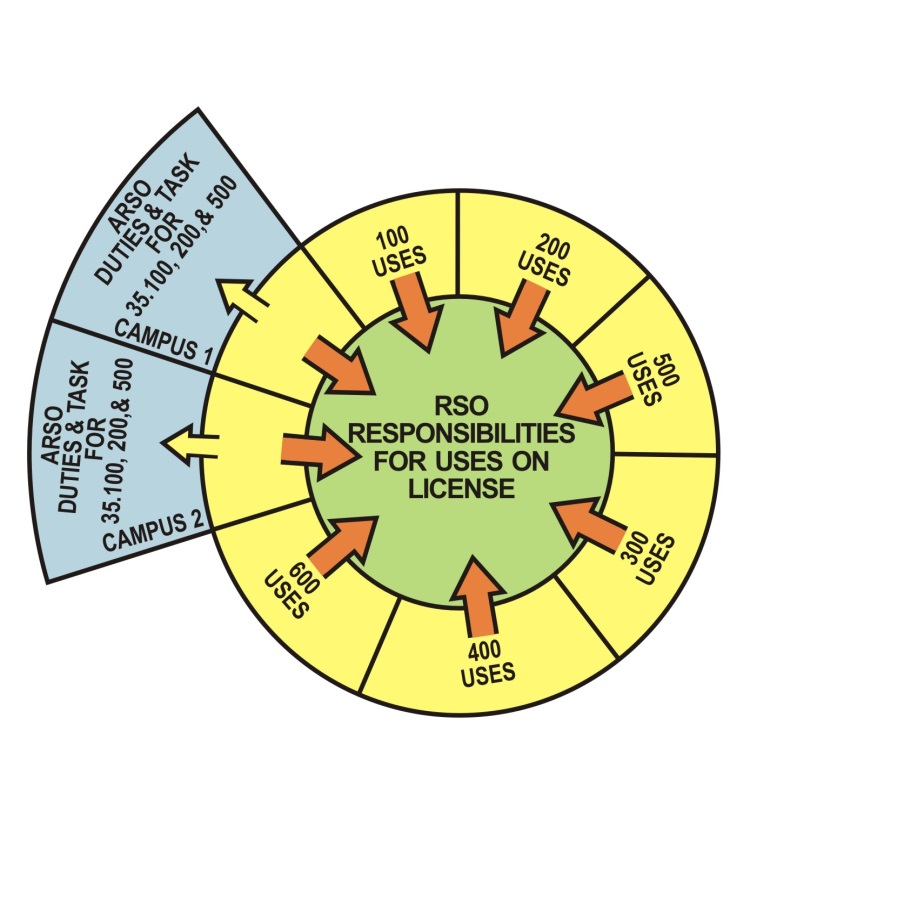
(b)
Figure 8.a1. Licensing Examples of Potential Radiation Safety
Officer (RSO) and Associate Radiation Safety Officer (ARSO)
arrangements: (a) a moderate sized program –
the RSO is responsible for the entire program and has direct
oversight over the 35.300 and 35.400 medical uses – a single
ARSO has oversight duties and tasks for 35.100, 35.200, and 35.500
medical uses and reports to the RSO. (b) a larger single campus program
– the RSO is responsible for the entire program – there
are two ARSOs with oversight duties and tasks over different
sections of the program and both report to the RSO. (c) A large multi-campus program –
the RSO is responsible for the entire program – there are two
ARSOs with oversight duties and tasks over the two smaller campuses.
Both ARSOs report to the RSO.
(c)
Associate Radiation Safety Officer
A licensee may choose to identify one or more individuals as ARSOs to support the RSO. The ARSOs could be assigned duties and tasks in the oversight of the radiation safety operations of designated sections of the licensed program, but the RSO retains responsibility for all sections of the program.
The ARSOs are required to complete the same training and experience requirements as the named RSO for their assigned sections of the radiation safety program. The RSO, with written agreement from licensee management, may assign duties and tasks to each ARSO that are limited to the types of use for which the ARSO is listed on the license. The ARSOs would perform duties and tasks in the oversight of the radiation safety operations of their assigned sections of the program, while reporting to the named RSO. The regulations continue to allow a licensee to name only one RSO on a license. Licensees with multiple operating locations or multiple types of uses can appoint a qualified ARSO at each location or for each type of byproduct material use. These individuals will be listed on the license as ARSOs. Their assigned sections of the program will also be listed.
Before the ARSO can be assigned duties and task in the oversight of the radiation safety operations of a different section of the program, the licensee must amend the license and provide documentation that the individual meets the training and experience requirements for the new duties and tasks.
Because the ARSOs have the same training and experience requirements as an RSO, the ARSOs will qualify to be named as the RSO on other licenses for the types of uses for which they are listed.
Requirements applicable to both RSO and ARSO
An AU, AMP, or ANP listed on any license or permit may be identified as an RSO or ARSO, consistent with the individual’s training and experience, allowing an increase in the number of qualified individuals available to serve as RSOs and ARSOs on NRC medical licenses. Additionally, both RSOs and ARSOs could serve as preceptors for individuals seeking to be named as the RSO or ARSO on a license.
If the individual was board certified by any of the boards listed in 35.57(a)(2) on or before October 24, 2005, the applicant must provide documentation that the individual used the materials and performed the medical uses before October 24, 2005 to meet the requirements to be an RSO or ARSO for those materials and uses. This documentation will be reviewed on a case-by-case basis to see if the time period of use, the materials used, and the types of use meet the criteria in the regulation. This provision of the rule lets these board certified individuals that were not named as an RSO but performing radiation safety duties and tasks prior to October 24, 2005, to be “grandfathered.”
Applicants are reminded of recentness of training requirements described in 10 CFR 35.59. Specifically, RSO and ARSO applicants must have successfully completed the applicable training and experience described in 10 CFR Part 35 within 7 years preceding the date of the application. Alternatively, RSO and ARSO applicants must have had related continuing education and experience since completing the required training and experience. This time provision applies to board certification as well as to other pathways to meeting requirements for training and experience.
Response from Applicant: Provide the following:
∙ Name
of the individualproposed
RSO.
AND
∙ Identify if applying for RSO or ARSO.
AND
∙ For a proposed ARSO, identify the section(s) of the licensee’s program for which the individual will be given duties and task in the oversight of radiation safety operations (e.g., 35.200 uses or 35.200 uses at one of the licensees alternate location)
AND
For an individual previously identified as an RSO or ARSO on an NRC or Agreement State license or permit:
∙ Previous license number (if issued by the NRC) or a copy of the license (if issued by an Agreement State) or a copy of a permit issued by an NRC master materials licensee on which the individual was named as the RSO or ARSO if requesting the same materials and medical uses;
AND
∙ After [DATE THAT IS 180 DAYS AFTER THE DATE OF PUBLICATION IN THE FEDERAL REGISTER], documentation of completion of the training requirements in § 35.50(d) for any new materials or new medical uses requested.
For an individual qualifying under
10 CFR 35.57 (a)(43):
(Note: This is only for a new medical use license requesting use of only accelerator-produced radioactive material, discrete sources of Ra-226, or both, for the same uses authorized under NRC’s waiver of August 31, 2005.)
∙ Documentation
that this individual functioned as an RSO for only
accelerator-produced radioactive materials, discrete sources of
Ra-226, or both, at
a Government agency or Federally recognized Indian Tribe before
November
30, 2007, or
at
all other locations of use before August 8, 2009, or an earlier date
as noticed by the NRC before
or
during
the effective period of NRC’s waiver of August 7, 2005;
AND
∙ Documentation that the individual performed as the RSO for the same medical uses requested.
For an individual qualifying under 10 CFR 35.50(a):
∙ Copy of certification by a specialty board whose certification process has been recognized1 by the NRC or an Agreement State under 10 CFR 35.50(a);
AND
∙ Description
of the training and experience specified in 10 CFR 35.50(de)
demonstrating that the proposed RSO or
ARSO
is qualified by training in the radiation safety, regulatory issues,
and emergency procedures as applicable to the types of use for which
the applicant seeks approval of an individual to serve as RSO or
ARSO;
AND
∙ Written
attestation, signed by a preceptor RSO, that the individual has
successfully completed the training and experience specified for
certification, as well as Completed the required training and
experience in radiation safety, regulatory issues, and emergency
procedures for the types of use for which the licensee seeks approval
and has achieved a level of radiation safety knowledge sufficient to
function independently as an RSO.
AND
∙ If applicable, description of recent related continuing education and experience as required by 10 CFR 35.59.
For an individual qualifying under 10 CFR 35.50(c)(1):
∙ Copy
of the certification(s) as a medical physicist by a board whose
certification process has been recognized2
by the NRC or an Agreement State under 10 CFR 35.51(a) and
description of the experience specified in 35.50(c)(1) demonstrating
that the proposed RSO or
ARSO
is qualified by experience
with the radiation safety aspects of similar types of use
of
byproduct materialapplicable
to the types of use
for which the applicant seeks approval of an individual to serve as
RSO or
ARSO
;
AND
∙ Description
of the training and experience specified in 10 CFR 35.50(de)
demonstrating that the proposed RSO or
ARSO
is qualified by training in radiation safety, regulatory issues, and
emergency procedures as
applicable to the types of use for which the applicant seeks approval
of an individual to serve as RSO or
ARSO;
AND
∙ Written
attestation, signed by a preceptor RSO, that the individual has
successfully completed the training and experience specified for
certification, as well as Completed the required training and
experience in radiation safety, regulatory issues, and emergency
procedures for the types of use for which the licensee seeks approval
and has achieved a level of radiation safety knowledge sufficient to
function independently as an RSO.
