Ssa_12032018
SSA_12032018.docx
The World Trade Center Health Program: Impact Assessment and Strategic Planning for Translational Research (Part 1, Formative Research: Focus Groups)
OMB: 0920-1252
The World Trade Center Health Program:
Impact Assessment and Strategic Planning for Translational Research
(Part 1, Formative Research: Focus Groups)
Supporting Statement – Section A
December 3, 2018
Program Official/Project Officer
LCDR Pattama Ulrich, RN, MPH
World Trade Center Health Program
DHHS/USPHS/CDC/NIOSH
513-223-0011
Table of Contents
Justification
Circumstances Making the Collection of Information Necessary
Purpose and Use of Information Collection
Use of Improved Information Technology and Burden Reduction
Efforts to Identify Duplication and Use of Similar Information
Impact on Small Businesses or Other Small Entities
Consequences of Collecting the Information Less Frequently
Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
Explanation of Any Payment or Gift to Respondents
Protection of the Privacy of Information Provided by Respondents
Institutional Review Board (IRB) and Justification for Sensitive Questions
Estimates of Annualized Burden Hours and Costs
Estimates of Other Total Annual Cost Burden to Respondents or Record Keepers
Annualized Cost to the Government
Explanation for Program Changes or Adjustments
Plans for Tabulation and Publication and Project Time Schedule
Reason(s) Display of OMB Expiration Date is Inappropriate
Exceptions to Certification for Paperwork Reduction Act Submissions
List of Attachments
Att. A Authorizing Legislation
Att. B 60-Day FRN
Att. C Consent Form
Att. D Focus Group Discussion Guide
Att. E Recruitment and Reminder emails
Att. F RAND IRB Determination
Att. G Brief Demographic Survey
Att. H NIOSH IRB Determination Form
Att. I Privacy Impact Assessment Form
SUPPORTING STATEMENT A
Submission for “The World Trade Center Health Program (WTCHP): Impact Assessment and Strategic Planning for Translational Research (Part 1, Formative Research: Focus Groups)”
• Goal of the assessment: NIOSH
has contracted with the RAND Corporation to evaluate progress toward
translational research funded through the World Trade Center Health
Program (WTCHP). This information collection request is for
the first stage of our assessment only. In
the first stage we will hold focus groups with different stakeholder
groups to explore their perspectives on translational research in
the context of the WTCHP. The results of these focus groups will
inform subsequent stages of the project that will be carried out in
ensuing years. • Intended
use of the resulting data: The results of this part of the
project will be used to: (1) gather data to
help inform an evaluation of the WTCHP and development of strategic
planning recommendations; (2) complement interviews conducted
in the other part of the qualitative WTCHP evaluation; and (3)
potentially inform selection of interview
participants based on an assessment of focus group participant
feedback across demographic characteristics. • Methods
to be used to collect the data: This part of the project will
entail a series of in-person and telephone/webinar-based focus
groups. • The
subpopulation to be studied: The focus groups will be conducted
with WTCHP researchers, research users, and the funder (NIOSH). • How data
will be analyzed: Data from the focus groups will be coded for
common themes using standard qualitative techniques.

A. Justification
1. Circumstances Making the Collection of Information Necessary
This is a request for a new information collection. The Centers for Disease Control and Prevention (CDC) is requesting a 1-year approval to collect information using focus groups.
The World Trade Center Health Program (WTCHP) was established by the James Zadroga 9/11 Health and Compensation Act of 2010, Public Law 111-347 (Attachment A- hereafter referred to as “the Zadroga Act”). Under subtitle C, the Zadroga Act requires the establishment of a research program on health conditions resulting from the 9/11 terrorist attacks. The WTCHP currently carries out a robust research program while providing critical monitoring and treatment services for its 75,000 members. Members include responders at the World Trade Center (WTC) and related sites and survivors who were in the New York City disaster area. The program maintains a research mission to identify physical and mental health conditions that may be related to the 9/11 terrorist attacks and define effective diagnostic procedures and treatments for WTC-related health conditions.
