Environmental Sampling Protocol
Att5_PFAS Environ Sampling EI Protocol _ FINAL .docx
ATSDR Exposure Investigations (EIs)
Environmental Sampling Protocol
OMB: 0923-0048
Protocol: Supplemental Exposure Investigation (EI) at Select PFAS Exposure Assessment Sites
Funded
and Sponsored by
Agency for Toxic Substances and Disease Registry (ATSDR)
Environmental Protection Agency (EPA)
Contents
Hampden County, MA…………………………………………………………………………………………………………………….8
New Castle County, DE…………………………………………………………………………………………………………………..9
Environmental Sampling Eligibility and Recruitment 9
Sample Collection Procedures 10
Informed Consent/Parental Permission/Assent Forms……………………………………………………………………10
Questionnaire………………………………………………………………………………………………………………………………..11
Sampling Strategy………………………………………………………………………………………………………………………….12
Table 2. Target quantitative Data Quality Indicatorsa 13
Limitations of the Exposure Investigation 16
Intended/Potential Use of Exposure Investigation Findings 16
Anticipated Risks and Benefits and Estimated Time Burden on Participants 17
Environmental Data Handling and Evaluation 18
Tables and Figures:
Figure 1: PFAS Conceptual Exposure Model
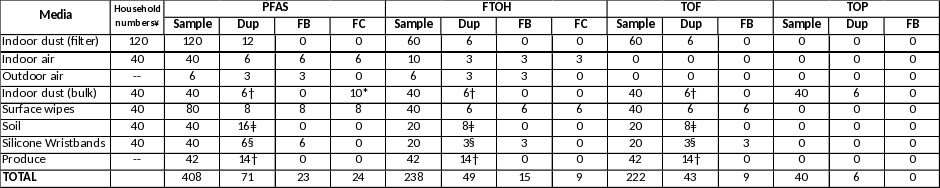
Table 1: Media and Sample Number: Environmental Exposure Investigation at EA Locations (Combined)
Table 2: Target Quantitative Data Quality Indicators
Table 3: Investigation Objective Statistical Methodology
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
Under Section 8006 of the Consolidated Appropriations Act, 2018, CDC/ATSDR was required to conduct statistically based biomonitoring exposure assessments (EAs) at “no less than eight current or former domestic military installations” that have or have had documented exposures to per- and polyfluoroalkyl substances (PFAS) in drinking water.
The intention of the EAs was to determine how exposure to PFAS in drinking water in communities near the military installations may have impacted levels of PFAS in serum and urine. In addition to serum and urine testing, tap water and indoor dust were also sampled in a subset of homes.
Exposure to PFAS can result from exposure to both drinking water and non-drinking water sources. Although there is information indicating additional sources of exposure to PFAS, information on the contribution of these sources to PFAS body burden levels is sparse. CDC/ATSDR and EPA are conducting this environmental sampling exposure investigation (EI) to identify potential non-drinking water contributors to PFAS body burdens.
This protocol describes an environmental sampling Exposure Investigation (EI) that will include sampling environmental media at the homes of a sub-set of participants from two EA communities. CDC/ATSDR will evaluate potential exposure inside the home by collecting samples that include indoor air, indoor dust (filtered through a vacuum and obtained as a bulk sample), and wet wipes for evaluating potential PFAS-containing product use. CDC/ATSDR will also evaluate potential exposure to PFAS via outdoor sources by sampling soil at residences and outdoor air within the community. In addition, a silicone wristband worn by some participants will be analyzed for PFAS to characterize PFAS exposure during daily activities. Samples of locally grown produce will be analyzed to evaluate dietary exposure to PFAS.
A questionnaire will be administered to all EA participants present in the home during the sampling to gather information about potential drinking water and non-drinking water PFAS exposure, such as use of household consumer products and the intake of food products that may contain PFAS. The results of the environmental sampling EI questionnaire will be evaluated in conjunction with the questionnaire administered during the EA.
A consent form will be required to conduct the environmental sampling and a consent/parental permission/assent form will be required to administer the EI questionnaire.
EAs were completed at eight locations throughout the United States. Two locations were chosen for inclusion in this investigation: Hampton County, MA and New Castle County, DE.
The goals of this environmental sampling EI are to evaluate:
Whether PFAS are detectable in various non-drinking water environmental media;
What the detectable levels of PFAS in environmental media are; and
Whether these detectable levels of PFAS may be associated with the existing measured body burden levels of PFAS identified during the EA.