AND
∙ If applicable, description of recent related continuing education and experience as required by 10 CFR 35.59.
For an individual qualifying under 10 CFR 35.57(a)(2):
∙ Copy of certification by a specialty board whose certification is listed in 10 CFR 35.57(a) (2);
AND
∙ Documentation demonstrating that the individual was using the requested materials and uses on or before October 24, 2005;
AND
∙ If applicable, description of recent related continuing education and experience as required by 10 CFR 35.59.
For an individual qualifying under 10 CFR 35.50(c)(2):
∙ Copy of the Commission
or Agreement State
licensee’s license,
permit issued by a Commission master material license, permit issued
by a Commission or Agreement State licensee of broad scope, or permit
issued by a Commission master material license broad scope permittee
indicating that the individual is an AU, AMP or ANP identified
on the licensee’s license and
has experience with the radiation safety aspects of similar types of
use of byproduct material for which the applicant seeks approval of
an individual to serve as RSO or
ARSO ;
AND
∙ Description of the training and
experience specified in 10 CFR 35.50(de)
demonstrating that the proposed RSO or
ARSO is qualified by training
in radiation safety, regulatory issues, and emergency procedures
applicable to the types of use for which the applicant seeks approval
of an individual to serve as RSO
AND
∙ Written
attestation, signed by a preceptor RSO, that the individual has
successfully completed the training and experience specified for
certification, as well as Completed the required training and
experience in radiation safety, regulatory issues, and emergency
procedures for the types of use for which the licensee seeks approval
and has achieved a level of radiation safety knowledge sufficient to
function independently as an RSO.
AND
∙ If applicable, description of recent related continuing education and experience as required by 10 CFR 35.59.
For an individual qualifying under 10 CFR 35.50(c)(3):
∙ Documentation of training and experience required to be named as an AU when simultaneously applying to be the AU and RSO on a new medical license;
AND
∙ Description of the training and experience specified in 10 CFR 35.50(d) demonstrating that the proposed RSO is qualified by training in radiation safety, regulatory issues, and emergency procedures as applicable to the types of use for which the applicant seeks approval of an individual to serve as RSO;
For an individual qualifying under 10 CFR 35.50(b):
∙ Description of the training and experience specified in 10 CFR 35.50(b)(1) demonstrating that the proposed RSO or ARSO is qualified by training and experience as applicable to the types of use for which the applicant seeks approval of an individual to serve as RSO or ARSO;
AND
∙ Description
of the training and experience specified in 10 CFR 35.50(de)
demonstrating that the proposed RSO or
ARSO
is qualified by training in radiation safety, regulatory issues, and
emergency procedures as applicable to the types of use for which the
applicant seeks approval of an individual to serve as RSO or
ARSO;
AND
∙ Written
attestation, signed by a preceptor RSO or
ARSO,
that the individual has successfully completed the training and
experience in 10 CFR 35.50(b)(1), as well as the required training
and experience in radiation safety, regulatory issues, and emergency
procedures for the types of use for which the licensee seeks approval
and is
ablehas
achieved a level of radiation safety knowledge sufficient
to function
independently
fulfill
the radiation safety-related duties
as an RSO or
as an ARSO
for a medical use licensee;
AND
∙ If applicable, description of recent related continuing education and experience as required by 10 CFR 35.59.
Notes:
∙ NRC
Form 313A (RSO), “Radiation Safety Officer and
Associate Radiation Safety Officer
Training, and
Experience,
and Preceptor Attestation [10 CFR 35.57,35.50],”
may be used to document training and experience for those individuals
qualifying under 10 CFR 35.50.
∙ The
licensee must notify the NRC within 30 days if, under 10 CFR 35.14,
an RSO or
ARSO
permanently discontinues his
or her
performance
of
duties under the license or has a name change; licensees must also
request an amendment to change an RSO or
ARSO
under 10 CFR 35.13.
∙ An
AU for medical uses, AMP, or ANP may be designated as the RSO or
ARSO
on the license if the individual has experience with the radiation
safety aspects of similar types of byproduct material use for which
he or she will
have has
RSO responsibilities or
ARSO duties and tasks
(see 10 CFR 35.50(c)(2)) and the RSO, as required by
10 CFR 35.24(g), has sufficient time, authority,
organizational freedom, resources, and management prerogative to
perform the duties.
∙ Descriptions of training and experience will be reviewed using the criteria listed above. The NRC will review the documentation to determine if the applicable criteria in 10 CFR Part 35, Subpart B, are met. If the training and experience do not appear to meet the criteria in Subpart B, the NRC may request additional information from the applicant or may request the assistance of the Advisory Committee on the Medical Uses of Isotopes (ACMUI) in evaluating such training and experience.
∙ The training and experience for the RSO of a medical use broad-scope license will be reviewed using the above criteria as well as criteria in 10 CFR Part 33.
[The following redline/strikeout revisions to Section 8.12 reflect changes to 10 CFR 35.57 grandfathering individuals that were board certified by boards listed in NRC regulations prior to October 25, 2005, and changes to 10 CFR 35.190, 35.290, 35.390, 35.392, 35.394, 35.396, 35.490, 35.491, and 35.690 revising the preceptor attestation requirements. The revisions to preceptor attestation requirements include rewording the attestation statement, removing the attestation requirement for most board certified individuals, and allowing residency program directors to provide attestation statements.]
8.12 ITEM 7: AUTHORIZED USERS (AUs)
Part 35 |
Applicability |
100 |
✓ |
200 |
✓ |
300 |
✓ |
400 |
✓ |
500 |
✓ |
600 |
✓ |
1000 |
✓ |
Regulations: 10 CFR 30.33(a)(3), 10 CFR 35.2, 10 CFR 35.11, 10 CFR 35.14, 10 CFR 35.27, 10 CFR 35.57, 10 CFR 35.59, 10 CFR 35.190, 10 CFR 35.290, 10 CFR 35.390, 10 CFR 35.392, 10 CFR 35.394, 10 CFR 35.396, 10 CFR 35.490, 10 CFR 35.491, 10 CFR 35.590, 10 CFR 35.690.
Criteria: Training and experience requirements for AUs for medical uses are described in 10 CFR 35.190, 10 CFR 35.290, 10 CFR 35.390, 10 CFR 35.392, 10 CFR 35.394, 10 CFR 35.396, 10 CFR 35.490, 10 CFR 35.491, 10 CFR 35.590, or 10 CFR 35.690.
Discussion: Although NRC does not define “AU” for nonmedical uses, for purposes of this discussion the term AU will be used to also mean individuals authorized for such nonmedical uses.
AU for Medical Uses: The responsibilities of AUs involved in medical use include the following:
∙ Radiation safety commensurate with use of byproduct material;
∙ Administration of a radiation dose or dosage and how it is prescribed;
∙ Direction of individuals under the AU’s supervision in the preparation of byproduct material for medical use and in the medical use of byproduct material;
∙ Preparation of written directives (WD), if required.
Applicants must meet recentness of training requirements described in 10 CFR 35.59. The AU applicants must have successfully completed the applicable training and experience criteria described in 10 CFR Part 35 within 7 years preceding the date of the application. Alternatively, applicants must have had related continuing education and experience since completing the required training and experience. This time provision applies to board certification as well as to other training pathways.
Section
35.57 of 10 CFR Part 35 provides that experienced AUs who
are named on a license or permit are not required to comply with the
training requirements in Subparts D through H to continue performing
those medical uses for which they were authorized before the
effective date of changes to the regulations in Section 35.57 (check
the regulations to determine this date).
For example, a physician who was authorized to use sodium iodine-131
for imaging and localization, involving greater than 30 microcuries
(a quantity for which a written directive is required under
10 CFR 35.40), a
use that was authorized under Section 35.200 prior to 2002,
would continue to be authorized for this use even
though it is currently authorized under Section 35.300 after 2002.
If the physician, dentist, or podiatrist has never been identified as an AU and was board certified by any of the boards listed in 35.57(b)(2) on or before October 24, 2005, the applicant must provide documentation that the individual used the materials and performed the medical uses before October 24, 2005 to meet the requirements to be an AU for those materials and uses. This documentation will be reviewed on a case-by-case basis to see if the time period of use, the materials used, and the types of medical use meet the criteria in the regulation. This provision of the rule “grandfathers” these board certified individuals that were never named as an AU but perform medical uses with the same materials prior to October 24, 2005.
In implementing the EPAct, the NRC “grandfathered” physicians, podiatrists, and dentists using only accelerator-produced radioactive materials, discrete sources of Ra-226, or both, for medical use, for the same uses performed before or under the NRC waiver of August 31, 2005. These individuals do not have to meet the requirements in 10 CFR 35.59, 35.190, 35.290, 35.390, 35.396, or 35.490. However, the applicant must document that the individual meets the criteria in 10 CFR 35.57(b)(3). This Section also states that physicians, dentists, and podiatrists who met certain criteria will qualify as AUs for those materials and uses performed before NRC’s waiver was terminated for them.