In 2016, NIOSH contracted with the RAND Corporation to conduct an independent evaluation of the WTCHP Research-to-Care (RTC) model including the research investments to date and the effectiveness with which the Program translates its research to different stakeholder groups. RAND was selected given the project team’s expertise with similar assessments and NIOSH’s requirement for an objective analysis. This work will ultimately provide guidance for the WTCHP on strategic directions, as well as produce generalizable knowledge about the translation of research into improved outcomes for individuals and populations exposed to disasters such as the 9/11 attacks. In the first stage of our assessment, we will hold a series of focus groups with different stakeholder groups to explore their perspectives on translational research in the context of the WTCHP. These focus groups are necessary to gather background information on the relationship between different stakeholders and the WTCHP that will complement interviews conducted in the other part of the qualitative WTCHP evaluation. The findings from the focus groups on their own will also inform the overall assessment of the WTCHP program. This information collection request is for the focus groups only. The interviews will start as soon as OMB approval is received, but potentially 6-9 months after the focus groups are conducted.
The RTC model (Figure 1) is the strategic framework employed by the WTCHP to prioritize, conduct, and assess research that informs excellence in clinical care for the population of responders and survivors affected by the 9/11 attack in New York City. The model assumes the collective involvement of WTCHP stakeholders, including members, researchers, clinicians, and program administrators. It accounts for a variety of inputs that can affect the progress and impact of WTCHP research, such as people and organizations, resources, and regulatory rules. The program supports a number of activities that aim to produce tangible outputs such as research findings on WTC-related conditions and healthcare protocols. Finally, the model anticipates short-, intermediate-, and long-term measurement of outcomes and serves as a communication tool for program planning and evaluation.
While the RTC model has guided WTCHP research since the Program’s inception, the WTCHP now needs evaluative work to determine whether research investments are resulting in maximal benefits for program stakeholders, and to plan for the program’s strategic priorities. The 2015 reauthorization of the Zadroga Act extended coverage to 2090. As a long-term initiative, periodic evaluation of the WTCHP is necessary to ensure a most effective program. The first period of research awarded under the WTCHP has recently come to a close; therefore, an evaluation of the effectiveness of the RTC model that has guided WTCHP research to care is timely. This evaluation is vital to program improvement and foundational in setting research priorities for continuing highly effective treatment as the program progresses. The ability of the WTCHP to serve its members and realize positive impacts on all of its stakeholders will depend heavily on effective translation of research activities and findings in a manner that is appropriate to diverse stakeholder groups.1 The focus groups proposed for this work will provide information on stakeholder views about three important translational science principles: the relevance, transparency, and usability of WTCHP research. The focus groups will complement data gathered during future in-depth interviews that will have a different emphasis than these focus groups. The future interviews will emphasize the themes of impact, long-term outcomes, and recommendations for future research investments and strategic direction.
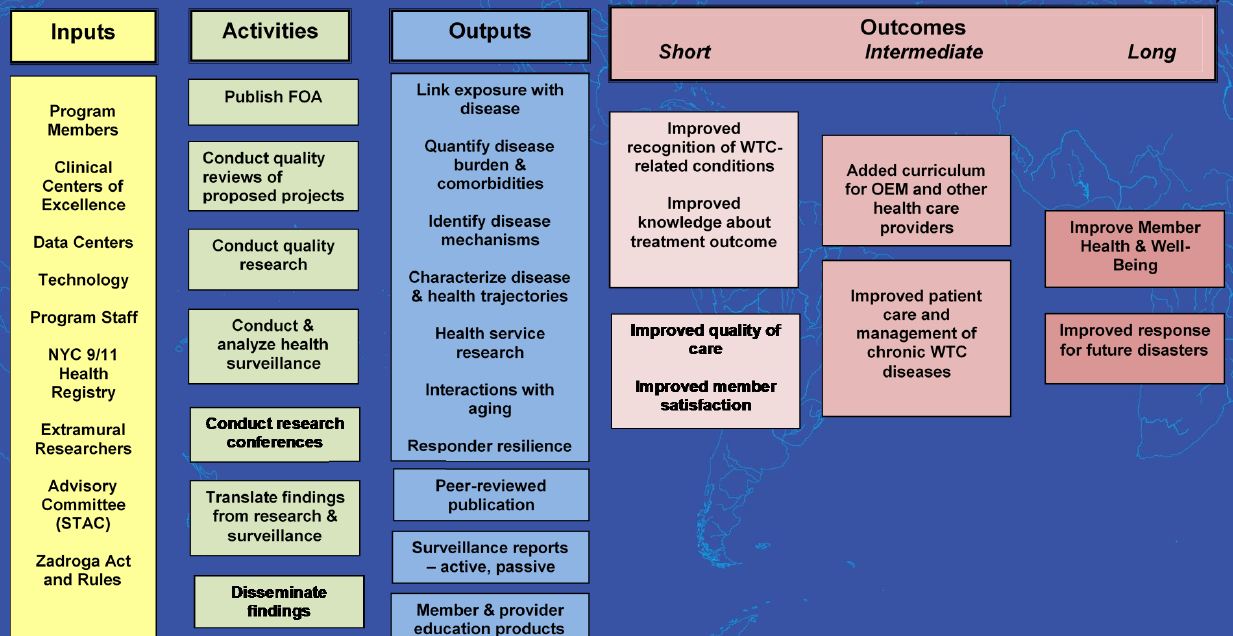
The present data collection is authorized by Section 51(a) of the Zadroga Act (42 USC Chapter 6A, Subchapter XXXI) (Attachment A).