CDC/ATSDR’s Office of Community Health and Hazard Assessment (OCHAA) conducts Exposure Investigations (EIs) when data gaps are identified at a site. CDC/ATSDR fills the data gaps by conducting biological and/or environmental sampling, with the results of the sampling allowing CDC/ATSDR to make better public health decisions.
In 2018, CDC/ATSDR was required to conduct no less than eight Exposure Assessments (EAs) in communities near military installations to evaluate the potential impacts on per-and polyfluoroalkyl substances (PFAS) body burden resulting from drinking water exposure [drinking water sources in the area exceeded EPA’s health advisory of 70 ppt (combined PFOA and PFOS) in the past]. The PFAS EAs are essentially EIs since they were intended to fill a data gap (e.g., PFAS in blood and urine resulting from drinking water exposure) and are considered to be non-research investigations. Given that random sampling was conducted at most of the EA locations (some sampling areas were small and everyone in the sampling area was welcome to participate), the results can be generalized to the sampling area. Sampling of tap water and indoor dust was also conducted at a subset of EA households.
The PFAS EA biological data were compared to nationally representative data, specifically, to data collected by CDC as part of its National Health and Nutrition Examination Survey (NHANES). This survey collects blood and urine samples and tests them for chemicals, including PFAS, from a representative sample of the civilian non-institutionalized U.S. population. PFAS levels in blood reported by NHANES indicate that PFAS are found in the blood of virtually all Americans (CDC 2021: Per- and Polyfluorinated Substances (PFAS) Factsheet | National Biomonitoring Program | CDC]. Serum PFAS levels at all EA locations, including MA and DE, were higher than NHANES levels for several PFAS, primarily PFHxS, PFOS and/or PFOA [ATSDR 2021: PFAS Exposure Assessment Site Locations | ATSDR (cdc.gov)].
PFAS have been used in many consumer products, including stain-, water- and grease-resistant products and materials treated with these products are used in everyday life. Figure 1 provides a Conceptual Exposure Model indicating how PFAS may be present in the environment as a result of anthropogenic sources (e.g., PFAS manufacturing, use of firefighting foam, incinerators), as well as the general use of PFAS-containing consumer products.
Figure
1: PFAS Conceptual Exposure Model
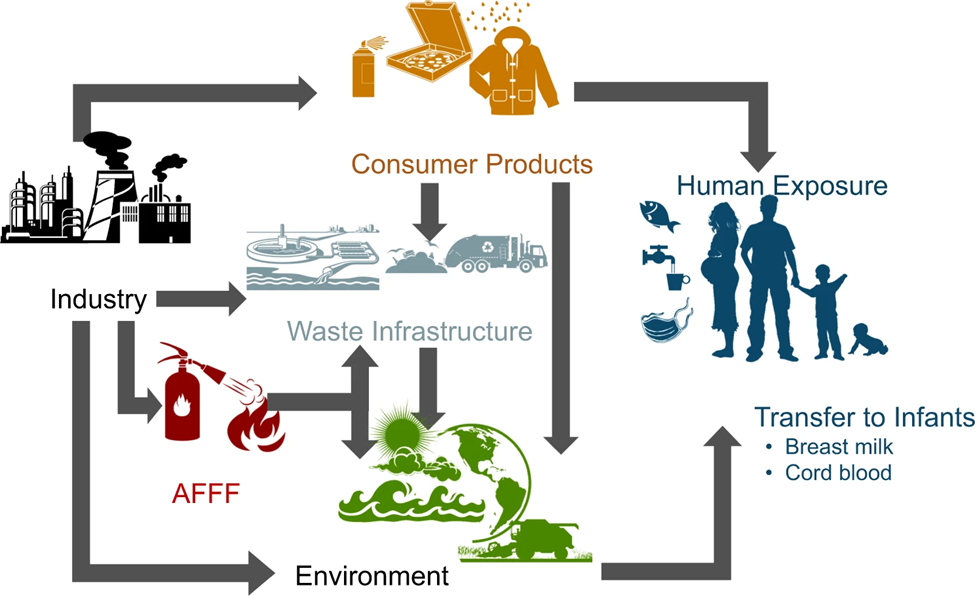
Note: AFFF = Aqueous Film Forming Foam
The goals of this environmental sampling EI are to evaluate:
Whether PFAS are detectable in various non-drinking water environmental media;
What the detectable levels of PFAS are in environmental media; and
Whether these detectable levels of PFAS may be associated with the existing measured body burdens of PFAS.