Technologists, therapists, or other personnel may use byproduct material for medical use under an AU’s supervision in accordance with 10 CFR 35.27, “Supervision,” and in compliance with applicable FDA, other Federal, and State requirements (10 CFR 35.7). Examples include FDA requirements for the conduct of certain types of clinical research after the submission of applications for Investigational New Drugs (IND) and under the auspices of a Radioactive Drug Research Committee (21 CFR 361.1).
There is no NRC requirement that an AU must render an interpretation of a diagnostic image or results of a therapeutic procedure. The NRC recognizes that the AU may or may not be the physician who interprets such studies. Additionally, NRC regulations do not restrict who can read and interpret diagnostic scans or the results of therapeutic procedures involving the administration of byproduct material to individuals.
An individual, who is qualified to be an AU but has not been named as an AU on a medical use license or permit may apply for and be authorized simultaneously as the RSO and the AU on the same new medical use license. The individual must have experience with radiation safety aspects of the types of use of byproduct material for which the individual is seeking simultaneous authorization and training specified in 35.50(d).
A licensee may request an AU on any medical use license or permit to be named as the RSO or ARSO for the same byproduct material and uses for which the AU is authorized.
AU for Nonmedical Uses: For in vitro studies, animal research, calibration of survey instruments, and other uses that do not involve the intentional exposure of humans, the list of proposed AUs should include the individuals who will actually be responsible for the safe use of the byproduct material for the requested use. This includes the individuals responsible for the production of PET radioactive drugs for noncommercial transfer to other medical users within a consortium (see Appendix AA).
An applicant should note which user will be involved with a particular use by referring to Items 5 and 6 of the application and providing information about the user’s training and experience.
Authorized nonmedical use or uses that do not involve the intentional exposure of humans (e.g., in vitro and animal research, calibration, dosimetry research) will be reviewed on a case-by-case basis.
Response from Applicant:
AU for Medical Uses: Provide the following:
∙ Name of the proposed AU and uses requested;
AND
∙ Medical, podiatry, or dental license number and issuing entity;
AND
For an individual previously identified as an AU on an NRC or Agreement State license or permit:
∙ Previous license number (if issued by the NRC) or a copy of the license (if issued by an Agreement State) or a copy of a permit issued by an NRC master materials licensee, a permit issued by an NRC or Agreement State broad-scope licensee, or a permit issued by an NRC Master Materials License broad-scope permittee on which the physician, dentist, or podiatrist was specifically named as an AU for the uses requested;
AND
∙ For an AU requesting a medical use not currently authorized on a license or permit, a description of the additional training and experience is needed to demonstrate the AU is also qualified for the new medical uses requested (e.g., training and experience needed to meet the requirements in 10 CFR 35.290(b), 35.396, 35.390(b)(1)(ii)(G) or 35.690(c)). A preceptor attestation may also be required. (For example, a preceptor attestation is needed for all individuals to meet the requirements of 10 CFR 35.396 and for individuals seeking authorization through the alternate training and experience pathway for 35.390 and 35.690.)
AND
∙ If applicable, description of recent related continuing education and experience as required by 10 CFR 35.59.
For an individual qualifying under 10 CFR 35.57(b)(3):
∙ Documentation that the physician,
dentist, or podiatrist used only accelerator-produced radioactive
materials, discrete sources of Ra-226, or both, for medical uses
performed at a
Government agency or Federally recognized Indian Tribe before
November 30, 2007, or at all other locations of use before August 8,
2009, or an earlier date as noticed by the NRC
before or during
the effective period of NRC’s waiver of August 31, 2005;
AND
∙ Documentation that the physician, dentist, or podiatrist used these materials for the same medical uses requested;
AND
∙ For an AU requesting a medical use
for which he or she is not currently authorized on a license or
permit, a description of the additional training and experience to
demonstrate the AU is also qualified for the new medical uses
requested
(e.g., training and experience needed to meet the requirements in
10 CFR 35.290(b), 35.396, 35.390(b)(1)(ii)(G) or
35.690(c)). A preceptor
attestation may also be required. (For example, a
preceptor attestation is needed for all individuals to meet the
requirements of 10 CFR 35.396 and for individuals seeking
authorization through the alternate training and experience pathway
for 35.390 and 35.690training,
experience, and attestations are needed to meet the requirements in
10 CFR 35.290(b), 35.396, 35.390(b)(1)(ii)(G) or
35.690(c).)
AND
∙ If applicable, description of recent related continuing education and experience as required by 10 CFR 35.59.
For an individual who was certified before October 24, 2005, by a board listed in 10 CFR 35.57(b)(2):
∙ Copy of certification issued before October 24, 2005, by a specialty board whose certification is listed in 10 CFR 35.57(b)(2);
AND
∙ Documentation demonstrating that the individual was using the requested materials and uses on or before October 24, 2005;
AND
∙ If applicable, description of recent related continuing education and experience as required by 10 CFR 35.59.
For an individual qualifying under 10 CFR Part 35, Subparts D, E, F, G, and/or H, who is board-certified:
∙ A copy of the certification(s) by a specialty board(s) whose certification process has been recognized3 by the NRC under 10 CFR Part 35, Subpart D, E, F, G, or H, as applicable to the use requested;
AND
∙ For a physician with a board certification recognized under 10 CFR 35.390, a description of the supervised work experience administering dosages of radioactive drugs required in 10 CFR 35.390(b)(1)(ii)(G) demonstrating that the proposed AU is qualified for the types of administrations for which authorization is sought;
AND
∙ For a physician with a board certification recognized under 10 CFR 35.390 for medical uses described in 10 CFR 35.200, a description of the supervised work experience eluting generator systems required in 10 CFR 35.290(c)(1)(ii)(G) demonstrating that the proposed AU is also qualified for imaging and localization medical uses;
AND
∙ For a physician with a board
certification recognized under 10 CFR 35.490 or
10 CFR 35.690 for medical uses
described in 10 CFR 35.396, a description of the training
and supervised work experience required
in10 CFR 35.396(b)(1)and (2) and
a copy of the attestation required in 10 CFR 35.396(b)(3)d)
to demonstrate qualifications for administering parenteral
administrations of unsealed byproduct material requiring a written
directive;
AND
∙ For an individual seeking authorization under 10 CFR Part 35, Subpart H, a description of the training specified in 10 CFR 35.690 (c) demonstrating that the proposed AU is qualified for the type(s) of use for which authorization is sought;
AND
∙ A written attestation,
signed by a preceptor physician AU, that the training and experience
specified for certification have been satisfactorily completed and
that a level of competency sufficient to function independently as an
AU for the medical uses authorized has been achieved. For
individuals seeking authorization under 10 CFR 35.390,
10 CFR 35.396, and 10 CFR 35.690, the
attestation must also include successful completion of the clinical
case work in 10 CFR 35.390(b)(1)(ii)(G), or training and
experience required by 10 CFR 35.396(d), or training for
10 CFR 35.600 types of use, as appropriate;
AND
∙ If applicable, a description of recent related continuing education and experience as required by 10 CFR 35.59.
For an individual qualifying under 10 CFR Part 35, Subparts D, E, F, G, and/or H, who is not board-certified:
∙ A description of the training and experience identified in 10 CFR Part 35, Subparts D, E, F, G, and H, demonstrating that the proposed AU is qualified by training and experience for the use(s) requested;
AND
∙ For an individual seeking authorization under 10 CFR Part 35, Subpart H, a description of the training specified in 10 CFR 35.690(c), demonstrating that the proposed AU is qualified for the type(s) of use for which authorization is sought;
AND
∙ A written attestation, signed by a
preceptor physician AU or
if applicable the residency program director,
that the above training and experience have been satisfactorily
completed and the
individual is able to that
a level of competency sufficient to function
independently fulfill
the radiation safety-related duties
as an AU for the requested
medical uses authorized has
been achieved;
AND
∙ If applicable, a description of recent related continuing education and experience as required by 10 CFR 35.59.
Notes:
∙ NRC Form 313A (AUD), “Authorized
User Training,
and
Experience and Preceptor Attestation (for uses defined under 35.100,
35.200, and 35.500) [10 CFR 35.57,
35.190, 35.290, and 35.590]”;
or NRC Form 313A (AUT), “Authorized User Training,
and
Experience and Preceptor Attestation (for uses defined under 35.300)
[10 CFR 35.57,
35.390, 35.392, 35.394, and
35.396]”; or NRC Form 313A (AUS), “Authorized User
Training,
and
Experience and Preceptor Attestation (for uses defined under 35.400
and 35.600) [10 CFR 35.57,
35.490, 35.491, and 35.690]”
may be used as appropriate to document training and experience for
those individuals qualifying under 10 CFR Part 35, Subparts D, E, F,
G, and/or H.
∙ Under
10 CFR 35.14, Llicensees
must notify the NRC within 30 days if an AU permanently discontinues
his or her duties under the license or has a name change under
10 CFR 35.14.
∙ Descriptions of training and experience will be reviewed using the criteria listed above. The NRC will review the documentation to determine if the applicable criteria in 10 CFR Part 35 are met. If the training and experience do not appear to meet the 10 CFR Part 35 criteria, the NRC may request additional information from the applicant or may request the assistance of the ACMUI in evaluating such training and experience.
Note to reviewers: Licenses will reflect any limitations on use for listed AUs (e.g., whether administrations in excess of 33 mCi of iodine-131 are allowed and specific uses under 10 CFR 35.600).