2. Purpose and Use of Information Collection
The purpose of the focus groups is to: (1) gather data to help inform an evaluation of the WTCHP and development of strategic planning recommendations; (2) complement data gathered during structured interviews with stakeholders that will be fielded with the same stakeholder categories; and (3) inform selection of future interview participants based on an assessment of participant feedback across demographic characteristics.
The focus groups will lay the groundwork for future more targeted and detailed data collection activities that will ultimately all be used to inform strategic planning recommendations to NIOSH that will be delivered at the end of the total project. In addition, this first step of research and engagement with the WTCHP stakeholders will provide us with insights into how best to communicate with and engage stakeholders at every stage of the RTC process, which is a critical aspect of future strategic planning recommendations.
One main protocol will be used to guide focus group sessions across all stakeholder categories (see below for discussion of stakeholder categories). The focus group questions have been constructed to be general and applicable to all stakeholder categories, allowing for group comparisons across questions (see Attachment E Focus Group Protocols).
In addition, a brief (2 minute) and anonymous demographic survey will be completed by focus group participants at the conclusion of the session, which will ask for participants’ age, gender, race, ethnicity, role within the WTCHP, and other relevant details about their role (e.g., clinician specialty, WTCHP member type). Collecting this information is important for accurately describing the sample of focus group participants, and furthermore, it will provide context for the qualitative data they provide; will lead to more nuanced analysis of their responses; and will illuminate any gaps or imbalances by demographic characteristic to address when recruiting for Part 2 of the qualitative work, the in-depth interviews.
Specific topics for the focus groups include:
Conceptualizations of research and “translational research”
Relevance of WTCHP research topics, potential gaps, and stakeholder priorities
Uses and usefulness of WTCHP research
Barriers to conduct and use of WTCHP research
Understanding of and perspectives on the relevance and usefulness of the Research-to-Care model
Focus group answers, particularly to the use of WTCHP-supported research and barriers to the use of such research in a translational fashion, will complement the semi-structured interviews’ more pointed probing on ways to overcome the challenges of translating research on the 9/11 attacks into better outcomes for WTCHP members. In addition, areas of alignment and divergence of opinions will be explored in more depth in the interviews that will be part of future research activities. Below we describe the stakeholder groups that will be targeted for focus group participation.
Funders (those affiliated with the WTCHP at NIOSH)
Provides NIOSH’s perspective on translational research efforts.
Researchers
WTC Health Registry staff: provides perspectives on surveillance of members’ health.
Principal investigators of WTCHP-supported research: provides important insights into research priorities and the process of communicating work to research users.
Research Users
Clinicians from the WTC Clinical Centers of Excellence: provide perspectives on quality of care and service delivery considerations from on-the-ground experience.
Leadership from the WTC Clinical Centers of Excellence and Data Center representatives: provides insights on how WTCHP-supported research is viewed by both health system leadership (who make decisions about clinical care) and leaders of the 3 Data Centers (who make decisions around clinical care as well as the flow of clinical data).
WTCHP members (responders and survivors): provides member perspectives on how research impacts personal care.
Others (policy makers, advisors of the program; non-profits; state/federal agencies that interact with the WTCHP): a group of stakeholders whose decision making affects the WTCHP as a whole.
Each focus group will consist of a defined stakeholder category, and will last approximately 2 hours. Focus groups will be conducted either in-person or via telephone/webinar depending on the stakeholder category and logistical/administrative considerations.