The serum results from the EA and the environmental sampling EI results will be interpreted using the results of the questionnaires from the EA as well as the EI to better understand water and non-drinking water exposures at two of the EA sites. Urine collected during the EA were only analyzed for a small number of participants (10%) and only one PFAS (PFBA) was detected at both MA and DE. The mean level of PFBA in urine at both locations was below the 95th percentile of NHANES for PFBA in urine. The data set for urine, therefore, is not considered to be robust enough for analysis in this environmental sampling EI.
The results of this environmental sampling EI may help individual participants better understand the magnitude of their non-drinking water environmental exposures to PFAS.
Procedures and Methods
Indoor and Outdoor PFAS sample collection
Given the widespread use of PFAS and their persistence in the environment, it is anticipated that PFAS associated with the use of household consumer products may be present both inside and outside the home and be available for exposure to residents. These potential exposures may be evaluated by sampling dust, indoor air and surface wipes. PFAS may be present in outdoor media, including soil and outdoor air, as a result of industrial and consumer use of products that contain PFAS and the use of PFAS-contaminated water outside the home (e.g, use of outside hose to water lawn). In addition, silicone wristbands worn by residents for a 7-day period of time may assess potential exposure of residents as they navigate their day, both inside and outside their home. Potential exposure in the diet may, in part, be evaluated by sampling locally grown produce. Potential exposure to PFAS by EA participants will also be evaluated using a questionnaire that will supplement the questionnaire completed during the EAs.
The primary criteria used to identify EA locations was the presence of PFAS in drinking water at levels above the EPA health advisory (i.e., 70 ppt for combined PFOA and PFOS) in the past. Water systems impacted by PFAS were remediated to levels below the health advisory in 2016 in MA and between 2014 and 2016 (dependent on the water system) in DE. The focus of the EAs was to evaluate the body burden of PFAS (serum and urine) in people known to be exposed to PFAS in their drinking water. This environmental sampling EI is focused on evaluating non-drinking water sources of PFAS exposure at two of the EA locations (see location selection discussion below) that were chosen using the following criteria:
The number of participants at the location,
The engagement of the participants within the community, to assist with reaching our recruitment goals,
The level of PFAS found in the drinking water, and
The presence of potential non-drinking water PFAS sources within the community, such as industrial sources in the area.
The Hampden County, MA (MA) location was chosen because it has a large number of households that were included in the EA (247 households); it has an engaged community that has high interest in PFAS; and the levels of PFAS found in drinking water are moderate, indicating that non-drinking water sources may be more significant contributors to PFAS body burden than in communities with high levels of PFAS in their drinking water. In the Hampden County area, significant industry associated with PFAS use or manufacturing has not been identified using Global Information System (GIS) tools provided by EPA and CDC/ATSDR.
The New Castle County, DE (DE) location (134 households) was chosen because the levels of PFAS in serum were elevated and were associated with elevated levels of PFAS in the drinking water. In addition, there are potential industrial sources of PFAS within the community that may be a source of PFAS exposure. In the New Castle County area, an increased number of industrial sources have been identified using Global Information System (GIS) tools provided by EPA and CDC/ATSDR, as compared to the Hampden County location. Therefore, it is anticipated that outdoor sources of PFAS, including outdoor air and soil, may be of greater concern for potential PFAS exposure than in the MA site.
Environmental Sampling Eligibility and Recruitment
Two EA locations, New Castle County, DE and Hampden County, MA, were chosen for the additional environmental sampling. An “invitation to participate” letter will be sent to the households of all EA participants that agreed in the EA consent form to be contacted for future PFAS work at both locations: 247 households in MA and 134 households in DE. The letter and other recruitment materials are provided in Appendix A. CDC/ATSDR will request that EA participants contact us to make an appointment once the invitation letter is received.