AU for Nonmedical Uses: Provide the following:
∙ Name of the proposed nonmedical use AU,
∙ Description of types, quantities, and proposed nonmedical uses for which the individual is responsible, and
∙ Description of individual’s educational and radiation safety training and experience with the types of materials and uses requested. This may include:
A copy of the NRC or Agreement State License listing the individual as an AU for the same types, quantities, and uses requested.
A permit issued by a Master Materials License licensee or broad-scope licensee or broad-scope permittee identifying the individual as an AU for the types, quantities, and uses requested.
Note: Authorized nonmedical use or uses that do not involve the intentional exposure of humans (e.g., in vitro and animal research, calibration, dosimetry research) will be reviewed on a case-by-case basis.
[The following redline/strikeout revisions to Section 8.13 reflect changes to 10 CFR 35.55 revising the wording of the attestation statement and removing the attestation requirement for board certified individuals.]
Part 35 |
Applicability |
100 |
✓ |
200 |
✓ |
300 |
✓ |
400 |
|
500 |
|
600 |
|
1000 |
✓ |
8.13 ITEM 7: AUTHORIZED NUCLEAR
PHARMACIST (ANP)
Regulations: 10 CFR 30.33(a)(3), 10 CFR 32.72(b)(2), 10 CFR 35.2, 10 CFR 35.11, 10 CFR 35.14, 10 CFR 35.27, 10 CFR 35.55, 10 CFR 35.57, 10 CFR 35.59.
Criteria: Training and experience requirements for
ANPs are described in 10 CFR 35.55.
Discussion: At many licensed medical facilities, an ANP is directly involved with the preparation of radiopharmaceuticals under the provisions of 10 CFR 35.100(b), 35.200(b), or 35.300(b). This may include the production of PET radioactive drugs under the provisions of 10 CFR 30.32(j).
Technologists, or other personnel, may prepare byproduct material for medical use under an ANP’s supervision in accordance with 10 CFR 35.27, “Supervision,” and in compliance with applicable FDA, other Federal, and State requirements (10 CFR 35.7). (Preparation of byproduct material for medical use may also be performed under the supervision of a physician who is an AU.)
Applicants are reminded that the recentness of training requirements described in 10 CFR 35.59 also apply to training and experience requirements in 10 CFR Part 35, Subpart B. Specifically, nuclear pharmacist applicants must have successfully completed the applicable training and experience criteria described in 10 CFR Part 35 within 7 years preceding the date of the application. Alternatively, nuclear pharmacist applicants must have had related continuing education and experience since initially completing the required training and experience. This time provision applies to board certification as well as to other training pathways for meeting requirements for training and experience.
In
implementing the EPAct, the NRC “grandfathered” nuclear
pharmacists using only accelerator-produced radioactive materials,
discrete sources of Ra-226, or both, in the practice of nuclear
pharmacy for the uses performed before or under the NRC waiver of
August 31, 2005. These individuals do not have to meet the
requirements of 10 CFR 35.59 or 10 CFR 35.55. The
applicant must, however, document that the individual meets the
criteria in 10 CFR 35.57(a)(43).
Section 35.57 also provides that nuclear pharmacists who met certain
criteria will qualify as ANPs for those materials and uses performed
before or under NRC’s waiver of August 31, 2005.
Response from Applicant: Provide the following:
∙ Name of the proposed ANP;
AND
∙ Pharmacist’s license number and issuing entity;
AND
For an individual previously identified as an ANP on an NRC or Agreement State license or permit or by a commercial nuclear pharmacy that has been authorized to identify ANPs:
∙ Previous license number (if issued by the NRC) or a copy of the license (if issued by an Agreement State) or a copy of a permit issued by an NRC master materials licensee, a permit issued by an NRC or Agreement State broad-scope licensee, or a permit issued by an NRC Master Materials License broad-scope permittee on which the individual was named an ANP or a copy of an authorization as an ANP from a commercial nuclear pharmacy that has been authorized to identify ANPs.
AND
∙ If applicable, description of recent related continuing education and experience as required by 10 CFR 35.59.
OR
For an individual qualifying under
10 CFR 35.57(a)(43):
∙ Documentation that the nuclear
pharmacist used only accelerator-produced radioactive material,
discrete sources of Ra-226, or both, in the practice of pharmacy at
a Government agency or Federally recognized Indian Tribe before
November 30,
2007, or at
all other locations of use before August 8, 2009, or at an earlier
date as noticed by the NRC before
or during the effective period of NRC’s waiver of August 31,
2005;
AND
∙ Documentation that the nuclear pharmacist used these materials for the same uses as requested.
AND
∙ If applicable, description of recent related continuing education and experience as required by 10 CFR 35.59.
OR
For an individual qualifying under 10 CFR 35.55(a):
∙ Copy of the certification of the specialty board whose certification process has been recognized1 under 10 CFR 35.55(a);
AND
∙ Written attestation,
signed by a preceptor ANP, that training and experience required for
certification have been satisfactorily completed and that a level of
competency sufficient to function independently as an ANP has been
achieved.
AND
∙ If applicable, description of recent related continuing education and experience as required by 10 CFR 35.59.
OR
For an individual qualifying under 10 CFR 35.55(b):
∙ Description of the training and experience specified in 10 CFR 35.55(b) demonstrating that the proposed ANP is qualified by training and experience;
AND
∙ Written attestation, signed by a
preceptor ANP, that the above training and experience have been
satisfactorily completed and that the
individual is able competency
sufficient to
function independently
fulfill the radiation safety-related duties
as an ANP has been achieved;
AND
∙ If applicable, description of recent related continuing education and experience as required by 10 CFR 35.59.
Notes:
∙ NRC Form 313A (ANP), “Authorized
Nuclear Pharmacist Training,
and
Experience,
and Preceptor Attestation [10 CFR 35.55]” may be used to
document training and experience for those individuals qualifying
under 10 CFR 35.55.
∙ Under 10 CFR 35.14, licensees must notify the NRC within 30 days if an ANP permanently discontinues his or her duties under the license or has a name change.
∙ Descriptions of training and experience will be reviewed using the criteria listed above. The NRC will review the documentation to determine if the applicable criteria in 10 CFR Part 35, Subpart B, are met. If the training and experience do not appear to meet the criteria in Subpart B, the NRC may request additional information from the applicant or may request the assistance of the ACMUI in evaluating such training and experience.
[The following redline/strikeout revisions to Section 8.14 reflect changes to 10 CFR 35.51 revising the attestation statement and removing the attestation requirement for board certified individuals.]
Part 35 |
Applicability |
100 |
|
200 |
|
300 |
|
400 |
✓ |
500 |
|
600 |
✓ |
1000 |
✓ |
8.14a ITEM 7: AUTHORIZED MEDICAL
PHYSICIST (AMP)
Regulations: 10 CFR 30.33(a)(3), 10 CFR 35.2, 10 CFR 35.14,
10 CFR 35.51, 10 CFR 35.57, 10 CFR 35.59, 10 CFR 35.433.
Criteria: Training and experience requirements for
AMPs are described in 10 CFR 35.51.
.
Discussion: While the AMP may not administer the dose, at licensed medical facilities conducting radiation therapy treatments, an AMP is directly involved with the calculation and other tasks associated with the administration of the radiation dose. A licensee performing ophthalmic radiation therapy treatments under 10 CFR 35.400 must ensure that certain tasks described in 10 CFR 35.433(b) are performed by either an AMP or an ophthalmic physicist. The American Association of Physicists in Medicine (AAPM) suggests that a medical physicist limit his or her involvement in radiation therapy to areas for which he or she has established competency.
Applicants are reminded of recentness of training requirements described in 10 CFR 35.59. Specifically, medical physicist and applicants must have successfully completed the applicable training and experience criteria described in 10 CFR Part 35 within 7 years preceding the date of the application. Alternatively, medical physicist applicants must have had related continuing education and experience since completing the required training and experience. This time provision applies to board certification as well as to other training pathways for meeting requirements for training and experience.
10 CFR 35.57 provides that experienced AMPs who were named on a license or permit are not required to comply with the training requirements in 10 CFR 35.51 to continue performing those uses for which they were authorized on or before [THE EFFECTIVE DATE OF THE Rule]. Section 35.57 also provides that physicists holding certain board certifications on or before October 24, 2005, are not required to comply with the training requirements in 10 CFR 35.51 for those materials and uses that they performed on or before October 24, 2005. All AMPs are required to meet the requirements of 10 CFR 35.51(c) after [THE EFFECTIVE DATE OF THE RULE] if they are seeking authorizations for new materials and medical uses.
If the medical physicist has never been identified as an AMP and was board certified by any of the boards listed in 35.57(a)(3) on or before October 24, 2005, the applicant must provide documentation that the individual used the materials and performed the medical uses before October 24, 2005 to meet the requirements to be an AMP for those materials and uses. This documentation will be reviewed on a case-by-case basis to see if the time period of use, the materials used, and the types of medical use meet the criteria in the regulation. This provision of the rule “grandfathers” these board certified individuals that were never named as an AMP but perform medical uses with the same materials prior to October 24, 2005.
In
implementing the EPAct, the NRC “grandfathered” medical
physicists using only accelerator-produced radioactive materials,
discrete sources of Ra-226, or both, for medical uses performed
before or under the NRC waiver of August 31, 2005. These individuals
do not have to meet the requirements of 10 CFR 35.59 or 10 CFR 35.51.
The applicant must, however, document that the individual meets the
criteria in 10 CFR 35.57(a)(43).