3. Use of Improved Information Technology and Burden Reduction
Depending on the timing of OMB approval, we anticipate conducting focus groups shortly after, most likely in the winter/early spring of 2019. If this occurs, results will be analyzed in the spring of 2019. If the timing of OMB approval coincides with one of the twice-yearly NIOSH-sponsored research meetings in NYC, we will plan to hold in-person focus groups with the stakeholder groups in attendance (NIOSH and principal investigators); the remainder of the focus groups will be held by webinar to minimize burden on the participants. For these webinars, RAND will employ electronic technology (e.g., video/webinar- or tele-conferencing) to conduct focus groups and maximize convenience.
4. Efforts to Identify Duplication and Use of Similar Information
This is the first evaluation of the WTCHP commissioned by NIOSH; therefore this work is novel and not duplicative and to NIOSH’s knowledge no information collected under this package is already in the possession of the federal government or other organizations involved with the WTCHP. NIOSH will make all reasonable effort to ensure that the information collection does not overlap with other data collection projects related to the WTCHP.
5. Impact on Small Businesses or Other Small Entities
No small businesses will be involved in this data collection.
6. Consequences of Collecting the Information Less Frequently
The proposed one-time information collection is needed in order to evaluate whether WTCHP research meets translational research principles of being relevant, useful, and transparent for all its stakeholders. Without evaluation, WTCHP research investments now and in the future, may not meet intended goals as laid out in the Research-to-Care model with consequences for the health and well-being of Program members. Burden to individuals participating in the evaluation will be minimized and only necessary questions are included in the collection instruments.
7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
There are no special circumstances associated with this information collection request. This request fully complies with the regulation 5 CFR 1320.5 and will be voluntary.
8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
The 60-day Federal Register notice was published on March 15, 2018 (Volume 83, Number 51, pp. 11535-11536). CDC did not receive public comments related to this notice.
9. Explanation of Any Payment or Gift to Respondents
There will be no payments or gifts to focus group participants.
10. Protection of the Privacy of Information Provided by Respondents
The CDC Privacy Act Officer reviewed this submission and determined that the Privacy Act does not apply. The Privacy Impact Assessment is attached (Attachment I).
The information to be collected during the focus group itself includes participants’ perspectives (in their own words) on the following topics:
Conceptualizations of research and “translational research”
Relevance of WTCHP research topics, potential gaps, and stakeholder priorities
Uses and usefulness of WTCHP research
Barriers to conduct and use of WTCHP research
Understanding of and perspectives on the relevance and usefulness of the Research-to-Care model
The brief demographic survey will ask the following questions:
Year of birth
Sex
Ethnicity
Race
WTCHP role in general terms (funder, researcher, research user)
If a WTCHP member, is the person a general responder, FDNY responder, or survivor
If a clinician, what is the person’s specialty
Number of years the person has been affiliated with the WTCHP
For both in-person and telephone/webinar focus groups, NIOSH and RAND will follow procedures for securing and maintaining privacy during all stages of information collection.
Participants will be advised of the nature of the information collection activity (their participation in a focus group), the length of time it will require (2 hours including a brief demographic survey that will take less than a minute to fill out), and that participation is purely voluntary. Participants will be assured that no penalties will be incurred if they wish not to respond to the information collection as a whole or to any specific questions. These procedures conform to ethical practices for collecting data from human subjects.
The proposed information collection has been reviewed and approved by RAND’s IRB (Attachment G). The NIOSH IRB has determined that the project is not research (Attachment H). A consent form will be provided to prospective participants in hardcopy form before in-person focus groups and electronically for telephone/webinar groups. Through the consent form, participants will receive information on the purpose and rationale of the project, explanation of what their participation will involve and how their data will be protected (Attachment D). Prior to the beginning of the information collection, a staff member will address any questions the participants have about the project.
All data will be stored in secure electronic files maintained by RAND and will be accessible only to staff directly involved in the project. All information will be maintained in a password protected secure location.
The proposed information collection will not involve collecting or sharing respondents’ personal identification or place of residence. No personally identifiable information will be collected (year of birth will be collected but not date); other information on the brief demographic survey is not PII. No IIF will be retained.
