Blood Pressure Methodology Study Phase 1
National Health and Nutrition Examination Survey
Att_12a_BP Method1 17-18 101816
Blood Pressure Methodology Study Phase 1
OMB: 0920-0950
Attachment 12a
Blood Pressure Methodology Phase 1
Form Approved OMB No. 0920-0950 Exp. Date xx/xx/20xx
Assurance of Confidentiality – All information which would permit identification of an individual, a practice, or an establishment will be held confidential, will be used only by NCHS staff, contractors, and agents only when required and with necessary controls, and will not be disclosed or released to other persons without the consent of the individual or establishment in accordance with section 308(d) of the Public Health Service Act (42 USC 242m) and the Confidential Information Protection and Statistical Efficiency Act (PL-107-347). By law, every employee as well as every agent has taken an oath and is subject to a jail term of up to five years, a fine of up to $250,000, or both if he or she willfully discloses ANY identifiable information about you.
NOTICE-Public reporting burden of this collection of information is estimated to average 30 minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to CDC/ATSDR Reports Clearance Officer; 1600 Clifton Road, MS D-74, Atlanta, GA 30333. ATTN: PRA (0920-0950).
Blood Pressure Methodology Phase 1
Justification for the Study
The ways of obtaining BP have changed remarkably in the last 20 years. Clinical BP, the category in which the National Health and Nutrition Examination Survey (NHANES) blood pressure (BP) method falls in, is no longer the only approach to obtaining accurate BP, both 24-hour ambulatory BP monitoring and home BP monitoring are acknowledged ways to obtain BP. All of these methods are presently use oscillometric automatic BP devices. Moreover, with the exception of the Framingham Heart Study and NHANES, other epidemiological studies use oscillometric automated devices to obtain BP values (1-6). Before a change can be made we need to better understand how BP measurements compare between the two types of devices and develop analytic methods to compare measurements obtained from the two devices. Measurements taken by the mercury device must be compared to those taken by a successor device so that secular trends of hypertension prevalence can be accurately maintained and followed.
We are proposing to conduct this study in the NHANES 2017-2018 survey cycle. A two phases study will be enable us to transition smoothly from the mercury to the automatic device. The first phase to be executed in NHANES 2017 will compare devices by selected covariates and the second phase to be executed in NHANES 2018 will compare high BP prevalence based on mean systolic and diastolic BP values obtained independently by the mercury device and Omron device. Whereas the first phase of the study will use data obtained from a selected sample, the second phase will be based on data of all eligible participants during one year of NHANES study.
It is expected that the final decision to change devices will be a decision to be derived jointly by National Heart Lung and Blood Institute (NHLBI), NHANES and the National Center for Health Statistics (NCHS). More specifically, after each phase of the study the results will be presented to appropriate stakeholders from NHLBI and NHANES/NCHS according to their decision we will decide on go-or-no-go to the next phase. The decision rules are described in the statistic section of the proposal.
Eligibility: All NHANES participants ages 6 and older who participate in the mobile examination center (MEC) blood pressure measurements component are eligible. The maximum number of respondents would be 702.
Informed Consent: Written informed consent will be obtained as part of the regular NHANES consent process for the examination in the MEC.
Exclusion Criteria: There are no exclusion criteria except those already in place for the regular NHANES Blood Pressure MEC component.
Data Collection: This study will follow Ni et al (2006) device comparison method study (7). The overall schema for our method study requires 5 minutes of rest prior to obtaining BP measurements from the reference or the test device, order randomized, followed by another 5 minutes of rest prior to obtaining BP measurements from the test (hereafter Omron) or the reference device (hereafter mercury). The interval between BP determinations for the mercury will be 30 seconds; whereas, the interval for the Omron device is 60 seconds. Following this schema will enable us to accomplish 3 objectives. First, to maintain the legacy protocol for the mercury reference device. Second, to obtain BP measurements at 60-second intervals with the Omron device aligns the protocol with national and international standards which require 60-second intervals between sequential BP test devices measurements (8-10). Lastly, provide us enough time to change cuffs between the mercury and the Omron. Figure 1 describes the device comparison study design.