Section 35.57 also provides that medical physicists who met certain
criteria will qualify as AMPs for those materials and uses performed
before or under NRC’s waiver of August 31, 2005. Note:
Although there may be a number of medical physicists working with
manual brachytherapy sources during the waiver, the NRC only requires
AMPs for the medical use of strontium-90 eye applicators, teletherapy
units, remote afterloader units, and gamma stereotactic radiosurgery
units. Because none of these devices are known to contain only NARM
material, the NRC expects few, if any, medical physicists to meet the
criteria in 10 CFR 35.57 of an AMP.
Response from Applicant: Provide the following:
∙ Name of the proposed AMP.
AND
For an individual previously identified as an AMP on an NRC or Agreement State license or permit:
∙ Previous license number (if issued
by the NRC) or a copy of the license (if issued by an Agreement
State) or a copy of a permit issued by an NRC master materials
licensee, a permit issued by an NRC or Agreement State broad-scope
licensee, or a permit issued by an NRC Master Materials License
broad-scope permittee on which the individual was specifically named
an AMP for the uses requested.
AND
∙ If applicable, a description of recent related continuing education and experience as required by 10 CFR 35.59.
OR
For an
individual qualifying under 10 CFR 35.57(a)(43):
∙ Documentation that the medical
physicist used only accelerator-produced radioactive material,
discrete sources of Ra-226, or both, for medical uses at
a Government agency or Federally recognized Indian Tribe before
November 30, 2007, or at all other locations of use before August 8,
2009, or an earlier date as noticed by the NRC
before or during the
effective period of NRC’s waiver of August 31, 2005;
AND
∙ Documentation that the medical
physicist used these materials for the same medical uses as
requested.
AND
∙ If applicable, a description of recent related continuing education and experience as required by 10 CFR 35.59.
OR
For an individual qualifying under 10 CFR 35.51(a):
∙ Copy of the certification(s) of the specialty board(s) whose certification process has been recognized2 under 10 CFR 35.51(a);
AND
∙ Description of the training and experience specified in 10 CFR 35.51(c) demonstrating that the proposed AMP is qualified by training in the types of use for which he or she is requesting AMP status, including hands-on device operation, safety procedures, clinical use, and operation of a treatment planning system;
AND
∙ Written
attestation,
signed by a preceptor AMP, that the required training and experience
required for certification, as well as the required training in 10
CFR 35.51(c) for the types of uses specified, have been
satisfactorily completed and that a level of competency sufficient to
function independently as an AMP has been achieved;
AND
∙ If applicable, a description of recent related continuing education and experience as required by 10 CFR 35.59.
OR
For an individual qualifying under 10 CFR 35.57(a)(3):
∙ Copy of the certification issued by the American Board of Radiology in therapeutic radiological physics, Roentgen ray and gamma ray physics, x-ray and radium physics, or radiological physics, or by the American Board of Medical Physics in radiation oncology physics, on or before October 24, 2005;
AND
∙ Documentation that the medical physicist performed the same medical uses as requested on or before October 24, 2005.
AND
∙ If applicable, a description of recent related continuing education and experience as required by 10 CFR 35.59.
OR
For an individual qualifying under 10 CFR 35.51(b):
∙ Description of the training and experience demonstrating that the proposed AMP is qualified by training and experience identified in 10 CFR 35.51(b)(1) for the uses requested;
AND
∙ Description of the training and experience specified in 10 CFR 35.51(c) demonstrating that the proposed AMP is qualified by training in the types of use for which the licensee seeks approval of an individual as AMP, including hands-on device operation, safety procedures, clinical use, and operation of a treatment planning system;
AND
∙ Written attestation, signed by a
preceptor AMP, that the proposed
AMP has satisfactorily completed
the training and experience required in 10 CFR 35.51(b)(1),
as well as the training in 10 CFR 35.51(c) for the types of
use specified, have been
satisfactorially completed and
is able that a level of
competency sufficient to
function independently
fulfill the
radiation safety-related duties as
an AMP for
each type of therapeutic medical unit for which the individual is
requesting authorized medical physicist status
has been achieved;
AND
∙ If applicable, a description of recent related continuing education and experience as required by 10 CFR 35.59.
Notes:
∙ NRC Form 313A (AMP), “Authorized
Medical Physicist and
Ophthalmic Physicist Training,
and
Experience and Preceptor Attestation [10
CFR 35.51,
35.57
(a)(3), and 35.433],”
may be used to document training and experience for those individuals
qualifying under 10 CFR 35.51.
∙ Under 10 CFR 35.14, licensees must notify NRC within 30 days if an AMP permanently discontinues his or her duties under the license or has a name change.
∙ Descriptions of training and experience will be reviewed using the criteria listed above. The NRC will review the documentation to determine if the applicable criteria in 10 CFR Part 35, Subpart B or F, are met. If the training and experience do not appear to meet the criteria in Subpart B or F, the NRC may request additional information from the applicant or may request the assistance of the ACMUI in evaluating such training and experience.
[The following redline addition in Section 8.14b reflects the addition of the ophthalmic physicist and the corresponding training requirements in 35.433.]
8.14b ITEM 7: OPHTHALMIC PHYSICIST
Part 35 |
Applicability |
100 |
|
200 |
|
300 |
|
400 |
✓ |
500 |
|
600 |
|
1000 |
|
Regulations: 10 CFR 30.33(a)(3), 10 CFR 35.2, 10 CFR 35.14, 10 CFR 35.59, 10 CFR 35.433.
Criteria: Training and experience requirements for ophthalmic physicists are described in 10 CFR 35.433(a)(2).
Discussion: A licensee performing ophthalmic radiation therapy treatments under 35.400 must ensure that either an AMP or an ophthalmic physicist performs certain tasks described in 10 CFR 35.433(b). These individuals perform the same tasks but have different training and experience requirements.
While the ophthalmic physicist may not administer the dose at licensed medical facilities conducting ophthalmic radiation therapy treatments, this individual is responsible for calculating the activity of each strontium-90 source that is used to determine treatment times. This individual will further assist the licensee in developing and implementing written procedures to provide high confidence that the administration is in accordance with the written directive.
Applicants are reminded of recentness of training requirements described in 10 CFR 35.59. Specifically, ophthalmic physicist applicants must have successfully completed the applicable training and experience criteria described in 10 CFR Part 35 within 7 years preceding the date of the application. Alternatively, ophthalmic physicist applicants must have had related continuing education and experience since completing the required training and experience.
Response from Applicant: Provide the following:
∙ Name of the proposed Ophthalmic Physicist;
AND
∙ Documentation of a master’s or doctor’s degree in physics, medical physics, other physical science, engineering, or applied mathematics from an accredited college or university;
AND
∙ Documentation of successful completion of 1 year of full time training in medical physics and an additional year of full time work experience in medical physics;
AND
∙ Documentation of training in:
∙ The creating, modifying, and completing of written directives;
∙ Procedures for administrations requiring a written directive; and
∙ Performing the calibration measurements of brachytherapy sources as detailed in § 35.432.
Notes:
∙ NRC Form 313A (AMP), “Authorized Medical Physicist and Ophthalmic Physicist, Training, Experience, and Preceptor Attestation [10 CFR 35.51, 35.57 (a)(3), and 35.433],” may be used to document training and experience ophthalmic physicist identified in 10 CFR 35.433.
∙ Under 10 CFR 35.14, licensees must notify NRC within 30 days if an ophthalmic physicist permanently discontinues his or her duties under the license or has a name change.
∙ Descriptions of training and experience will be reviewed using the criteria listed above. The NRC will review the documentation to determine if the applicable criteria in 10 CFR 35.433(a)(2), are met. If the training and experience do not appear to meet the criteria in 10 CFR 35.433(a)(2), the NRC may request additional information from the applicant or may request the assistance of the ACMUI in evaluating such training and experience.
[The following redline additions to Section 8.19 address the addition of the ophthalmic physicist, changes made to 10 CFR 35.12 to clarify information needed for 10 CFR 35.1000 medical uses, and reminds applicant that there are calibration and use provisions similar to those for therapy units in 10 CFR 35.400 and 35.600 for certain 10 CFR 35.1000 medical uses.]
8.19 ITEM 9: THERAPY UNIT
Part 35 |
Applicability |
100 |
|
200 |
|
300 |
✓* |
400 |
|
500 |
|
600 |
✓* |
1000 |
✓ |
Regulations: 10 CFR 30.33(a)(2), 10 CFR 35.12, 10 CFR 35.27,
10 CFR 35.432, 10 CFR 35.630, 10 CFR 35.632, 10 CFR 35.633,
10 CFR 35.635, 10 CFR 35. 642, 10 CFR 35.643,
10 CFR 35.645, 10 CFR 35.2432, 10 CFR 35.2630,
10 CFR 35.2632, 10 CFR 35.2642, 10 CFR 35.2643, 10 CFR 35.2645. ,
*Special
requirements re: brachytherapy and LDR afterloader sources and Sr-90
sources.
Criteria: The above regulations contain NRC requirements,
including recordkeeping requirements, for verification and
periodic spot-checks of source activity or output. To perform
these measurements, the applicant must possess appropriately
calibrated dosimetry equipment. For manual brachytherapy sources and low dose-rate (LDR) remote afterloader sources, licensees may use source activity or output determined by the manufacturer, provided that the manufacturer’s measurements meet applicable requirements. Similar provisions are included in licensing guidance for certain therapy 35.1000 medical uses. See NRC’s web site (http://www.nrc.gov/materials/miau/med-use-toolkit.html) for specific information.