The proposed collection will not impact the respondents’ privacy. All collected information will remain secure. Collected information includes: focus group transcripts which will be entered into appropriate data management systems for qualitative analysis (the researchers will use Dedoose software), with all personal identifying information deleted following information verification and cleaning: and 2) responses to the anonymous brief demographic survey, which will be entered into an Excel spreadsheet that will be password protected; researchers will have no ability to link individuals who participated in focus groups. Final de-identified electronic data (transcripts and summary reports) will be maintained by NIOSH. Analyses will not include any IIF regarding participants.
All focus group participants will give consent using Attachment D Informed Consent form. Recruitment will also be done using the language in the consent form. RAND will collect and analyze the project specific data. Other than sending initial recruitment emails, NIOSH will not be in contact with project participants (and will only have access to de-identified response data). All information provided by participants will be treated in a secure manner and will not be disclosed unless otherwise compelled by law. Participants will be informed prior to participation that their responses will be treated in a secure manner.
11. Institutional Review Board (IRB) and Justification for Sensitive Questions
Both the RAND IRB and the CDC IRB have determined that IRB approval is not required. (Attachment G, RAND IRB documentation and Attachment H, NIOSH IRB documentation).
The 9/11 attacks were a traumatic event that were the impetus for the formation of the WTCHP and are the context for the current project. However, our focus group questions focus not on the event itself but rather on stakeholder relations with and perspectives on the WTCHP. The focus group questions are not designed to be sensitive in nature, and most of the stakeholders have had a longstanding engagement with the WTCHP so are likely accustomed to thinking about and engaging with their memories of the 9/11 attacks. We acknowledge that for many stakeholders, the WTCHP may be their employer or the funder of their research. To minimize psychological distress, the moderator and information collection instructions will inform participants that they do not have to respond to any questions they do not want to answer and that they may stop participating at any time.
12. Estimates of Annualized Burden Hours and Costs
We outline the estimated burden hours and respondent costs for the proposed project in Table 12-A. The recruitment strategy for the project is based on three broad categories: WTCHP Funders, WTCHP Researchers, and WTCHP Research Users. Across the three types of focus group respondents we estimate a minimum of 96 and maximum of 120 participants. Since 10 participants are Federal employees acting within the scope of their job responsibilities (WTCHP Funders), these individuals are excluded from the burden estimate, and the burden table is based on the remaining 110 participants.
Within the categories of Researcher and Research User, focus group recruitment and participation will be organized by functional role (e.g., WTCHP researcher / Center of Excellence leadership or data manager / clinician / member, etc.). These roles include a mix of employees (private sector or state/local government) and private citizens. For purposes of burden estimation we have further reclassified the functional roles into groups that correspond to categories of Affected Public. We assume that the preponderance of Researchers, leadership from WTC Clinical Centers of Excellence, and Other Stakeholders are based in academic institutions or other units of state and local government. We assume that WTCHP clinicians are predominantly from the private sector, and we assume that WTCHP members (responders and survivors) are either private citizens or current/former employees of state and local government.
Each respondent will participate in a focus group that will last approximately 2 hours (see Attachment E). A Brief Demographic Survey will be completed at the conclusion of each focus group discussion and is included in the 2-hour estimate (see Attachment F).
Burden estimates are based on RAND’s prior experience with similar discussion guides and a pilot test conducted June 20, 2018 with internal RAND researchers and staff. The annual total burden hours are estimated to be 220 hours.
Table 12-A: Estimated Annualized Burden Hours
Type of Respondents |
Form Name |
Number of Respondents |
Number of Responses per Respondent |
Average Burden per Response (in hours) |
Total Burden (in hours) |
Principal Investigators of WTCHP-Funded Research |
Focus Group Discussion Guide and Brief Demographic Survey |
30 |
1 |
2 |
60 |
Leadership from WTC Clinical Centers of Excellence and Other Stakeholders |
Focus Group Discussion Guide and Brief Demographic Survey |
20 |
1 |
2 |
40 |
WTC Health Registry staff |
Focus Group Discussion Guide and Brief Demographic Survey |
10 |
1 |
2 |
20 |
Clinicians Caring for WTCHP Members |
Focus Group Discussion Guide and Brief Demographic Survey |
20 |
1 |
2 |
40 |
WTCHP Responders and Survivors (State/local govt) |
Focus Group Discussion Guide and Brief Demographic Survey |
15 |
1 |
2 |
30 |
WTCHP Responders and Survivors (private citizens) |
Focus Group Discussion Guide and Brief Demographic Survey |
15 |
1 |
2 |
30 |
Total |
|
110 |
|
220 |
|
Table 12-B: Estimated Annualized Burden Cost
The total annualized cost burden is estimated to be a maximum of $7,044, based upon mean hourly wages from the May 2018 Weekly and Hourly Earnings from the New York State Department of Labor.