CDC/ATSDR estimates a response rate of approximately 30% for this EI. For the EAs in MA, the response rate was approximately 8% (3000 household requests and 247 homes enrolled) and in DE, the response rate was approximately 4.5% (3000 requests and 134 homes enrolled). There were, however, many addresses that were not valid (e.g, invalid address, empty homes, commercial properties) and homes that were interested in participating but were not eligible due to time of residency (participants had to be living in the sampling frame for at least a year prior to water mitigation). Given that the households we did enroll reflect an interested portion of the population, we are estimating a response rate of approximately 30% for this investigation.
CDC/ATSDR will collect a filtered dust sample and administer questionnaires at a maximum of 80 households in MA and 40 households in DE, which represent approximately 30% of EA households in each community. If more than the proposed maximum of households are interested in being included in the EI, the participants will be chosen based on a first-come-first-serve basis; this is indicated in the invitation letter. If fewer than the proposed maximum number of households respond to the invitation letter, EA participants that have not responded will be randomly selected and contacted by phone to invite them to participate.
A subset of 20 households at each location will be chosen to participate in a more robust sampling effort (bulk dust, indoor air, wet wipes, soil, silicone wristband) in addition to the filtered dust sample and questionnaire. The 20 households to be scheduled for the additional sampling will be chosen on a first-come first-serve basis during the recruitment period. When a participant calls to be included in the sampling, they will be offered the additional sampling, and the first 20 households to agree will be asked questions to ensure that all additional media can be collected (Appendix A).
If the participant answers “no” to any of the questions regarding the more robust sample collection, they are still eligible to complete the one-appointment dust sampling. CDC/ATSDR will continue to offer the more robust sample collection to participants who call until 20 households are identified at each location. After the 20 households are chosen, the rest of the participants will only be offered dust sampling in their homes. The number and type of each environmental sample to be collected in the participants’ households is described below.
All participating households will receive a follow-up letter confirming their participation and sampling appointments, and a reminder phone call prior to their appointments (Appendix A). Appropriate consent forms (Appendix B) and questionnaires (Appendix C) will be provided with the confirmation letter to allow participants to complete the forms prior to the sampling appointment. This will provide time for all EA participants in the home to complete the appropriate forms so they do not need to be present during the sampling.
CDC/ATSDR will provide all participants with a $20 gift card per household per appointment as a token of appreciation for their participation; a total of $40 will be provided to the subset of 20 households where two appointments are required to complete the sampling. The additional amount for the two-appointment households is also intended to cover any additional charge the participant may incur resulting from the use of their electricity during the indoor air sampling. Participants will be provided the gift card(s) at each appointment.
Informed Consent/Parental Permission/Assent Forms
The consent/parental permission/assent forms completed during the EA included a provision that allows CDC/ATSDR to use the results of the EA sampling, both biological and environmental, along with the results of the EA questionnaire for future PFAS analysis. This information will be used along with the results of the environmental sampling EI questionnaire to evaluate potential non-drinking water exposure to PFAS. The consent/parental permission/assent forms signed as part of this environmental sampling EI will ask participants to again consent to the use of the EA results (Appendix B).
A Privacy Act Statement and appropriate consent/parental permission/assent forms are provided in Appendix B. When the sampling appointment is made, the number of EA participants that provided a blood sample during the EA, and who live in the home, will be identified. The appropriate number of consent/parental permission/assent forms and questionnaires for the household will be mailed with the appointment reminder letters. All EA participants in the household will be asked to complete the consent forms and questionnaires prior to the first sampling appointment. When the EI team arrives for the sampling appointment, the consent forms and questionnaires will be verified and completed at the home if they are incomplete.
One adult resident in the EA household will sign a consent form to conduct the environmental sampling (either just dust sampling or the full suite of indoor and outdoor sampling) and for completion of both a household and personal exposure questionnaire (Appendix B2).
Additional adults in the household will only need to consent to complete the personal exposure questionnaire (Appendix B3) since the other adult in the household consented to the sampling and completion of the household questionnaire. For children younger than 18 years old, a parental permission form and assent form (for children between 12 and 17 years old) will be completed to allow them to complete the personal exposure questionnaire (Appendix B4 and B5).
Consent forms include the purpose of the assessment; procedures for sample collection; benefits and risks of participation; and contact information should participants have additional questions. All forms are written at an appropriate reading level. All signed consent forms will be secured by the CDC/ATSDR lead in the field and securely archived at CDC/ATSDR.