Discussion: Except for manual brachytherapy sources and LDR remote afterloader sources, where the source output or activity is determined by the manufacturer in accordance with 10 CFR Part 35, the applicant must possess a calibrated dosimetry system (e.g., Farmer chamber, electrometer, well-type ionization chamber) that will be used to perform calibration measurements of sealed sources to be used for patient therapy. Dosimetry systems and/or sealed sources used to calibrate the licensee’s dosimetry systems must be traceable to NIST or to a laboratory accredited by AAPM, pursuant to 10 CFR 35.630. The licensee must maintain records of calibrations of dosimetry equipment for the duration of the license.
The licensee’s AMP must perform full calibrations of sealed sources and devices used for therapy in accordance with published protocols currently accepted by nationally recognized bodies (e.g., AAPM, ACR, ANSI). (Note: Calibration by an AMP is not required for manual brachytherapy sources, except for calculating the activity of strontium-90 sources.) The licensee’s AMP or ophthalmic physicist must calculate the activity of each strontium-90 source that is used to determine the treatment times for ophthalmic treatments. In addition, the licensee must perform spot-check measurements of sealed sources and devices used for therapy in accordance with written procedures established by the AMP (10 CFR 35.642, 10 CFR 35.643, and 10 CFR 35.645). If the licensee seeks authorization for a medical use under 35.1000, the licensing guidance on NRC’s website (http://www.nrc.gov/materials/miau/med-use-toolkit.html) should be reviewed to determine if calibration and use procedures need to be submitted for that 35.1000 medical use. Calibration procedures described by the AAPM or any published protocol approved by a nationally recognized body, as applicable, may be used.
The calibration procedures should address, in part, the method used to determine the exposure rate (or activity) under specific criteria (i.e., distances used for the measurement, whether the measurement is an “in air” measurement or done using a phantom configuration of the chamber with respect to the source(s) and device, scatter factors used to compute the exposure rate, etc.).
Full calibrations must be performed before first medical use1, whenever spot-check measurements (if required) indicate that the output differs by more than 5% from the output obtained at the last full calibration corrected mathematically for decay, following replacement of the sources or reinstallation of the unit in a new location not previously described in the license, following any repairs of the unit that include removal of sealed sources or major repair of the components associated with the source exposure assembly, and at intervals as defined in 10 CFR 35.632, 10 CFR 35.633, and 10 CFR 35.635. Manual brachytherapy sources must be calibrated only initially, prior to use.
For sealed sources used in therapy, and in particular, for new types of use, licensees should select dosimetry equipment that will accurately measure the output or the activity of the source. Contact a licensing specialist at an NRC Regional Office for additional assistance.
Response from Applicant: Provide the following:
∙ The applicant must provide the procedures required by 10 CFR 35.642, 10 CFR 35.643, and 10 CFR 35.645, if applicable to the license application.
∙ The applicant for a medical use under 35.1000 must provide the procedures for 10 CFR35.642, 10 CFR 35.643, and 10 CFR 35.645 required by 10 CFR 35.12(b)(2) that are described in the licensing guidance posted for that 35.1000 medical use on NRC’s website (http://www.nrc.gov/materials/miau/med-use-toolkit.html), or explain why the procedure is not provided.
References:
∙ AAPM Task Group No. 21, “A Protocol for the Determination of Absorbed Dose from High
Energy Photon and Electron Beams”;
∙ AAPM Task Group No. 40, “Comprehensive QA for Radiation Oncology,” AAPM Report
No. 54, “Stereotactic Radiosurgery”;
∙ AAPM Task Group No. 56, “Code of Practice for Brachytherapy Physics.”
Copies
of these documents and many other documents from AAPM referenced in
this guide may be obtained from Medical Physics Publishing (MPP),
4513 Vernon Boulevard,
Madison, WI 53705-4964 or ordered
electronically from http://www.medicalphysics.org.
[The following redline additions to Section 8.20 reflect clarifications made to 10 CFR 35.12 for information needed for 10 CFR 35.1000 medical uses, and remind applicants that there are equipment and facility descriptions similar to those for medical uses in 10 CFR 35.300, 35.400, and 35.600 for certain 10 CFR 35.1000 medical uses.]
Part 35 |
Applicability |
100 |
✓ |
200 |
✓ |
300 |
✓ |
400 |
✓ |
500 |
✓ |
600 |
✓ |
1000 |
✓ |
8.20 ITEM 9: OTHER EQUIPMENT AND
FACILITIES
Regulations: 10 CFR 20.1101, 10 CFR 20.1801,
10 CFR 30.33(a)(2), 10 CFR 30.34, 10 CFR 35.12,
10 CFR 35.315, 10 CFR 35.415, 10 CFR 35.457,
10 CFR 35.615, 10 CFR 35.647, 10 CFR 35.657.
Criteria: Facilities and equipment must be adequate to
protect health and minimize danger to life or property.
Discussion: The applicant should describe, in Item 9 of the application, other equipment and facilities available for safe use and storage of byproduct material listed in Item 5 of this application. This description should be identified as Attachment 9.4.
The applicant must describe additional facilities and equipment for PET radionuclide and radiopharmaceutical therapy programs to safely receive, use, store, and dispose of radioactive material. The applicant should focus on facilities to be used for radioactive drug therapy administration and patient accommodations (e.g., private room with private bath). The most widely used source of radiopharmaceutical therapy is I-131 sodium iodide. If the radionuclide is administered in volatile liquid form, it is important to place the patient dosage in a closed environment (e.g., a fume hood). Also note there are hazards associated with volatile iodine in pill form; applicants should consider this in establishing their radiological controls. When patients are treated with I-131 sodium iodide, sources of contamination include airborne I-131, urine, perspiration, saliva, and other secretions.
For PET radionuclide use and PET radioactive drug production areas, the applicant should focus on the need for (1) additional shielding, (2) hot cells containing remote handling devices, (3) other remote handling devices that may be needed when handling and storing the higher energy emissions of these materials, and (4) special delivery systems if the applicant prepares its own PET radionuclides or has them delivered by a direct transfer tube or system from a PET radionuclide producer. Applicants synthesizing PET radioactive drugs should also focus on volatility issues and releases.
For teletherapy, GSR, and high dose-rate (HDR) facilities, the licensee shall require any individual entering the treatment room to ensure, through the use of appropriate radiation monitors, that radiation levels have returned to ambient levels. One method of meeting the requirements of 10 CFR 35.615(c) is a beam-on radiation monitor permanently mounted in each therapy treatment room that is equipped with an emergency power supply separate from the power supply for the therapy unit. Such beam-on monitors can provide a visible indication (e.g., flashing light) of an exposed or partially exposed source. Applicants may propose an alternative to a permanently mounted monitor.
Section 10 CFR 35.615(d) requires that, except for LDR units, each licensee shall construct or equip each treatment room so as to permit continuous observation of the patient while the patient is in the treatment room. If a shielded viewing window will be used, the thickness, density, and type of material used should be specified. If a closed-circuit television system (or some other electronic system) will be used to view the patient, the backup system or procedure to be used in case the electronic system malfunctions should be specified, or the applicant must commit to suspending all treatments until the electronic system is repaired and functioning again. The communications system should allow the patient to communicate with the unit operator in the event of medical difficulties. An open microphone system can be used to allow communication without requiring a patient to move to activate controls.
The regulations require adequate equipment and controls to maintain exposures of radiation to workers ALARA and within regulatory limits. Section 10 CFR 35.615(b), in part, requires that each door leading into the treatment room be provided with an electrical interlock system to control the on-off mechanism of the therapy unit. The interlock system must cause the source(s) to be shielded if the door to the treatment room is opened when the source is exposed. The interlock system must also prevent the operator from initiating a treatment cycle unless the treatment room entrance door is closed. Further, the interlock must be wired so that the source(s) cannot be exposed after interlock interruption until the treatment room door is closed and the on-off control for the source(s) is reset at the console.
Due to the unique characteristics of pulsed dose-rate (PDR) remote afterloaders and the lack of constant surveillance of their operation, a more sophisticated alarm system is essential to ensure the patient is protected during treatment. In addition to the above, consider the following:
∙ The PDR device control console is not accessible to unauthorized personnel during treatment.
∙ A primary care provider checks the patient to ensure that the patient’s device has not been moved, kinked, dislodged, or disconnected.
∙ A more sophisticated interlock/warning system is normally installed for PDR devices. This system should perform the following functions or possess the following characteristics:
– The signal from the PDR device and the signal from the room radiation monitor should be connected in such a manner that an audible alarm sounds if the room monitor indicates the presence of radiation and the device indicates a “safe” or retracted position.
– The alarm circuit should also be wired in such a manner that an audible alarm is generated for any device internal error condition that could indicate the unintended extension of the source. This would constitute a circuit that generates the audible alarm when either the “source retracted and radiation present” or the appropriate internal error condition(s) exists.
– The “source safe and radiation present” signal should also be self-testing. If a “source not safe” input is received without a corresponding “radiation present” signal, the circuit should generate an interlock/warning circuit failure signal that will cause the source to retract. Reset this circuit manually before attempting to continue treatment.
– The audible alarm should be sufficiently loud to be clearly heard by the facility’s responsible device/patient monitoring staff at all times.