Type of Respondents |
Form Name |
Number of Respondents |
Average Hourly Wage |
Total Burden (in hours) |
Total Cost |
Principal Investigators of WTCHP-Funded Research |
Focus Group Discussion Guide and Brief Demographic Survey |
30 |
$30.72 |
60 |
$1,843 |
Leadership from WTC Clinical Centers of Excellence and Other Stakeholders; WTC Health Registry staff |
Focus Group Discussion Guide and Brief Demographic Survey |
30 |
$30.72 |
60 |
$1,843 |
Clinicians Caring for WTCHP Members |
Focus Group Discussion Guide and Brief Demographic Survey |
20 |
$37.84 |
40 |
$1,514 |
WTCHP Responders and Survivors (State/local govt) |
Focus Group Discussion Guide and Brief Demographic Survey |
15 |
$30.72 |
30 |
$922 |
WTCHP Responders and Survivors (private citizens) |
Focus Group Discussion Guide and Brief Demographic Survey |
15 |
$30.72 |
30 |
$922 |
Total |
|
$7,044 |
|||
13. Estimates of Other Total Annual Cost Burden to Respondents or Record Keepers
There is no cost or burden to the participants other than their time to participate in the data collection.
14. Annualized Cost to the Government
The total cost to the Federal Government is $32,887.
Staff (FTE) |
Average Hours per Collection |
Average Hourly Rate |
Average Cost |
Data collection costs |
|
NA |
$5,031 |
Social Science Analyst, GS 15 (Analysis and project management costs) |
440 |
63.31 |
$27,856 |
Estimated Total Cost of Information Collection |
$32,887 |
||
15. Explanation for Program Changes or Adjustments
This is a new information collection.
16. Plans for Tabulation and Publication and Project Time Schedule
As noted in A.3., depending on the timing of OMB approval, we anticipate conducting focus groups shortly after, most likely in the winter/early spring of 2019. If this occurs, results will be analyzed in the spring of 2019. If the timing of OMB approval coincides with one of the twice-yearly NIOSH-sponsored research meetings in NYC, we will plan to hold in-person focus groups with the stakeholder groups in attendance (NIOSH and principal investigators); the remainder of the focus groups will still be held by webinar to minimize burden on the participants. We do not plan to publish results of the focus groups as a discrete product, as they are intended to complement Part 2 of the qualitative research. The results will be used to inform later parts of the project and may be integrated into future project reports.
Table 16-A: Project Time Schedule
Activity |
Time Schedule |
Recruitment emails sent to focus group participants |
1 week after OMB approval |
Information/data collection |
1-2 months after OMB approval |
Analyses |
3-4 months after OMB approval |
Standard qualitative techniques will be used for data analysis. Focus group transcripts will be entered into qualitative research software and independently coded for common themes by two researchers. To ensure different coders are interpreting the literature as similarly as possible, we will: (1) develop descriptive codebooks that give clear meanings to the use of different codes; (2) perform intercoder agreement checks prior to analyses where both analysts read the same text, code independently, and discuss areas of disagreement; and (3) perform supervisory reviews at regular time intervals and issue course corrections if necessary. The codebooks will allow for pre-specified themes around the main areas of known interest (e.g., relevance, usability, transparency of WTCHP research), and will also allow for the identification of new themes that arise from the assessment.
17. Reason(s) Display of OMB Expiration Date is Inappropriate
The display of the OMB expiration date is not inappropriate.
18. Exceptions to Certification for Paperwork Reduction Act Submissions
There are no exceptions to the certification.
1 Concannon, Thomas W., et al. "A new taxonomy for stakeholder engagement in patient-centered outcomes research." Journal of General Internal Medicine 27.8 (2012): 985-991.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Julie Brown |
| File Modified | 0000-00-00 |
| File Created | 2021-01-20 |
© 2026 OMB.report | Privacy Policy