Figure 1
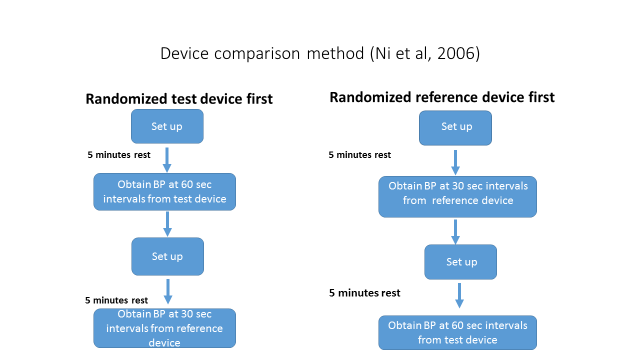
The study will require a two-stage randomization schema; specifically, the first randomization will assign the order of devices used in the randomized arms: mercury device first or the Omron device first. The second randomization will be to determine who, among the two technicians, will be the active observer, see Figure 2.
The active observer will perform the pre-measurement procedures. The pre-measurement procedures consist of all procedures prior to BP observations, specifically, positioning the participants, placing and changing the appropriate BP cuff on the upper arm, obtaining the maximum inflation level (MIL) in mercury condition, and initiating the 5-minute wait.
During the measurement procedure in the mercury condition, the active observer will be responsible for placing the stethoscope bell over the brachial artery, controlling the deflation valve and auscultating the BP using a double-headed stethoscope, observing the mercury column, and recording the 3 reference readings (systolic K1 and diastolic K5) at 30-second intervals. The active observer will be masked from the passive observer readings and Omron device readings. During the measurement procedure in Omron device condition, the active observer will make sure that the Omron device is in “hide” mode, initiate the Omron devices readings in automatic mode, and ensure that the 3 consecutive BP readings are done in 60-second intervals.
The passive observer has two tasks, first simultaneously with the active observer he/she will auscultate the BP using a double-headed stethoscope, observing the mercury column, and will record the 3 mercury readings (systolic K1 and diastolic K5) at 30-second intervals while being masked from the active observer readings and test device readings.
The total component time is expected to be 20-30 minutes. Once the 6 BP measurements are captured, the results will be recorded (double-keyed) by the revealer, who is trained to record the pressures from the machine so as to mask the results from the two observers.
Report of Findings: Findings from the blood pressure methodology study will not be reported to participants. Participants will receive blood pressure results from their regular NHANES blood pressure measurements.
References
Ginsberg HN. The ACCORD (Action to Control Cardiovascular Risk in Diabetes) Lipid Trial
What we learn from subgroup analyses DIABETES CARE, VOLUME 34, SUPPLEMENT 2, MAY 2011 doi: 10.2337/dc11-s203
Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, Fine LJ, Goff DC Jr, Johnson KC, Killeen AA, Lewis CE, Oparil S, Reboussin DM, Rocco MV, Snyder JK, Williamson JD, Wright JT Jr, Whelton PK; SPRINT Study Research Group. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014 Oct;11(5):532-46. doi: 10.1177/1740774514537404.
National Heart, Lung, and Blood Institute (NHLBI) 2014 The Coronary Artery Risk Development in Young Adults Study (CARDIA). Retrieved from http://www.nhlbi.nih.gov/research/resources/obesity/population/index.htm
Zohoori N, Pulley L, Jones C, Senner J, Shoob H, Merritt RK. Conducting a statewide health examination survey: the Arkansas Cardiovascular Health Examination Survey (ARCHES). Prev Chronic Dis. 2011; 8(3): A67.
Nieto FJ, Peppard PE, Engelman CD, McElroy JA, Galvao LW, Friedman EM, Bersch AJ, Malecki KC. The Survey of the Health of Wisconsin (SHOW), a novel infrastructure for population health research: rationale and methods. BMC Public Health. 2010 23; 10:785.
Oregon Health Study Group (2009-2010). Oregon health study. Available at: http://www.nber.org/oregon/documents/survey/in-person/inperson-survey-protocols.pdf
Ni H, Wu C, Prineas R, Shea S, Liu K, Kronmal R, Bild D. Comparison of Dinamap PRO-100 and mercury sphygmomanometer blood pressure measurements in a population-based study. Am J Hypertens. 2006; 19(4): 353-60.
Association for the Advancement of Medical Instrumentation (AAMI). Non-invasive sphygmomanometers —Part 2: Clinical investigation of automated measurement type. Retrieved from http://my.aami.org/aamiresources/previewfiles/8106002_1306_preview.pdf
Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005:45(1);142–161.
Canadian Hypertension Education Program 2015 guidelines. Retrieved from https://www.hypertension.ca/en/chep
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | CDC INSTITUTIONAL REVIEW BOARD (IRB) |
| Author | vlt0 |
| File Modified | 0000-00-00 |
| File Created | 2021-01-20 |
© 2026 OMB.report | Privacy Policy