– No provisions for bypassing this alarm circuit or for permanently silencing the alarm should be made to the circuit as long as the room radiation monitor is indicating the presence of radiation. If any circuitry is provided to mute the audible alarm, such circuitry should not mute the alarm for a period of more than 1 minute. Controls that disable this alarm circuit or provide for silencing the alarm for periods in excess of 1 minute should be prohibited.
If the alarm circuit is inoperative for any reason, licensees should prohibit further treatment of patients with the device until the circuit has been repaired and tested. If the alarm circuit fails during the course of a patient treatment, the treatment in progress may continue as long as continuous surveillance of the device is provided during each treatment cycle or fraction.
Applicants may submit information on alternatives to fixed shielding as part of their facility description. This information must demonstrate that the shielding will remain in place during the course of patient treatment.
For patient rooms where LDR remote afterloader use is planned, neither a viewing nor an intercom system is required. However, the applicant should describe how the patient and device will be monitored during treatment to ensure that the sources and catheter guide tube are not disturbed during treatment and to provide for prompt detection of any operational problems with the LDR device during treatment.
Similar provisions regarding equipment and facilities are included in licensing guidance for certain 35.1000 medical uses. See NRC’s web site (http://www.nrc.gov/materials/miau/med-use-toolkit.html) for specific information.
Response from Applicant: Follow the guidance in Section 5.2 to determine if the response to this section includes security-related sensitive information and needs to be marked accordingly.
For PET radionuclide use, PET radioactive drug production, and radiopharmaceutical therapy programs, describe the additional facilities and equipment for these uses.
For manual brachytherapy facilities, provide a description of the emergency response equipment.
For teletherapy, GSR, and remote afterloader facilities, provide a description of the following:
∙ Warning systems and restricted area controls (e.g., locks, signs, warning lights and alarms, interlock systems) for each therapy treatment room;
∙ Area radiation monitoring equipment;
∙ Viewing and intercom systems (except for LDR units);
∙ Steps that will be taken to ensure that no two units can be operated simultaneously, if other radiation-producing equipment (e.g., linear accelerator, X-ray machine) is in the treatment room;
∙ Methods to ensure that whenever the device is not in use or is unattended, the console keys will be inaccessible to unauthorized persons; and
∙ Emergency response equipment.
For 35.1000 medical uses, review the licensing guidance posted for that 35.1000 medical use on NRC’s website (http://www.nrc.gov/materials/miau/med-use-toolkit.html), and provide the appropriate descriptions of other equipment and facilities, or explain why the descriptions are not provided.
[The following redline additions to Section 8.21 remind applicants that there are minor radiation safety program change provisions similar to those in 10 CFR 35.26 for certain 10 CFR 35.1000 medical uses.]
Part 35 |
Applicability |
100 |
✓ |
200 |
✓ |
300 |
✓ |
400 |
✓ |
500 |
✓ |
600 |
✓ |
1000 |
✓ |
PROGRAM
Regulations: 10 CFR 20.1101, 10 CFR 20.2102, 10 CFR 30.33,
10 CFR 30.34(e), 10 CF R 35.24, 10 CFR 35.26, 10 CFR 35.610,
10 CFR 35.2024, 10 CFR 35.2026.
Criteria: The regulations in 10 CFR 20.1101 state that each
licensee must develop, document, and implement a Radiation Protection Program commensurate with the scope of the
licensed activity. The program must be sufficient to ensure
compliance with the provisions of 10 CFR Part 20 regulations. The licensee is responsible for the conduct of all licensed activities and the acts and omissions of individuals handling licensed material. Under 10 CFR 30.34(e), the NRC may incorporate into byproduct materials licenses, at the time of issuance or thereafter, additional requirements and conditions that it deems appropriate or necessary to protect health or to minimize danger to life and property. Licensee management’s authorities and responsibilities for the Radiation Protection Program are described in 10 CFR 35.24, while 10 CFR 35.26 sets forth four circumstances in which the licensee may revise its Radiation Protection Program without NRC approval. For example, no NRC approval is required when the revision does not require a license amendment. Applicants for 35.1000 medical uses may apply for license conditions that will permit the licensee to revise its radiation safety program for that 35.1000 medical use to conform to revised licensing guidance posted on NRC’s web site (http://www.nrc.gov/materials/miau/med-use-toolkit.html) without additional NRC approvals. The circumstances for this approval are similar to those in 10 CFR 35.26.
Discussion: Applicants/licensees must abide by all applicable regulations, develop, implement, and maintain procedures when required, and/or provide requested information about the proposed Radiation Protection Program during the licensing process. Tables C.1 and C.2 in Appendix C may be helpful in determining what information should be provided when requesting a license. If the licensee has authority for the production of PET radioactive drugs under 10 CFR 30.32(j), the radiation production program must include radiation safety issues associated with this nonmedical use.
Response from Applicant: Respond to subsequent sections of this document regarding Item 10 of the application.
Applicants for 35.1000 medical uses may apply for approval to revise, without further NRC approval, the radiation safety program for that 35.1000 medical use to conform to revised licensing guidance posted on NRC’s web site (http://www.nrc.gov/materials/miau/med-use-toolkit.html).
[The following redline additions to Section 8.22 remind applicants that safety and emergency procedures required in 10 CFR 35.12 may be required for 10 CFR 35.1000 medical uses.]
Part 35 |
Applicability |
100 |
|
200 |
|
300 |
|
400 |
|
500 |
|
600 |
✓ |
1000 |
✓ |
8.22 ITEM 10: SAFETY PROCEDURES AND
INSTRUCTIONS
Regulations: 10 CFR 30.34(j), 10 CFR 35.12(c)(2),
10 CFR 35.610, 10 CFR 35.642, 10 CFR 35.643,
10 CFR 35.645.
Criteria: When applying for authorization under
10 CFR 30.32(j) to produce PET radioactive drugs for noncommercial
distribution to other medical use licensees in the consortium, the applicant must develop, document, and implement certain procedures. See Appendix AA for discussion and response from applicant.
Before using materials under 10 CFR 35.600, the applicant must develop, document, submit, and implement written safety procedures for emergency response. Section 10 CFR 35.610 requires, in part, that written procedures be developed, implemented, and maintained for responding to an abnormal situation involving a remote afterloader unit, a teletherapy unit, or a gamma stereotactic radiosurgery unit. The procedures needed to meet 10 CFR 35.610 must include:
∙ Instructions for responding to equipment failures and the names of the individuals responsible for implementing corrective actions,
∙ The process for restricting access to and posting of the treatment area to minimize the risk of inadvertent exposure, and
∙ The names and telephone numbers of AUs, AMPs, and the RSO to be contacted if the unit or console operates abnormally.
A copy of these procedures must be physically located at the therapy unit console. The instructions must inform the operator of procedures to be followed if the operator is unable to place the source(s) in the shielded position, or remove the patient from the radiation field with controls from outside the treatment room.
Before using materials under certain 35.1000 medical uses, the applicant must develop, document, submit, and implement written safety procedures for emergency responses. The licensing guidance on NRC’s web site (http://www.nrc.gov/materials/miau/med-use-toolkit.html) for 35.1000 medical uses provides specific information for each 35.1000 medical use.
Discussion: The applicant must establish and follow written procedures for emergencies that may occur (e.g., a therapy source fails to retract or return to the shielded position, or a GSR couch fails to retract). A copy of the manufacturer’s recommendations and instructions should be given to each individual performing therapy treatments or operating the therapy device. Practice drills, using nonradioactive (dummy) sources (when possible), must be practiced annually or more frequently, as needed. The drills should include dry runs of emergency procedures that cover stuck or dislodged sources and applicators (if applicable), and emergency procedures for removing the patient from the radiation field. Team practice may also be important for adequate emergency coordination for such maneuvers as removing a patient from a malfunctioning GSR unit and manual movement of the patient treatment table. These procedures, designed to minimize radiation exposure to patients, workers, and the general public, should address the following points, as applicable to the type of medical use:
∙ When the procedures are to be implemented, such as any circumstance in which the source becomes dislodged, cannot be retracted to a fully shielded position, or the patient cannot be removed from the beam of radiation.
∙ The actions specified for emergency source recovery or shielding that primarily consider minimizing exposure to the patient and health care personnel while maximizing the safety of the patient.
∙ The step-by-step actions for single or multiple failures that specify the individual(s) responsible for implementing the actions. The procedures should clearly specify which steps are to be taken under different scenarios. The procedure should specify situations in which surgical intervention may be necessary and the steps that should be taken in that event.
∙ Location of emergency source recovery equipment, specifying what equipment may be necessary for various scenarios. Emergency equipment should include shielded storage containers, remote handling tools, and if appropriate, supplies necessary to surgically remove applicators or sources from the patient and tools necessary for removal of the patient from the device.
∙ Radiation safety priorities, such as giving first consideration to minimizing exposure to the patient, usually by removing the patient from the room (rather than using tools to attempt to return the source to the off position). Note: If the first step of the emergency procedures for teletherapy units specifies pressing the emergency bar on the teletherapy unit console, the applicant is advised that this action may cause the source to return to the off position but may also cut power to the entire teletherapy unit or to the gantry or the couch.
∙ Instructing the staff to act quickly and calmly, and to avoid the primary beam of radiation.
∙ Specifying who is to be notified.
∙ Requirements to restrict (lock, as necessary) and post the treatment area with appropriate warning signs as soon as the patient and staff are out of the treatment room.
Response from Applicant:
Provide procedures required by 10 CFR 35.610.