Questionnaires and the appropriate consent forms will be mailed to participants’ households with the appointment confirmation letter (Appendix C). This will provide time for all household participants to complete the questionnaire so they will not be required to be present in the home during the sampling. The questionnaires will be provided as follows:
One adult EA participant of the home will be administered two questionnaires:
Household questionnaire (Appendix C1): includes questions regarding water use inside and outside the home and the use of water filters. It also includes questions about characteristics of the home, including square footage, the nature of flooring materials, the use of products in the home that may contain PFAS (stain-resistant, water-resistant and grease-resistant products), and indoor cleaning and outdoor watering practices. The household questionnaire applies to all people in the household and is only required to be completed by one person.
Personal questionnaire (Appendix C2): includes questions regarding personal exposure, such as personal water use, the time spent outdoors and dietary intake of locally grown produce and convenience food.
Completion of the questionnaires will be verified when the EI team comes to the home to complete the environmental sampling. The hard copy questionnaires will be collected by EI team members and kept secure. The questionnaire results will be recorded using the Epi Info suite of software tool when the sampling is completed. If clarification of any questionnaire answer is needed after the sampling has been completed, ATSDR may contact the participant by phone for clarification.
Table 1 provides the number of total samples, including Quality Control (QC) samples, that will be taken during the Exposure Investigation to evaluate non-drinking water exposure. The table includes the total number of samples to be taken at the 80 homes in MA and the 40 homes in DE. Appendix D, the Sampling and Analysis Plan (SAP), provides detailed sample collection procedures and analysis methods to be used for each sampled medium.
To evaluate potential indoor sources of PFAS, a maximum of 120 households (80 in MA and 40 in DE) will be sampled for indoor dust using a vacuum and filter setup that was used to collect indoor dust during the EAs. From the 120 homes, a maximum of 40 homes (20 homes at each location) will be chosen for additional analysis to better evaluate both indoor (indoor air, bulk dust, surface wipes) and outdoor (soil) sources of PFAS. The bulk dust sample will be taken from the participant’s vacuum cleaner (bagged or bagless) during the first visit to the home. In addition, one adult in each of the 20 households will be provided two to three silicone wristbands to evaluate personal exposure to PFAS while the participant is within or away from home. The wristbands will be provided to the person that signed the environmental sampling consent form.
To evaluate potential community exposure to PFAS, a maximum of three samples of outdoor air at each location will be taken at a central location within the community that is not associated with an EA household. Up to two simultaneous, 7-day samples will be taken using the same methodology used to evaluate indoor air, to allow for comparison. In addition, another 7-day sample may be taken at the outdoor sampling location using a higher capacity pump to allow for lower detection limits.
Finally, 21 samples of locally grown produce at each location, obtained from local markets and grocery store, will be sampled to evaluate the presence of PFAS in up to 7 types of produce; the team will attempt to gather 3 samples of each type of produce.
In addition to a standard set of PFAS, the analysis will include evaluation of three additional sets of PFAS analytes (FTOH, TOF and TOP – see below) that will assist in evaluating fluorotelomer alcohols and PFAS precursors or breakdown products to provide a more complete evaluation of PFAS exposure. These PFAS analyses are considered to be exploratory, and the goal is to evaluate the presence or absence of a range of PFAS and their concentrations in several exposure media. The analyses for which sample numbers are provided in Table 1 include:
PFAS – provides a list of PFAS that the laboratory is able to evaluate; the list will vary depending on the medium to be sampled
Fluorotelomer Alcohols (FTOH) - provides an evaluation of a set of PFAS, which may also be precursors for other PFAS
Total Organic Fluorine (TOF) - provides the total organic fluorine content of the sample
Total Oxidizable Precursor (TOP) - provides an evaluation of PFAS precursors (completed for bulk dust samples only)
Quality Control (QC) samples to be taken include duplicates, triplicates, or split samples, where appropriate, and field blanks and control samples. The field control samples are samples that are provided by the laboratory and contain a known amount of PFAS to evaluate analyte recovery.
Sample collection teams will likely include personnel from both CDC/ATSDR and EPA. Each team will include at least one CDC/ATSDR staff member who will be responsible for the privacy and security of the consent forms and the questionnaires.
