Part 4- Final Rule Guidance to OMB for 2017- 10 CFR Part 30, 32, and 35
Part 4 of final guidance sent to OMB for 2017 final Part 35 rule.docx
10 CFR 35, Medical Use of Byproduct Material
Part 4- Final Rule Guidance to OMB for 2017- 10 CFR Part 30, 32, and 35
OMB: 3150-0010
PART 2
Supplemental Guidance for NUREG-1556,
Volume 13, Revision 1, Consolidated Guidance
About Materials Licenses: Program-Specific
Guidance About Commercial Radiopharmacy
Licenses
The following redline/strikeout changes for Section 8.7.2 reflect the changes to 10 CFR 35.55 removing the preceptor attestation requirement from the nuclear pharmacist board certification pathway and changes to the attestation statement for the alternate training and experience pathway.
8. AUTHORIZED NUCLEAR PHARMACIST (ANP)
Regulations:10 CFR 32.72 (b)(2), (4), and (5); 10 CFR 35.2; 10 CFR 35.55(a) and (b); and 10 CFR 35.59.
Criteria: The ANP must be a State-licensed or State-registered pharmacist with adequate training and experience.
Discussion: Each commercial nuclear pharmacy must have an ANP to prepare or supervise the preparation of radioactive drugs for medical use. An individual who is not qualified to be an ANP may work under the supervision of an ANP.
The criteria for a pharmacist to work as an
ANP at a commercial radiopharmacy are described in 10 CFR 32.72(b)(2)
and (4).
This section of the regulation refers to the training for an ANP,
which includes the definition of an ANP in 10 CFR 35.2 (which in turn
includes the board certification requirements in 10 CFR 35.55(a));
the training and experience criteria for the alternate pathway
described in 10 CFR 35.55(b); and the recentness of training criteria
in 10
CFR 35.59 that requires the successful completion of training within 7 years preceding the date of the application. Additional training and experience may be necessary if the time interval is greater than 7 years. Applicants may find it convenient to present this documentation using NRC Form 313A (ANP) in Appendix G. Each hour of training may be listed only once, (i.e., under the most applicable category). The recentness of training requirements apply to board certification as well as to other recognized training pathways.
In implementing the EPAct, NRC “grandfathered” nuclear pharmacists by permitting the licensee to designate a pharmacist as an ANP, if the pharmacist used only accelerator-produced radioactive materials, discrete sources of Ra-226, or both, in the practice of nuclear pharmacy for the uses performed before November 30, 2007, or under the NRC waiver of August 31, 2005. These individuals do not have to meet the requirements of 10 CFR 32.72(b)(2)(i) or (ii). However, the applicant must document that the individual meets the criteria in 10 CFR 32.72(b)(4).
On-the-job training may not be counted toward the hours listed above unless it was obtained as part of a formal training course. A "formal" training course is one that incorporates the following
elements:
A detailed description of the content of the course is maintained on file at the sponsoring institution and can be made available to NRC upon request;
Evidence that the sponsoring institution has examined the student’s knowledge of the course content is maintained on file at the institution and can be made available to NRC upon request. This evidence of the student’s overall competency in the course material should include a final grade or percentile; and
A permanent record that the student successfully completed the course is kept at the institution.
Response from Applicant: For each proposed ANP, provide the following:
Name of the proposed ANP.
AND
Pharmacist’s license number and issuing entity.
For an individual previously identified as an ANP on an NRC or Agreement State license or permit or by a commercial nuclear pharmacy that has been authorized to identify ANPs (10 CFR 32.72(b)(2)(i)):
Previous license number (if issued by NRC) or a copy of the license (if issued by an Agreement State) or a copy of a permit issued by an NRC master materials licensee, a permit issued by an NRC or Agreement State broad-scope licensee, or a permit issued by an NRC Master Material License broad-scope permittee on which the individual was named an ANP or a copy of an authorization as an ANP from a commercial nuclear pharmacy that has been authorized to identify ANPs,
OR
For an individual qualifying under 10 CFR 32.72(b)(4):
• Documentation that the individual was a nuclear pharmacist preparing only radioactive drugs containing accelerator-produced radioactive material,
AND
• Documentation that the individual practiced at a pharmacy, a Government agency or Federally recognized Indian Tribe before November 30, 2007, or at all other pharmacies before August 8, 2009, or an earlier date as noticed by the NRC,
OR
For an individual qualifying under 10 CFR 35.55(a):
Copy of the certification(s) of the specialty board whose certification process has been recognized2 under 10 CFR 35.55(a),
AND
Written attestation, signed by a preceptor ANP, that training and experience required forcertification have been satisfactorily completed and that a level of competency sufficientto function independently as an ANP has been achieved,
AND
• If applicable, description of recent related continuing education and experience as required by 10 CFR 35.59.
OR
For an individual qualifying under 10 CFR 32.72(b)(2)(ii):
Description of the training and experience specified in 10 CFR 35.55(b) demonstrating that the proposed ANP is qualified by training and experience,
AND
Written attestation, signed by a preceptor ANP, that the individual has satisfactorily completed the
training and experiencerequiredments in 10 CFR 35.55(b)(1)certificationhavehas been satisfactorily completedand is ablethat a level ofcompetencysufficienttofunctionindependently fulfill the radiation safety-related duties as an ANPhas beenachieved,
AND
If applicable, description of recent related continuing education and experience as required by 10 CFR 35.59.
Notes:
NRC Form 313A (ANP), "Authorized Nuclear Pharmacist Training and Experience and Preceptor Attestation [10 CFR 35.55]," may be used to document training and experience for those individuals qualifying under 10 CFR 35.55(a) or (b).
Descriptions of training and experience will be reviewed using the criteria listed above. The NRC will review the documentation to determine if the applicable criteria in 10 CFR
32.72(b)(2) are met. If the training and experience do not appear to meet the criteria in Subpart B, the NRC may request additional information from the applicant or may request the assistance of the Advisory Committee on the Medical Uses of Isotopes (ACMUI) in evaluating such training and experience.
The following redline/strikeout changes to Section 8.10.6 reflect revisions to 10 CFR 30.34(g) and 35.204 adding new molybdenum-99/technetium-99m generator elution test frequencies and new reporting requirements when the Molybdenum99/technetium-99m and strontium-82/rubidium-82 generator eluates exceed breakthrough values.
8.10.6 SAFE USE OF RADIONUCLIDES AND EMERGENCY PROCEDURES
Regulations: 10 CFR 20.1101, 10 CFR 20.1801, 10 CFR 20.1802, 10 CFR 20.2201, 10 CFR 20.2202, 10 CFR 20.2203, 10 CFR 30.34(g), 10 CFR 30.50, 10 CFR 19.11(a)(3).
Criteria: Licensees are required to do the following:
Keep radiation doses to workers and members of the public ALARA;
Ensure security of licensed material; and
Make the required notifications of events to NRC.
