NIST, ITL, Measuring the Accuracy of Facial Forensics Comparisons - Informed Consent Form
0693-0043-NIST-MeasuringAccuracy-FacialForesnsics-Informed Consent.docx
NIST Generic Clearance for Usability Data Collections
NIST, ITL, Measuring the Accuracy of Facial Forensics Comparisons - Informed Consent Form
OMB: 0693-0043
CR Informed Consent
Version February 21, 2019
Informed Consent to Participate in Research
Principal Investigator: P. Jonathon Phillips, Ph.D.
Study Title: Measuring the accuracy of facial forensics comparisons
Study Site(s): Gaithersburg and other locations as determined by Principal Investigator
Key Information This is a brief summary of key information to describe the research study you are being invited to participate in. You will find more detailed information explained later in this document. |
Risks or Discomforts; Reasonable, expected benefits This research is considered to be minimal risk, which means that the risks associated with this study are the same as what you face every day. There is also a very small risk that someone who is not authorized could get access to the data we have stored about you. However, we describe how we will protect your privacy and confidentiality in a later section of this consent form. |
Introduction
You are being asked to take part in a research study. Research studies include only people who choose to take part. This document is called an informed consent form. Please read this information carefully and take your time making your decision. Ask the researcher or study staff to discuss this consent form with you, please ask him/her to explain any words or information you do not clearly understand. The nature of the study, risks, inconveniences, discomforts, and other important information about the study are listed below.
The person who is in charge of this research study is P. Jonathon Phillips, Ph.D. This person is called the Principal Investigator (PI). However, other NIST research staff may be involved and can act on behalf of the person in charge.
This research is being sponsored by the Federal Bureau of Investigation (FBI).
Purpose of the study
There are many other aspects of face recognition. To gain a more
complete understanding of the face recognition ability of facial
forensic examiners, we are conducting a study consisting of three
experiments that test different aspects of face recognition. The
three experiments do not necessarily test the professional skills
that facial examiners use when making comparisons in their
laboratory.
Why are you being asked to take part?
We are asking you to take part in this research study because you might fit into one of the four categories: a forensic facial examiner, a forensic facial reviewer, a face super-recognizer, or a fingerprint examiner without experience or training in facial forensic examination. You must also have your employer’s permission if you will participate in study activities during your normal working hours.
Study Procedures:
If you take part in this study, you will be asked to:
Schedule an interview with a NIST researcher. At the start of the interview, we will give you the consent form which includes a description of the experiments.
Participate in a private interview in which we will:
Review the goals of the project.
Explain the consent form.
Answer any questions you may have.
Ask you if you want to sign the consent form. You will choose to sign or not to sign the form. The NIST researcher who explained the consent form to you signs the form and sends the form to you by email.
Ask you a series of screening questions to determine if you fit into one of the categories of people who are eligible to participate in this study. If you do not fit into one the categories, your participation in the study will end at this step and your data will be destroyed.
Participate in three experiments. Each experiment will be taken on a NIST computer provided by the NIST researcher.
One experiment measures your ability to remember faces.
This experiment contains a practice section where you will learn how the experiment is run.
In each trial, you will be asked to memorize up to 6 faces at a time. The faces will be shown for up to 20 seconds.
Then a set of faces will be presented. You will be asked to identify a face that you just memorized.
The other two experiments will ask you to compare two faces that are shown on your screen.
Each experiment consists of at most 156 pairs of faces.
On the screen, two faces will be presented side-by-side for up to 30 seconds.
Rate the similarity between each pair on the following scale:
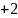 :
Sure they are the same.
:
Sure they are the same.
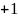 :
Think they are the same.
:
Think they are the same.
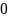 :
Do not know.
:
Do not know.
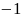 :
Think they are not the same.
:
Think they are not the same.
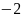 :
Sure they are not the same.
:
Sure they are not the same.A pair of faces will be displayed until you enter a rating or for 30 seconds. If you take more than 30 seconds to make a decision, the faces will disappear from the screen and you will need to enter a rating. The next pair of images will not be shown until you enter a rating.
You may withdraw from this study until you click the final submit button on the third experiment you take.
Please do not consult or discuss this study with friends, family, or colleagues from your institution or other institutions.
We will keep this data and your previous data to perform detailed analysis and for re-analysis and meta-analysis at a later date.
Total Number of Participants
The study is being administered by NIST and up to 300 individuals will take part in this study.