See Appendix AA for responses required by 10 CFR 30.32(j).
If appropriate, review 35.1000 medical use licensing guidance on NRC’s website (http://www.nrc.gov/materials/miau/med-use-toolkit.html), and provide safety and emergency procedures requested for the particular 35.1000 medical use, or describe why the procedures are not needed.
Part 35 |
Applicability |
100 |
|
200 |
|
300 |
|
400 |
|
500 |
|
600 |
✓ |
1000 |
✓ |
8.27 ITEM 10: INSTALLATION,
MAINTENANCE, ADJUSTMENT, REPAIR,
AND INSPECTION OF THERAPY
DEVICES CONTAINING SEALED
SOURCES
Regulations: 10 CFR 20.1101, 10 CFR 30.32, 10 CFR 30.34,
10 CFR 35.605, 10 CFR 35.655, 10 CFR 35.2605,
10 CFR 35.2655.
Criteria:
In accordance with 10 CFR 35.605 and 10 CFR 35.655,
licensees must ensure that therapy devices containing sealed sources
are installed, maintained, adjusted, repaired, and inspected by
persons specifically licensed to conduct these activities. The above
activities should be conducted according to the manufacturers’
written recommendations and instructions and according to the SSDR.
In addition, 10 CFR 35.655 requires that teletherapy and
GSR units be fully inspected and serviced during source replacement.
The interval between each full-inspection servicing shall not
exceedor at intervals
not to exceed 5 years for
teletherapy units and 7 years for gamma stereotactic radiosurgery
units, whichever
comes first, to ensure that
the source exposure mechanism and
other safety components
functions properly.
Maintenance is necessary to ensure that the
device functions as designed,
and source integrity
and safety components are is
not compromised and
that the device functions as designed.
Similar
provisions are included in licensing guidance for certain therapy
35.1000 medical uses. See NRC’s web site
(http://www.nrc.gov/materials/miau/med-use-toolkit.html)
for specific information.
Discussion: Maintenance and repair includes installation, replacement, and relocation or removal of the sealed source(s) or therapy unit that contains a sealed source(s). Maintenance and repair also includes any adjustment involving any mechanism on the therapy device, treatment console, or interlocks that could expose the source(s), reduce the shielding around the source(s), affect the source drive controls, or compromise the radiation safety of the unit or the source(s).
The NRC requires that maintenance and repair (as defined above) be performed only by persons specifically licensed by NRC or an Agreement State to perform such services. Most licensee employees do not perform maintenance and repair because they do not have the specialized equipment and technical expertise to perform these activities. Applicants requesting authorization to possess and use LDR remote afterloaders should review 10 CFR 35.605 before responding to this item. Section 10 CFR 35.605 allows for an AMP to perform certain service activities with regard to LDR remote afterloader units.
Response from Applicant: No response is necessary if the licensee contracts with personnel who are licensed by NRC or an Agreement State to install, maintain, adjust, repair, and inspect the specific therapy device possessed by the licensee. However, if the applicant requests that an employee who is trained by the manufacturer be authorized to perform the aforementioned activities, the applicant must provide sufficient information to allow the NRC to evaluate and approve such authorization (see CFR 35.605 and 10 CFR 35.655). This should include the following:
∙ Name of the proposed employee and types of activities requested,
AND
∙ Description of the training and experience demonstrating that the proposed employee is qualified by training and experience for the use requested,
AND
∙ Copy of the manufacturer’s training certification and an outline of the training in procedures to be followed.
Note: The applicant should specify only those installation, maintenance, inspection, adjustment, and repair functions, as described in a certificate or letter from the manufacturer of the device, that document the employee’s training in the requested function(s).
[The following redline addition to Section 9 reflects changes to 10 CFR 35.13 requiring a licensee to amend the license before permitting an individual to work as an ARSO or before assigning a current ARSO to oversee a new section of the radiation protection program.]
Part 35 |
Applicability |
100 |
✓ |
200 |
✓ |
300 |
✓ |
400 |
✓ |
500 |
✓ |
600 |
✓ |
1000 |
✓ |
A LICENSE
Regulations: 10 CFR 30.37, 10 CFR 30.38, 10 CFR 35.13.
The NRC now has regulatory authority over sealed sources
and devices containing accelerator-produced radioactive
material and discrete sources of Ra-226, under the new
definition of byproduct material resulting from the EPAct.
Licensees may need license amendments for such purposes as
to authorize use of these materials, to revise their Radiation Safety Programs to meet new requirements, or to provide new facility diagrams. The NRC issued a waiver on August 31, 2005, that permitted licensees to continue to use the newly defined byproduct material until the waiver was terminated on August 8, 2009. Licensees in Government agencies, Federally recognized Indian tribes, Delaware, the District of Columbia, Puerto Rico, the U.S. Virgin Islands, Indiana, Wyoming, and Montana who possess and use accelerator-produced radioactive material or discrete sources of Ra-226, or both, may continue to use these materials for medical use or prepare PET radioactive drugs for noncommercial distribution to other consortium members until the date of NRC’s final licensing determination, provided the licensee submits an amendment application within 6 months after November 30, 2007. Other licensees should check with the appropriate NRC Regional Office to determine when they have to submit their license amendments.
Licensees are responsible for applying for amendments to licenses and for keeping them up-to-date. Furthermore, to continue a license after its expiration date, the licensee must submit an application for a license renewal at least 30 days before the expiration date (10 CFR 2.109, 10 CFR 30.36(a)).
Under 10 CFR 35.13, a licensee is required to apply for and receive a license amendment before several activities can occur, including:
∙ Receipt or use of byproduct material for a type of use permitted by 10 CFR Part 35, but not authorized on the licensee’s current Part 35 license;
∙ Permitting anyone to work as an AU
for medical uses, AMP, ophthalmic
physicist, or ANP, unless the
individual meets one of the exceptions listed in 10 CFR 35.13(b).
(information required to document training and experience may be
provided on the appropriate NRC Form 313A series of forms for change
or addition of AU for medical uses, AMP, ophthalmic
physicist, ANP, or
RSO,
or ARSOs);
∙ Changing the RSO;
∙ Permitting an individual to work as an ARSO, or before the RSO assigns a current ARSO duties and tasks in the oversight for a new section of the radiation protection program.
∙ Receiving byproduct material in excess of the amount, or receiving radionuclides or forms different than, currently authorized on the NRC license;
∙ Changing an area or address of use identified in the application or on the license. This includes additions and relocations of areas where PET radionuclides are produced or additions or relocations of a radionuclide delivery line from the PET radionuclide production area to a 10 CFR 35.100 or 10 CFR 35.200 medical use area. However, other changes and additions to the 10 CFR 35.100 and 10 CFR 35.200 medical use area do not require a license amendment and can be made, provided NRC is notified as required by 10 CFR 35.14 within 30 days following the changes, and
∙ Revising procedures required by 10 CFR 35.610, 35.642, 35.643, and 35.645, when the revision reduces the level of radiation safety.
∙ Receiving a sealed source from a different manufacturer or of a different model number than authorized by the license unless the sealed source is used in manual brachytherapy, is listed in the SSDR, for the quantity and for an isotope authorized by the license.
In case of a medical emergency requiring an expedited license amendment, contact the materials licensing staff at the appropriate NRC Regional Office.
For both renewal and amendment requests, applicants should do the following:
∙ Use the most recent guidance in preparing an amendment or renewal request,
∙ Submit in
duplicate either an NRC
Form 313 or a letter requesting an amendment or renewal, and
∙ Provide the license number.
APPENDIX
B
NRC Form 313A Series
“Medical Use Training and Experience and Preceptor Attestation”
[The most current revisions to the NRC Form 313A series may be found at: http://www.nrc.gov/reading-rm/doc-collections/forms/#NRC. Copies of the versions of the forms that conform to the final rule amending 10 CFR Part 35 in 2016 are provided below.]
11 Applicants that have their own cyclotrons and produce PET radionuclides that they use to produce PET radioactive drugs for their own use under the appropriate provisions of 10 CFR Part 35 may have different shielding or special equipment requirements than most medical use applicants who receive unit doses, multi-dosage vials, or generators from drug manufacturers or commercial nuclear pharmacies that are packaged in self-shielding radiation transport shields. Information needed for the different shielding or special equipment requirements can be found in Section 9.
11 The names of board certifications that have been recognized by the NRC or an Agreement State are posted on NRC’s Web site http://www.nrc.gov/materials/miau/med-use-toolkit.html.
22 The names of board certifications that have been recognized by the NRC or an Agreement State are posted on the NRC’s Web site http://www.nrc.gov/materials/miau/med-use-toolkit.html.
33 The names of board certifications that have been recognized by the NRC or an Agreement State are posted on the NRC’s Web site http://www.nrc.gov/materials/miau/med-use-toolkit.html.
11 The names of board certifications that have been recognized by the NRC or an Agreement State are posted on the NRC’s Web site http://www.nrc.gov/materials/miau/med-use-toolkit.html.
22 The names of board certifications that have been recognized by the NRC or an Agreement State are posted on the NRC’s Web site http://www.nrc.gov/materials/miau/med-use-toolkit.html.
11 For brachytherapy sources, “first medical use” is defined as the first use following the effective date of the revised 10 CFR Part 35, October 24, 2002.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | DBH |
| File Modified | 0000-00-00 |
| File Created | 2021-01-20 |
© 2026 OMB.report | Privacy Policy