Discussion:
Licensees are responsible for the security and safe use of all
licensed material from the time it arrives or is produced at their
facility until its use, transfer, and/or disposal. Licensees should
develop written procedures to ensure safe use of licensed material.,
and tThe
procedures should also include operational and administrative
guidelines, as
well as procedures to assure reports of events are complete and made
in a timely manner in accordance with reporting requirements.
See Appendix P
of this NUREG. The written
procedures should provide reasonable assurance that only
appropriately trained personnel will handle and use licensed material
without undue hazard to workers or members of the public.
General Safety Procedures
The written procedures should include the following elements:
Contamination controls;
Waste disposal practices;
Personnel and area monitoring (including limits);
Use of protective clothing and equipment;
Safe handling of radioactive materials;
Recording requirements;
• Reporting requirements; and
Responsibilities.
These procedures should include policies for:
Frequency of personnel monitoring;
Performing molybdenum-99 breakthrough measurements on each
the firsteluateafterreceiptof a molybdenum-99/technetium-99m generator;Reporting to NRC and the distributor when there is more than 0.15 kilobecquerel of molybdenum-99 per megabecquerel of technetium-99m (0.15 microcurie of molybdenum-99 per millicurie of technetium-99m) in the eluate;
Use of appropriate shielding (see Figure 8.8);
Frequent glove changes to minimize exposure to the individual and to avoid spread of contamination in the facility; and
Special procedures for higher risk activities, such as use of radioiodine and repair of chemistry synthesis equipment for PET radiopharmaceuticals.
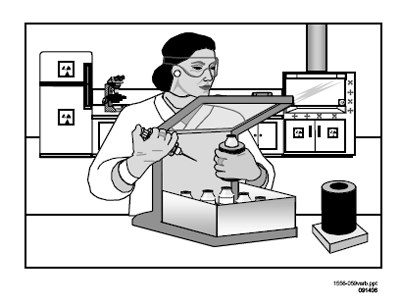
Figure 8.8 Use of Appropriate Shielding.
Applicants should also develop radionuclide-specific procedures based on the respective hazards associated with the radionuclides. General safety guidelines are described in Appendix Q. Applicants should use these guidelines to aid in the development of their own procedures for the safe use of radionuclides.
Furthermore, applicants that produce radioactive materials using an accelerator should also refer to the safety procedures found in NUREG-1556, Vol. 21, “Consolidated Guidance About Materials Licenses: Program-Specific Guidance About Possession Licenses for Production of Radioactive Materials Using an Accelerator.”
Licensees should determine if they have areas that require posting in accordance with 10 CFR 20.1902, unless they meet the exemptions listed in 10 CFR 20.1903. Also, containers of licensed material (including radioactive waste) must be labeled in accordance with 10 CFR 20.1904, unless they meet the exemptions in 10 CFR 20.1905.
Emergency Procedures
Accidents and emergencies can happen during any operation with radionuclides, including their receipt, transportation, use, transfer, and disposal. Such incidents can result in contamination or release of material to the environment and unintended radiation exposure to workers and members of the public. In addition, loss or theft of licensed material, and fires involving radioactive material, can adversely affect the safety of personnel and members of the public. Applicants should therefore develop and implement procedures to minimize, to the extent practical, the potential impact of these incidents on personnel, members of the public, and the environment.
Applicants should establish written procedures to handle events ranging from a minor spill to a major accident that may require intervention by outside emergency response personnel. These procedures should include provisions for immediate response, after-hours notification, handling of each type of emergency, equipment, and the appropriate roles of staff and the RSO. In addition, the licensee should develop procedures for routine contacts with its local fire department officials to inform them of its operations and identify locations of radioactive materials and elevated radiation levels in the event of their response to a fire. Except for minor spills or releases of radioactivity that can be controlled and cleaned up by the user, licensee staff should have a clear understanding of their limitations in an emergency with step-by-step instructions and clear direction of whom to contact. The licensee should establish clear delineations between minor contamination events, minor spills, and major spills and events.
Emergency spill response materials should be strategically placed in well-marked locations for use by all trained staff. All equipment should be periodically inspected for proper operation and replenished as necessary. Appendix Q includes model emergency procedures. Applicants may adopt these procedures or develop their own, incorporating the safety features included in these model procedures.
Certain incidents and emergencies require notification of NRC. Appendix T provides a list of major NRC reporting and notification requirements relevant to commercial radiopharmacies. Response from Applicant: Submit the following statement:
"We have developed and will implement and maintain written procedures for the safe use of radioactive materials that address:
Facility and personnel radioactive contamination minimization, detection, and control;
Performing molybdenum-99 breakthrough measurements on each
the firsteluate from aafter receipt of themolybdenum-99/technetium-99m generator;Reporting under the requirements in 10 CFR 30.34(g) if there is more than 0.15 kilobecquerel of molybdenum-99 per megabecquerel of technetium-99m (0.15 microcurie of molybdenum-99 per millicurie of technetium-99m) in an eluate; and
Use of protective clothing and equipment by personnel
that meet the requirements in 10 CFR 20.1101, 10 CFR 20.1801, 10 CFR 20.1802, 10 CFR 30.34(g), and 10 CFR 19.11(a)(3), as applicable;" AND
"We have developed and will implement and maintain written procedures for identifying and responding to emergencies involving radioactive material, including:
Lost, stolen, or missing licensed material;
Exposures to personnel and the public in excess of NRC regulatory limits;
Releases of licensed materials in effluents and the sanitary sewer in excess of NRC regulatory limits;
Excessive radiation levels or radioactive material concentrations in restricted or unrestricted areas;
Radioactive spills and contamination;
Fires, explosions, and other disasters with the potential for the loss of containment of licensed material; and
Routine contacts with local fire departments and local law enforcement agencies (LLEA),
that meet the requirements in 10 CFR 20.1101, 10 CFR 20.2201-2203, and 30.50, and other requirements, as applicable;"
The following redline/strikeout revisions to Section 8.10.8 reflect the conforming
changes needed for commercial nuclear pharmacies that prepare radiopharmaceuticals used primarily for their alpha-emitting radiation characteristics in response to the revision of 10 CFR 35.390(b)(1)(G)(3) to specifically include these radionuclides.
8.10.8 DOSAGE MEASUREMENT SYSTEMS
Regulation: 10 CFR 32.72(c).
Criteria: Commercial radiopharmacy licensees must possess and use instrumentation capable of accurately measuring the radioactivity in radioactive drugs.
Discussion: Due to the potential for radiopharmacy errors to adversely affect their customers (medical facilities) and their customers’ patients, each dosage of a radioactive drug must be measured prior to transfer to provide high confidence that the correct amount of the radioactive drug is transferred in accordance with the customer’s request.
The applicant must have procedures for the use of the instrumentation, including the measurement, by direct measurement or by a combination of measurement and calculation, of the amount of radioactivity in dosages of alpha-, beta-, gamma-, or photon-emitting radioactive drugs prior to their transfer for commercial distribution.