Voluntary Participation / Withdrawal
You should only take part in this study if you want to volunteer. You should not feel that there is any pressure to take part in the study. You are free to participate in this research or withdraw at any time. There will be no penalty if you stop taking part in this study. A decision not to participate will not affect your job status.
You may withdraw from the follow-up study until you click the final submit button on the third experiment. If you wish to withdraw from the study, you will need to tell the NIST researcher. If you choose to withdraw, all of your data from this portion, including any completed experiments, will be destroyed.
Benefits
The potential benefits of participating in this research study include contributing to the body of knowledge of psychology and forensics sciences in understanding how facial forensic examiners process faces. In addition, the knowledge gained from this experiment could contribute to develop more accurate procedures for performing facial forensic identification.
NIST does not own all these additional experiments, and these experiments are still used in the field. Therefore, we will not be releasing the answers for these experiments.
Risks or Discomfort
This research is considered minimal risk. That means that the risks associated with this study are the same as what you may encounter every day (e.g., working on an office computer, or taking an online survey). There are no known additional risks to those who take part in this study.
Compensation
You will receive no payment or other compensation for taking part in this study.
Costs
It will not cost you anything to take part in the study.
Privacy and Confidentiality
We will keep your study records private and confidential. Certain people may need to see your study records. Anyone who looks at your records must keep them confidential. These individuals include:
The NIST research team, including the Principal Investigator, study coordinator, and all other NIST research staff.
Certain government people who need to know more about the study, and individuals who provide oversight to ensure that we are doing the study in the right way.
Any agency of the federal, state, or local government that regulates this research such as the FBI and Office for Human Research Protections (OHRP) within the Department of Health and Human Services (DHHS).
You will be assigned a study number in order to link your performance across the three experiments. Concurrently with publishing the results of the study the key linking study numbers to subjects’ identities (name) will be destroyed.
A master list linking your personally identifiable information to the study number will be kept in an encrypted disk on a government computer located in a locked office at NIST or on a government encrypted disk in a locked cabinet at NIST. The master list will be kept since there is the potential for this data to be useful many years beyond the point of study completion. The file that includes your study code and other study information is called the de-identified data set. The de-identified data set will be made available to other researchers including Prof. Alice J. O’Toole of the University of Texas at Dallas, Dr. David White of the University of New South Wales, Australia, and Dr. Eilidh Noyes of the University of Huddersfield, UK.
Your identity will be protected to the extent permitted by law, including the Freedom of Information Act. We may publish what we learn from this study. If we do, we will not include your name. We will not knowingly publish anything that would let people know who you are. Total confidentiality cannot be guaranteed, since all security measures have vulnerabilities and may be compromised.
Future use of research data and/or specimens
We will keep all data, including identifiable data, for future research. Future research will be reviewed by an IRB that has the responsibility to make sure research studies are conducted in an ethical manner that protects the rights and welfare of subjects. For future research, only the de-identified data set will be shared outside NIST; no one outside the study team will receive the master list.
Relevant research results
We will not provide participants with their individual scores or results.
You can get the answers to your questions, concerns, or complaints
If you have any questions, concerns or complaints about this study, or experience an unanticipated problem, or research-related injury, call P. Jonathon Phillips, Ph.D., at 301-975-5348.
If you have questions about your rights as a participant in this study, or have complaints, concerns or issues you want to discuss with someone outside the research team, call the Human Subjects Protection Office at (301) 975-5445.
You will receive a copy of this signed consent form.
Consent to Take Part in this Research Study
I freely give my consent to take part in this study. I understand that by signing this form I am agreeing to take part in research. I have received a copy of this form to take with me.
_____________________________________________ ____________
Signature of Person Taking Part in Study Date
_____________________________________________
Printed Name of Person Taking Part in Study
Statement of Person Obtaining Informed Consent
I have carefully explained to the person taking part in the study what he or she can expect from their participation. I confirm that this research subject speaks the language that was used to explain this research and is receiving an informed consent form in their primary language. This research subject has provided legally effective informed consent.
______________________________________________ ____________
Signature of Person obtaining Informed Consent Date
______________________________________________
Printed Name of Person Obtaining Informed Consent
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Subject | USF IRB Form, informed consent, template, adult, social, behavioral |
| Author | California Family Health Council, John Arnaldi, Norma Epley |
| File Modified | 0000-00-00 |
| File Created | 2021-01-15 |
© 2026 OMB.report | Privacy Policy