These procedures must ensure that the dose calibrator, or other dose measurement system, functions properly. This is accomplished by performing periodic checks and tests prior to first use, followed by checks at specified intervals, and following repairs that could affect system performance. Equipment used to measure dosages that emit gamma, alpha, or beta radiation must be calibrated for the applicable radionuclide being measured. For most photon-emitters, activity measurement is a fairly straightforward determination; however, for low-energy photon emitters, beta-emitters, and alpha-emitters, a correction factor is often necessary to accurately determine the activity. There are inherent technical difficulties to overcome in the determination and application of low-energy photon-, beta-, and alpha-correction factors. These difficulties include dependence on geometry, lack of an industry standard for materials used in the manufacture of both vials and syringes, and lack of a National Institute of Standards and Technology (NIST)- traceable standard for all radionuclides currently in use. If radiopharmacies intend to initially distribute ( i.e., measure, prepare, and label) low-energy photon-, beta-, and alpha-emitting radionuclides, the applicant must provide the calculation to demonstrate its ability to accurately dispense such materials. If the applicant intends to use low-energy photon-, beta-, and alpha- correction factors supplied by the instrument manufacturer, or other entity, it should include a means for ensuring the accuracy of the supplied factor. If radiopharmacy applicants intend to only redistribute low-energy photon-, beta-, and alpha-emitting radionuclides that have been previously prepared and distributed by other persons licensed pursuant to 10 CFR 32.72, then the correction factor calculation is not required.
Licensees must assay patient dosages in the same type of vial and geometry as used to determine the correct dose calibrator settings. The use of different vials or syringes may result in measurement errors, for example, due to the variation of bremsstrahlung created by interaction between beta particles and the differing dosage containers. Licensees are reminded that beta emitters should be shielded using a low-atomic-numbered material to minimize the production of bremsstrahlung, followed by a high-atomic-numbered material thick enough to attenuate the bremsstrahlung intensity.
For each dose measurement system, specific periodic tests must be performed, as appropriate to the system, to ensure correct operation. Typically, all systems must be checked each day of use for constancy to ensure continued proper operation of the system. In addition, other appropriate tests may include accuracy (for the range of energies to be measured), linearity (for the range of activities to be measured), and geometry dependence (for the range of volumes and product containers).
The applicant should ensure that it possesses a sufficient number of such instruments to allow for periods when instruments are out of service for repair and calibration.
Appendix O contains a model procedure for dose calibrator testing.
Response from Applicant: The applicant shall describe the types of systems (measurement or combination of measurement and calculation) it intends to use for the measurement of alpha-, beta-, gamma-, and photon-emitting radioactive drugs;
AND
For each dose measurement system used to measure the amount of radioactivity in alpha-, beta-, gamma-, or photon-emitting radioactive drugs, state: "We have developed, and will implement and maintain a written procedure for the performance of dose measurement system checks and tests that meets the requirements in 10 CFR 32.72(c)";
AND
If applicable, e.g., when dose calibrators are used to measure photon emissions associated with beta or alpha emissions, the applicant must include a sample calculation for determining low-energy photon-, beta-, and alpha-correction factors for dose calibrators with ionization chambers;
Radiopharmacies that intend to initially
distribute (i.e., measure, prepare, and label) low-energy
photon-, beta-,
and alpha-emitting
radionuclides must provide the calculations
to demonstrate its ability to accurately dispense such materials;
however, a
correction factor calculations
isare
not required if radiopharmacy applicants intend to only redistribute
low-energy
photon-, beta- or
alpha-emitting radionuclides
that were previously prepared and distributed by others who are
licensed pursuant to 10 CFR 32.72.
OR
If applicable, the applicant must include a means for ensuring the accuracy of low-energy photon-, beta-, and alpha-correction factors supplied by the instrument manufacturer, or other entity.
Redline/strikeout revisions are shown below for several sections of Appendix C. An explanation is provided in at the beginning of each section.
APPENDIX C
Suggested Format for Providing Information Requested in Items 5 through 11 of NRC Form 313
[The following redline/strikeout revisions to the Item 7, “Individual(s) responsible for radiation safety program and their training and experience,” section of Appendix C reflect the change to 10 CFR 35.55 removing the preceptor attestation requirement from the nuclear pharmacist board certification pathway and changes to the attestation statement for the alternate training and experience pathway. The Item 7, “Individual(s) responsible for radiation safety program and their training and experience,” section of Appendix C starts on page C-5 of the printed copy of NUREG-1556, Vol. 13, Rev. 1.]
Item No. |
Title and Criteria |
Yes |
Description Attached |
|||
Item Number and Title |
Suggested Response |
Yes |
Description Attached |
|||
7. Individual(s) Responsible for Radiation Safety Program and Their Training and Experience |
An organizational chart describing the management structure, reporting paths, and the flow of authority between executive management and the RSO. |
❒ |
❒ |
|||
7.1 RSO Name of Proposed RSO: _______________ |
A copy of the license (NRC or Agreement State) that authorized the uses requested and on which the individual was specifically named as the RSO, ANP, or AU; |
❒ |
❒ |
|||
OR |
|
|
||||
Description of the training and experience demonstrating that the proposed RSO is qualified by training and experience as applicable to commercial nuclear pharmacies. |
❒ |
❒ |
||||
7.2 Authorized Nuclear Pharmacist(s) Name(s) of Proposed ANP(s): _____________
|
For each proposed ANP: |
|
|
Name of the proposed ANP. |
❒ |
❒ |
|
AND |
|
|
|
Pharmacist’s license number and issuing entity; |
❒ |
❒ |
|
AND |
|
|
|
For an individual previously identified as an ANP on an NRC or Agreement State license or permit or by a commercial nuclear pharmacy that has been authorized to identify ANPs (10 CFR 32.72(b)(2)(i)): |
|
|
|
Previous license number (if issued by NRC) or a copy of the license (if issued by an Agreement State) or a copy of a permit issued by an NRC master materials licensee, a permit issued by an NRC or Agreement State broad scope licensee, or a permit issued by an NRC Master Materials License broad scope permittee on which the individual was named an ANP or a copy of an authorization as an ANP from a commercial nuclear pharmacy that has been authorized to identify ANPs. |
❒ |
❒ |
|
OR |
|
|
|
For an individual qualifying under 10 CFR 32.72(b)(4): |
|
|
|
|
❒ |
❒ |
|
AND |
|
|
|
|
❒ |
❒ |
|
OR |
|
|
|
For an individual qualifying under 10 CFR 35.55(a): |
|
|
|
|
❒ |
❒ |
|
|
|
|
|
AND |
|
|
|
|
❒ |
❒ |
|
OR |
|
|
|
For an individual qualifying under 10 CFR 32.72(b)(2)(ii): |
|
|
|
|
❒ |
❒ |
|
AND |
|
|
|
|
❒ |
❒ |
|
AND |
|
|
|
|
❒ |
❒ |
|
Notes: |
|
|
|
NRC Form 313A (ANP), “Authorized Nuclear Pharmacist Training and Experience and Preceptor Attestation [10 CFR 35.55]” may be used to document training and experience for those individuals qualifying under 10 CFR 35.55(a) or (b). |
|
|
|
|
Descriptions
of training and experience will be reviewed using the criteria
listed above. The NRC will review the documentation to determine
if the applicable criteria in |
|
|
7.3 Authorized User(s) Name(s) of Proposed AU(s): __________________ |
For each proposed AU: |
|
|
Name of the proposed AU. |
❒ |
❒ |
|
AND |
|
|
|
|
❒ |
❒ |
|
AND |
|
|
|
|
❒ |
❒ |
|
OR |
|
|
|
|
❒ |
❒ |
|
OR |
|
|
|
|
❒ |
❒ |
|
[The following redline/strikeout revisions to the Item 10, “Radiation Safety Program, Safe Use of Radionuclides and Emergency Procedures,” section of Appendix C reflect the changes to 10 CFR 35.204 increasing the frequency for performing the Mo-99 breakthrough test and 10 CFR 35.3204 Mo-99 breakthrough reporting requirements. The Item 10, “Radiation Safety Program, Safe Use of Radionuclides and Emergency Procedures,” section of Appendix C starts on page C-12 of the printed copy of NUREG-1556, Vol. 13, Rev. 1.] |
|
|
|
10. |
|
|
||
|
Safe Use of Radionuclides and Emergency Procedures We have developed and will implement and maintain written procedures for the safe use of radioactive materials that address:
that meet the requirements in 10 CFR 20.1101, 10 CFR 20.1801, 10 CFR 20.1802, 10 CFR 30.34(g), and 10 CFR 19.11(a)(3), as applicable; AND We have developed and will implement and maintain written procedures for identifying and responding to emergencies involving radioactive material, including:
limits;
|
|
|
|
|
• routine contacts with local fire departments and local law enforcement agencies. that meet the requirements in 10 CFR 20.1101, 10 CFR 20.2201-2203, and 10 CFR 30.50 and other requirements, as applicable.
|
|
[The following redline revisions to the Item 10, “Radiation Safety Program, Dosage Measurements Systems,” section of Appendix C reflect conforming changes for the radiopharmacy from the revision of 10 CFR 35.390 with the addition of alpha emitters. The Item 10, “Radiation Safety Program, Dosage Measurements Systems,” section of Appendix C starts on page C-13 of the printed copy of NUREG-1556, Vol. 13, Rev. 1.]
Dosage Measurement Systems Describe the types of systems (measurement or combination of measurement and calculation) to be used for the measurement of alpha-, beta-, gamma-, and photon-emitting radioactive drugs; AND For each dose measurement system used to measure the amount of radioactivity in alpha-, beta-, gamma-, or photon-emitting radioactive drugs, state: "We have developed, and will implement and maintain a written procedure for the performance of dosage measurement system checks and tests that meets the requirements in 10 CFR 32.72(c)"; AND If applicable, include a sample calculation for determining low-energy photon-,beta-, and alpha-correction factors for dose calibrators with ionization chambers; OR If applicable, include a means for ensuring the accuracy of low-energy photon-, beta-, and alpha-correction factors supplied by the instrument manufacturer or other entity.
|
|
|
Redline/strikeout revisions are shown below for several sections of Appendix D. An explanation is provided in at the beginning of each section.
APPENDIX D
Checklist for License Application
[The following redline/strikeout revision to the D.5 Items 5 & 6 : “Materials to be possessed and proposed uses,” section of Appendix D corrects a citation in 10 CFR Part 35 for calibration and reference sources. The D.5 Items 5 & 6 :” Materials to be possessed and proposed uses,” section of Appendix D starts on page D-1 of the printed copy of NUREG-1556, Vol. 13, Rev. 1.]
D.5 ITEMS 5 & 6 : MATERIALS TO BE POSSESSED AND PROPOSED USES
Yes |
No |
Radioisotope |
Form or Mfg/Model No. |
Quantity |
Purpose of Use |
Specify Other Uses Not Listed on SSD Certificate |
|
|
Byproduct Materials with Atomic No. 1-83 |
Any |
__________millicuries per nuclide, 1 curie total possession, except as noted: |
10 CFR 32.72 and 10 CFR 30.41 |
------------
|
|
|
Molybdenum-99 |
Any |
__________curies |
10 CFR 32.72 and 10 CFR 30.41 |
------------
|
|
|
Technetium-99m |
Any |
__________curies |
10 CFR 32.72 and 10 CFR 30.41 |
------------
|
|
|
Iodine-131 |
Any |
__________millicuries |
10 CFR 32.72 and 10 CFR 30.41 |
------------
|
|
|
Fluorine-18 |
Any |
__________millicuries |
10 CFR 32.72 and 10 CFR 30.41 |
------------
|
|
|
Iodine-123 |
Any |
__________millicuries |
10 CFR 32.72 and 10 CFR 30.41 |
------------
|
|
|
Xenon-133 |
Any |
__________curies |
10 CFR 32.72 and 10 CFR 30.41 |
------------
|
|
|
Any Byproduct Material in a Brachytherapy Source, as listed |
Sealed Sources |
___________millicuries |
10 CFR 32.74 and 10 CFR 30.41 |
|
|
|
in 10 CFR 35.400 |
|
|
|
------------ [ ] Uses are: |
|
|
Any Byproduct Material in a sealed source for diagnosis, as listed in 10 CFR 35.500 |
Sealed Sources |
_____________curies per source and curies total |
10 CFR 32.74 and 10 CFR 30.41 |
------------
|
|
|
Any byproduct material listed in 10 CFR 31.11(a) |
Prepackage d units for in vitro diagnostic tests |
_____________millicuri es |
10 CFR 31.11 |
------------
|
|
|
Any byproduct material authorized under
10 CFR 35.65 a) |
Sealed Sources |
______________millicu ries |
Calibration and checking of the licensees instruments and 10 CFR 32.74 and 10 CFR 30.41 |
------------
|
|
|
Depleted Uranium |
Metal |
_____________kilogra ms |
Shielding for molybdenum99/technetium- 99m generators |
------------
|
|
|
Cesium-137 |
Sealed sources in compatible device as specified in |
Not to exceed maximum activity per source as specified in Sealed Source and Device Registry Sheet |
Instrument calibration |
|
|
|
|
Sealed Source and Device Registry Sheet |
|
|
|
|
|
Other (specify) |
|
|
|
|
[The following redline/strikeout revisions to D.6 Item 7: “Training and experience,” section of Appendix D reflect the changes to 10 CFR 35.55 removing the preceptor attestation requirement from the nuclear pharmacist board certification pathway and changes to the attestation statement for the alternate training and experience pathway. The D.6 Item 7: “Training and experience,” section of Appendix D starts on page D-4 of the printed copy of NUREG-1556, Vol.
13, Rev. 1.]
D.6 ITEM 7:TRAINING AND EXPERIENCE,
Item Number and Title |
Suggested Response |
Yes |
Alternative Procedures Attached |
7. Individual(s) Responsible for Radiation Safety Program and Their Training and Experience |
An organizational chart describing the management structure, reporting paths, and flow of authority between executive management and the RSO. |
|
|
Radiation Safety Program And Their Training And Experience 7.1 Radiation Safety Officer (RSO) Name:_________________ |
A copy of the license (NRC or Agreement State) that authorized the uses requested and on which the individual was specifically named as the RSO, an ANP, or an AU; OR Description of the training and experience demonstrating that the proposed RSO is qualified by training and experience as applicable to commercial nuclear pharmacies. |
|
|
Radiation Safety Program and Their Training and Experience 7.2 Authorized Nuclear Pharmacist(s) Name(s):_______________ |
AND
AND For an individual previously identified as an ANP on an NRC or Agreement State license or permit or by a commercial |
|
|
|
nuclear pharmacy that has been authorized to identify ANPs (10 CFR 32.72(b)(2)(i)):
OR For an individual qualifying under 10 CFR 32.72(b)(4):
AND
|
|
|
|
OR For an individual qualifying under 10 CFR 35.55(a):
CFR 35.55(a);
AND
For an individual qualifying under 10 CFR 32.72(b)(2)(ii):
10 CFR 35.55(b) demonstrating that the proposed ANP is qualified by training and experience; AND
|
|
|
|
required AND
Notes:
Pharmacist Training and Experience and Preceptor Attestation [10 CFR 35.55]" may be used to document training and experience for those individuals qualifying under 10 CFR 35.55(a) or (b).
32.72(b)(2) are met. If the training and experience do not appear to meet the criteria, the NRC may request additional information from the applicant or may request the assistance of the ACMUI in evaluating such training and experience. |
|
|
Radiation Safety Program and Their Training and Experience 7.3 Authorized User(s) Name(s):_________________ |
For each proposed AU: AND
AND
AND
OR
OR
|
|
|
|
[The following redline/strikeout revisions to D.10 Item 10.6: “Safe Use of Radionuclides and Emergency Procedures,” section of Appendix D reflect the changes to 10 CFR 35.204 increasing the frequency of the Mo-99 breakthrough test and 10 CFR 30.34(g) reporting requirements for Mo-99 breakthrough. The D.10 Item 10.6: “Safe Use of Radionuclides and Emergency Procedures,” section of Appendix D starts on page D-9 of the printed copy of NUREG-1556, Vol. 13, Rev. 1.] |
|
|
Safety Program 10.6 Safe Use of Radionuclides and Emergency Procedures |
We have developed and will implement and maintain written procedures for the safe use of radioactive materials that address:
measurements
on
that meet the requirements in 10 CFR 20.1101, 10 CFR 20.1801, 10 CFR 20.1802, 10 CFR 30.34(g), and 10 CFR 19.11(a)(3), as applicable. AND We have developed and will implement and maintain written procedures for identifying and responding to emergencies involving radioactive material, including:
|
|
|
|
that meet the requirements in 10 CFR 20.1101, 10 CFR 20.2201, 20.2202, 20.2203, and 10 CFR 30.50 and other requirements, as applicable. |
|
|
|
[The following redline revisions to D.10 Item 10.8, “Dosage Measurement,” section of Appendix D reflect the conforming radiopharmacy changes resulting from adding alpha emitters in 10 CFR 35.390. D.10 Item 10.8, “Dosage Measurement,” section of Appendix D starts on page D-10 of the printed copy of NUREG-1556, Vol. 13, Rev. 1.] |
|
|
10.8 Dosage Measurement Systems |
Describe the types of systems (measurement or combination of measurement and calculation) to be used for the measurement of alpha-, beta-, gamma-, and photonemitting radioactive drugs; AND For each dosage measurement system used to measure the amount of radioactivity in alpha-, beta-, gamma, or photon-emitting radioactive drugs, state: "We have developed, and will implement and maintain, a written procedure for the performance of dosage measurement system checks and tests that meets the requirements in 10 CFR 32.72(c)"; AND If applicable, include a sample calculation for determining low-energy photon-,beta-, and alpha-correction factors for dose calibrators with ionization chambers; OR If applicable, include a means for ensuring the accuracy of low-energy photon-,beta-, and alpha-correction factors supplied by the instrument manufacturer or other entity. |
|
|
The following redline/strikeout revisions and the revised NRC Form 313A (ANP) in Appendix G reflect the changes to the training and experience requirements in 10 CFR 32.72, and 10 CFR 35.55 for the Authorized Nuclear Pharmacist.
APPENDIX G
Formats for Documenting Training and Experience for
Individuals Responsible for Radiation Protection Program
Table G-1 Authorized User or Radiation Safety Officer Training in Basic Radioisotope Handling Techniques
Name (Last, First, Initial) |
|
|||||||
Location of Training |
Dates |
Title |
Total Hours |
Breakdown of Course in Clock Hours |
||||
RPP |
BH |
IR |
INST |
REG |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTALS |
|
|
|
|
|
RPP - Radiation Protection Principles
BH - Biological Hazards
IR - Ionizing Radiation Units & Characteristics
INST - Radiation Detection Instrumentation
REG - NRC Regulations and Standards
Table G-2 Authorized User and Radiation Safety Officer Experience in Handling Radioisotopes
(Actual use of radioisotopes under the supervision of an authorized user or Radiation Safety Officer, respectively)
Name (Last, First, Initial) |
|
|
|||
Isotope(s) used |
Maximum amount used at any one time |
Location of use |
Purpose of use* |
Total Hours of Experience |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* Description of experience
Shipping, receiving, and performing related radiation surveys.
Using and performing checks for proper operation of dose calibrators, survey meters, and other instruments used to measure photon- and high-energy betaemitting radionuclides.
Using and performing checks for proper operation of instruments used to measure alpha- and low energy beta-emitting radionuclides.
Calculating, assaying, and safely preparing radioactive materials.
Use of procedures to prevent or minimize contamination and/or use of proper decontamination procedures.
Documentation of Training and Experience to Identify an Individual on a License as an Authorized Nuclear Pharmacist.
Experienced Authorized Nuclear Pharmacists
An applicant or licensee that is adding an experienced Authorized Nuclear Pharmacist (ANP) to its commercial radiopharmacy license only needs to provide evidence that the individual is listed on a license issued by the NRC or Agreement State, a permit issued by an NRC Master Materials Licensee, a permit issued by an NRC or Agreement State broad-scope licensee, or a permit issued by an NRC master materials broad-scope permittee, and that the individual meets the recentness of training criteria described in 10 CFR 35.59. The applicant also may provide evidence that the individual is listed on an NRC or Agreement State commercial nuclear pharmacy license or identified as an ANP by a commercial nuclear pharmacy authorized to identify ANPs. For individuals who have been previously authorized by, but not listed on, the commercial nuclear pharmacy license, medical broad-scope license, or master materials license medical broad-scope permit, the applicant should submit either verification of previous authorizations granted or evidence of acceptable training and experience.
Experienced Nuclear Pharmacists Who Only Used Accelerator-Produced
Nuclear Materials, or Discrete Sources of Radium-226, or Both, for Medical or Nuclear Pharmacy Uses
During the implementation of the EPAct, NRC "grandfathered" nuclear pharmacists that used only accelerator-produced radioactive materials, discrete sources of radium-226 (Ra-226), or both, for nuclear pharmacy uses under the NRC waiver of August 31, 2005, when using these materials for the same uses. Nuclear pharmacists that used accelerator-produced radionuclides or discrete sources of Ra-226 during the effective period of the waiver do not have to meet the requirements of 10 CFR 35.59, or the training and experience requirements in 10 CFR Part 35, Subpart B for those materials and uses.
The applicant or licensee that is adding one of these experienced individuals to its commercial nuclear pharmacy license should document that the individual used only accelerator-produced radionuclides, or discrete sources of Ra-226, for nuclear pharmacy uses during the effective period of the waiver and that the materials were used for the same uses requested. This documentation may be, but is not restricted to, evidence that the individual was listed on an Agreement State or non-Agreement State license or permit authorizing these materials for the requested uses.
Applications that Include Individuals for Authorized Nuclear Pharmacist Recognition by NRC
Applicants should submit NRC Form 313A (ANP) to show that the individual meets the correct training and experience criteria in 10 CFR Part 35, Subpart B. There are two primary training and experience routes to qualify an individual as an ANP. The first is by means of certification by a board recognized by NRC and listed on the NRC website (http://www.nrc.gov/materials/miau/med-use-toolkit.html) as provided in 10 CFR 35.55(a).
The second route is by meeting the structured educational program, supervised work experience, and preceptor attestation requirements in 10 CFR Part 35.55(b), Subpart B.
Recentness of Training
The required training and experience, including board certification, described in 10 CFR Part 35 must be obtained within the 7 years preceding the date of the application, or the individual must document having had related continuing education, retraining, and experience since obtaining the required training and experience. Examples of acceptable continuing education and experience include the following:
Successful completion of classroom and laboratory review courses that include radiation safety practices relative to the practice of nuclear pharmacy, and
Practical experience in nuclear pharmacy under the supervision of an ANP at the same or another licensed facility that is authorized as a nuclear pharmacy.
General Instructions and Guidance for Filling Out NRC Form 313A Series
If the applicant wishes to identify a license and it is an Agreement State license, the applicant should provide a copy of the license. If the applicant wishes to identify a Master Materials License permit, the applicant should provide a copy of the permit. If the applicant wishes to identify an individual (i.e., supervising individual or preceptor) who is authorized under a broad-scope license or broad-scope permit of a Master Materials License, the applicant should provide a copy of the permit issued by the broad-scope licensee/permittee. Alternatively, the applicant may provide a statement signed by the Radiation Safety Officer or chairperson of the Radiation Safety Committee similar to the following: "__________(name of supervising individual or preceptor) is authorized under _______________(name of licensee/permittee) broad-scope license number__________ to use_________(materials) during ____________( time frame)".
INTRODUCTORY INFORMATION
Name of Individual
Provide the individual's complete name so that NRC can distinguish the training and experience received from that received by others with a similar name.
Note: Do not include personal or private information (e.g., date of birth, social security number, home address, personal phone number) as part of your qualification documentation.
State or Territory where Licensed
Note that the NRC requires pharmacists to be licensed by a State or territory of the United States, the District of Columbia, or the Commonwealth of Puerto Rico to practice pharmacy.
Requested Authorization(s)
Check all authorizations that apply and fill in the blanks as provided.
Part I. Training and Experience
There are always multiple pathways provided for each training and experience section. Select the applicable one.
Item 1. Board Certification
The applicant or licensee may use this pathway if the proposed nuclear pharmacist is certified by a board recognized by NRC (to confirm that NRC recognizes that board's certifications, see NRC's web page http://www.nrc.gov/materials/miau/med-usetoolkit.html.
Note:
An individual that is board eligible will not be considered for this pathway until the individual is actually board-certified. Further, individuals holding other board certifications will also not be considered for this pathway.
The applicant or licensee must provide a copy of the board certification
andcompleted attestationas indicated on the attached NRC Form 313A (ANP).As indicated on the form, additional information is needed if the board certification was greater than 7 years ago.
Item 2. Structured Educational Program for a Proposed Authorized Nuclear Pharmacist
This pathway is used for those individuals not listed on the license as an ANP, who do not meet the requirements for the board certification pathway.
The regulatory requirements refer to a structured educational program consisting of both (a) classroom and laboratory training, and (b) supervised practical experience in nuclear pharmacy. All hours credited to classroom and laboratory training must relate directly to radiation safety and safe handling of byproduct material and be allocated to one of the topics in 10 CFR 35.55 (b)(1)(i).
The proposed ANP may receive the required classroom and laboratory training, and supervised practical experience at a single training facility or at multiple training facilities; therefore, space is provided to identify each location and date of training or experience. The date should be provided in the month/day/year (mm/dd/yyyy) format.
Under the "classroom and laboratory training," provide the number of clock hours spent on each of the topics listed in the regulatory requirements.
The proposed ANP may obtain the required "classroom and laboratory training" in any number of settings, locations, and educational situations. For example, at some medical teaching/university institutions, a course may be provided for that particular need and taught on consecutive days. In other training programs, the period may be a semester or quarter as part of the formal curriculum. Also, the classroom and laboratory training may be obtained using a variety of other instructional methods. Therefore, NRC will broadly interpret "classroom and laboratory training" to include various types of instruction, including online training, as long as it meets the specific clock hour requirements and the subject matter relates to radiation safety and safe handling of byproduct material for the uses requested.
Under the "supervised practical experience" section of the form, provide the number of clock hours for each topic. The supervised practical experience topics for the nuclear pharmacists include all the basic elements in the practice of nuclear pharmacy. Therefore, all the hours of supervised experience are allocated to these topics.
Note: As indicated on the form, additional information is needed if the training and/or supervised practical experience was completed more than 7 years ago.
Part II. Preceptor Attestation
The
NRC defines the term “preceptor” in 10 CFR 35.2,
“Definitions,” to mean “an individual who provides,
directs, or verifies training and experience required for an
individual to become an authorized user, an authorized medical
physicist, an authorized nuclear pharmacist, or a Radiation Safety
Officer.” While the supervising individual for the practical
experience in nuclear pharmacy may also be the preceptor, the
preceptor does not have to be the supervising individual as long as
the preceptor directs or verifies the training and experience
required. The preceptor must provide an attestation in writing
regarding the training and experience of a pharmacist
applying through the structured educational program for a proposed
authorized nuclear pharmacist. The preceptor must attest
that the individual has satisfactorily completed the appropriate
training and experience criteria and is
ablehas
achieved
a level of competency
sufficient
to function
independently fulfill
the radiation safety-related duties as an authorized nuclear
pharmacist.
This preceptor also has to meet specific requirements.
The
NRC Form 313A (ANP) Part II - Preceptor Attestation has two sections.
The preceptor must complete both sections;,
i.e.,
The preceptor must selects either the
board
certification or
including the structured
educational program,
when filling out
attestation,
the
first
section on this page. The second and final sections of the page
request specific
information
about the
preceptor’s authorization to use licensed material,
and in
addition
to
the
preceptor’s signature.


The following redline revisions in Appendix H reflect the new reporting Mo99 breakthrough requirements in 10 CFR 30.34(g).
APPENDIX H
Typical Duties and Responsibilities of the Radiation Safety Officer
The RSO’s duties and responsibilities include ensuring radiological safety and compliance with NRC and DOT regulations, and with the conditions of the license (see Figure H.1). Typically, these duties and responsibilities include ensuring that:
General surveillance is provided over all activities involving radioactive material, including routine monitoring, special surveys, and responding to events;
Incidents are responded to, investigated, their cause(s) and appropriate corrective action(s) are identified, and timely corrective action(s) are taken;
Proper authorities are notified of incidents such as damage, fire, or theft;
Proper NRC and manufacturer/distributor notification is made when required (e.g., over exposures; leaking sources).
Proper NRC and distributor notification is made when there is more than 0.15 kilobecquerel of molybdenum-99 per megabecquerel of technetium-99m (0.15 microcurie of molybdenum-99 per millicurie of technetium-99m) in an eluate))
Corrective actions are developed, implemented, and documented when violations of regulations or license conditions or program weaknesses are identified;
All activities are immediately terminated following any unsafe condition or activity that is found to be a threat to public health and safety;
He or she is the primary source of radiation protection information for personnel at all levels of responsibility;
All radiation workers are properly trained;
Procedures for the safe use of radioactive materials are developed and implemented;
The license's procedures and controls, based upon sound radiation protection principles, are periodically reviewed to ensure that occupational doses and doses to members of the public are as low as is reasonably achievable (ALARA). Documentation is maintained to demonstrate, by measurement or calculation, that the total effective dose equivalent to the individual member of the public likely to receive the highest dose from the licensed operation does not exceed the annual limit;
General surveillance is provided over all activities involving radioactive material, including routine monitoring, special surveys, and responding to events.
Incidents are responded to, investigated, their cause(s) and appropriate corrective action(s) are identified, and timely corrective action(s) are taken.
Proper authorities are notified of incidents such as damage, fire, or theft.
Corrective actions are developed, implemented, and documented when violations of regulations or license conditions or program weaknesses are identified.
All activities are immediately terminated following any unsafe condition or activity that is found to be a threat to public health and safety.
He or she is the primary source of radiation protection information for personnel at all levels of responsibility.
All radiation workers are properly trained.
Procedures for the safe use of radioactive materials are developed and implemented.
The licensee's procedures and controls, based upon sound radiation protection principles, are periodically reviewed to ensure that occupational doses and doses to members of the public are as low as is reasonably achievable (ALARA). Documentation is maintained to demonstrate, by measurement or calculation, that the total effective dose equivalent to the individual member of the public likely to receive the highest dose from the licensed operation does not exceed the annual limit.
Prospective evaluations are performed of occupational exposures, and those individuals likely to receive, in one year, a radiation dose in excess of 10% of the allowable limits are provided personnel monitoring devices.
When necessary, personnel monitoring devices are used and exchanged at the proper intervals, and records of the results of such monitoring are maintained.
The performance of fume hoods and gloveboxes used for volatile radioactive material work are monitored for proper operation.
The receipt, opening, and delivery of all packages of radioactive material arriving at the nuclear pharmacy are overseen and coordinated.
An inventory of all radioactive materials is maintained and the types and quantities of radionuclides at the facility are limited to the forms and amounts authorized by the license.
Sealed sources are leak-tested at required intervals.
There is effective management of the radioactive waste program, including effluent monitoring.
Packaging and transport of radioactive material is in accordance with all applicable DOT requirements.
An up-to-date license is maintained and amendment and renewal requests and notifications of new ANPs are submitted in a timely manner.
Radiation Safety Program audits are performed at least annually and documented.
He or she acts as liaison to NRC.
All required records are properly maintained.
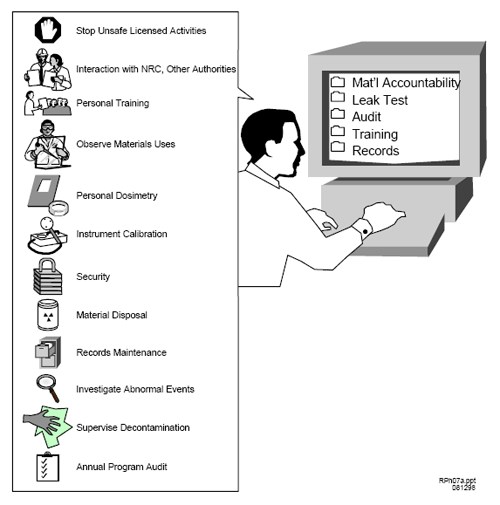
Figure H.1 Typical Duties and Responsibilities of the RSO.
Redline/strikeout revisions are shown below for one section of Appendix I. An explanation is provided in at the beginning of this section.
APPENDIX I.
Suggested Commercial Radiopharmacy Audit Checklist
[The following redline revisions to the “Notification and reports,” section of Appendix I reflect the addition of new mo-99 breakthrough reporting requirements in 10 CFR 30.34(g). The “Notification and reports,” section of
Appendix I starts on page I-7 of the printed copy of NUREG-1556, Vol. 13, Rev. 1.]
Notification and Reports
Was any radioactive material lost or stolen? Were reports made? [10 CFR 20.2201, 10 CFR 30.50]
Did any reportable incidents occur? Were reports made? [10 CFR 20.2202, 10 CFR 30.34(g), 10 CFR 30.50]
Did any overexposures or high radiation levels occur? Reported? [10 CFR 20.2203, 10 CFR 30.50]
Were any contaminated packages or packages with surface radiation levels exceeding 200 mrem received? Reported to NRC?
If any events (as described in items A through D above) did occur, what was root cause? Were appropriate notifications made and corrective actions taken?
F.Is the management/RSO aware of telephone number for NRC Emergency Operations Center? [(301) 816-5100]
Redline/strikeout revisions are shown below for one section of Appendix Q. An explanation is provided in at the beginning of this section.
APPENDIX Q
General Topics for Safe Use of Radioisotopes and Model Emergency Procedures
[The following redline revision to the “General Topics for Safe Use of
Radioisotopes,” section of Appendix Q reflects the increased frequency of Mo-99 breakthrough measurements in 10 CFR 35.204 and new reporting requirements for Mo-99 breakthrough in 10 CFR 30.34. The “General Topics for Safe Use of Radioisotopes,” section of Appendix Q starts on page Q-1 of the printed copy of NUREG-1556, Vol. 13, Rev. 1.]
General Topics for Safe Use of Radioisotopes
Each licensee using radioactive material should establish general rules for the safe use of the material so that workers know what is required. Typical instructions should include:
Wear a laboratory coat or other protective clothing at all times when working with radioactive materials;
Use syringe shields and vial shields when preparing and handling radioactive drugs;
Measure all radiopharmaceuticals prior to transfer;
Measure the molybdenum-99 content of each generator eluate. Do not transfer those radiopharmaceuticals for human medical use that will contain more than 0.15 microcuries of molybdenum-99 per millicurie of technetium-99m at the time of administration, and report each eluate that exceeds this limit at time of measurement to NRC and the distributor;
Wear disposable gloves at all times when handling radioactive materials and change gloves frequently to minimize the spread of contamination;
Before leaving the hot lab, monitor hands, shoes, and clothing for contamination in a low-background area, allowing sufficient time for instrument response;
Do not eat, drink, smoke, or apply cosmetics in any area where licensed material is stored or used;
Do not store food, drink, or personal effects in areas where licensed material is stored or used (see Figure Q.1). Personal items brought into the restricted area (radios, compact discs, notepads, books, etc.) should be surveyed for contamination before removal from the area;
Food and beverages used in the preparation of radiopharmaceuticals should be clearly labeled "Not for personal consumption" if stored with radioactive materials;
Wear personnel monitoring devices, if required, at all times while in areas where licensed materials are used or stored;
Dispose of radioactive waste only in designated, labeled, and properly shielded receptacles;
Never pipette by mouth;
Store radioactive solutions in clearly labeled containers; and
Secure all licensed material when it is not under the constant surveillance and immediate control of the user(s).
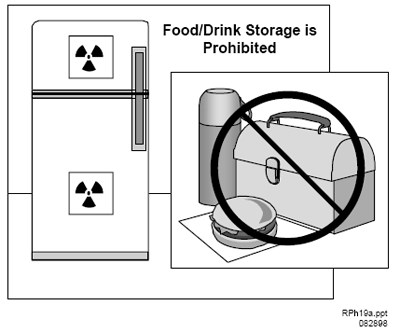
Figure Q.1 Storage of Food and Drink. Food or drink for personal consumption should not be stored in refrigerators with radioisotopes.
The following redline revisions in Appendix H reflect the new reporting
Mo-99 breakthrough requirements in 10 CFR 30.34(g) and 10 CFR 30.50(b)(2).
APPENDIX T
NRC Incident Notifications
NRC Incident Notifications
Table T.1 Typical Notifications Required for Radiopharmacy Licensees
Event |
Telephone Notification |
Written Report |
Regulatory Requirement |
Theft or loss of material |
immediate |
30 days |
10 CFR 20.2201(a)(1)(i) |
Whole body dose greater than 0.25 Sv (25 rems) |
immediate |
30 days |
10 CFR 20.2202(a)(1)(i) |
Extremity dose greater than 2.5 Sv (250 rems) |
immediate |
30 days |
10 CFR 20.2202(a)(1)(iii) |
Intake of five times the annual limit on intake |
immediate |
30 days |
10 CFR 20.2202(a)(2) |
Removable contamination exceeding the limits of 10 CFR 71.87(i) - (beta/gamma/low toxicity alpha - 22 dpm/cm2; all other alpha - 2.2 dpm/cm2) |
immediate |
none |
10 CFR 20.1906(d)(1) |
External radiation levels exceeding the limits of 10 CFR 71.47 - (any point on the surface - 2 mSv/hr (200 mrem/hr)) |
immediate |
none |
10 CFR 20.1906(d)(2) |
Whole body dose greater than 0.05 Sv (5 rems) in 24 hours |
24 hours |
30 days |
10 CFR 20.2202(b)(1)(i) |
Extremity dose greater than 0.5 Sv (50 rems) in 24 hours |
24 hours |
30 days |
10 CFR 20.2202(b)(1)(iii) |
Intake of one annual limit on intake |
24 hours |
30 days |
10 CFR 20.2202(b)(2) |
Occupational dose greater than the applicable limit in 10 CFR 20.1201 |
none |
30 days |
10 CFR 20.2203(a)(2)(i) |
Dose to individual member of public greater than 1 mSv (100 mrems) |
none |
30 days |
10 CFR 20.2203(a)(2)(iv) |
Defect in equipment that could create a substantial safety hazard |
2 days |
30 days |
10 CFR 21.21(d)(3)(i) |
Report molybdenum-99 content of a generator eluate that is more than 0.15 microcuries of molybdenum-99 per millicurie of technetium-99m |
Within 7 days |
30 days |
10 CFR 30.34(g) and 10 CFR 35.3204(a) |
Filing petition for bankruptcy under 11 U.S.C. |
none |
immediately after filing petition |
10 CFR 30.34(h) |
Expiration of license |
none |
60 days |
10 CFR 30.36(d) |
Decision to permanently cease licensed activities at entire site |
none |
60 days |
10 CFR 30.36(d) |
Decision to permanently cease licensed activities in any separate building or outdoor area that is unsuitable for release for unrestricted use |
none |
60 days |
10 CFR 30.36(d) |
No principal activities conducted for 24 months at the entire site |
none |
60 days |
10 CFR 30.36(d) |
No principal activities conducted for 24 months in any separate building or outdoor area that is unsuitable for release for unrestricted use |
none |
60 days |
10 CFR 30.36(d) |
Event that prevents immediate protective actions necessary to avoid exposure to radioactive materials that could exceed regulatory limits |
immediate |
30 days |
10 CFR 30.50(a) |
An unplanned contamination event involving greater than 5 times the ALI, and half-life greater than 24 hours requiring access to be restricted for more than 24 hours |
24 hours |
30 days |
10 CFR 30.50(b)(1) |
Equipment is disabled or fails to function as designed when required to prevent |
24 hours |
30 days |
10 CFR 30.50(b)(2) |
radiation exposure in excess of regulatory limits |
|
|
|
Unplanned fire or explosion that affects the integrity of any licensed material or device, container, or equipment with licensed material |
24 hours |
30 days |
10 CFR 30.50(b)(4) |
Note: Telephone notifications shall be made to NRC Operations Center, at 301-8165100 or 301-951-0550.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | DBH |
| File Modified | 0000-00-00 |
| File Created | 2021-01-20 |
© 2026 OMB.report | Privacy Policy





 Not applicable
Not applicable