Table 1: Media and Sample Number: Environmental Exposure Investigation at EA Locations (Combined)
¥ A total of 80 homes will be sampled in MA and 40 in DE. 20 homes at each location will be sampled for filtered dust and all other media
Dup = Duplicate; FB = Field Blank; FC = Field Control
*Lab will purchase 10g of NIST SRM 2585 to prepare 10 field controls for PFAS in the bulk dust sample
** two wipe samples will be taken at each of the 20 homes at each location
† split sample and not a duplicate for bulk dust and produce samples
ǂ triplicate analysis for incremental sampling protocol
§ if only a portion of the wristband is used for the analyses, the laboratory will extract a second portion of the wristband as a duplicate sample
Data Quality Indicator goals for precision, accuracy and completeness are provided in Table 2.
Table 2. Target Quantitative Data Quality Indicatorsa
Metric |
Precision |
Accuracy |
% Completeness |
|
Collection |
Analysis |
|||
PFAS (per- and polyfluoroalkyl substances) |
± 25% |
70 – 130% |
90 |
95 |
FTOH (fluorotelomer alcohols) |
± 25% |
70 – 130% |
90 |
95 |
TOF (total organic fluorine) |
± 25% |
N/Ab |
90 |
95 |
TOPc (total oxidizable preccursors) |
± 25% |
N/Ab |
90 |
|
aCollection completeness is based on the number of samples attempted for collection and analysis. Since the investigation design is not intended to be representative or generalizable, completeness is not based on the overall design goals for numbers of participants.
bNot applicable; no standards available for spike preparation.
cTotal oxidizable precursor analyses scheduled only for bulk dust samples.
The following sections provide a brief overview of the sampling and analysis process for each medium; further details are in the Sampling and Analysis Plan (SAP) in Appendix D. The Health and Safety Plan for completion of the environmental sampling EI is included in Appendix E.
In addition to exposure to PFAS in the drinking water, indoor exposure to PFAS may include exposure to indoor air, dust, and surface residue.
Measurements of indoor air may provide an indication of airborne PFAS that may be attached to particulates (dust) as well as some species that may be more volatile and be present in the vapor phase. Measurements of household dust may act (to some extent) as a proxy for the presence of the analytes in products used in the household and as a descriptor of direct exposures (dust ingestion). The evaluation of all these media is exploratory with a primary goal of assessing presence/absence and concentrations in indoor media and may also yield information about how levels of PFAS in indoor media are correlated with PFAS serum concentrations.
Indoor dust and residue
Indoor dust and residue resulting from the use of PFAS-containing products within the home will be evaluated using three different collection methods:
Filtered composite sample using the same method used for the PFAS EAs, with a sample being collected at all participants’ homes (80 in MA and 40 in DE);
Bulk sample from a vacuum cleaner (bagged or bagless) to provide a larger sample to allow for a more robust evaluation (20 households in both locations); and
Wipe samples within the home to evaluate dust as well as residues that may result from the use of PFAS-related products. Two wet wipe samples will be collected at each home (20 households in each location) on surfaces that may be associated with residue or dust build-up. One sample will be taken on the kitchen counter (to assess PFAS from food preparation and the use of cleaning and sealant products) and one will be taken in a closet/mud room area (to assess the presence of PFAS from clothing).
All samples will be provided to the laboratory and analyzed in accordance to procedures and methods provided in Appendix D.
Indoor air
Twenty homes at each location will be evaluated for PFAS in indoor air. An integrated indoor air sample will be collected from the main living space of the home over a course of 7 days. CDC/ATSDR personnel or their contractors will set up the indoor air collection apparatus at an initial appointment and will collect it at a second appointment 7 days later.
The air samples will be collected on filters attached to a pump that operates at approximately 4-5 liters/minute to allow for maximum collection potential with a minimum level of noise for as little disruption to the household as possible. The sample and all appropriate QC samples will be sent to the laboratory for analysis for constituents provided in Appendix D.
CDC/ATSDR will collect surface soil samples at 20 households at each environmental sampling EI location using an incremental sampling methodology (ISM) approach. This sampling method involves collecting and combining many equal mass increments of soil (i.e., increment samples) across a specific area or volume of soil (e.g., an exposure unit) into a single representative sample for laboratory analysis (i.e., bulk ISM sample). The combined sample is sieved and ground to obtain a consistent particle size and then subsampled and processed by the laboratory following specific protocols. Due to the sampling density afforded by collecting many increments, ISM samples can provide more precise and representative estimates of an exposure unit’s average contaminant concentrations than other soil sampling approaches.
All samples will be provided to the laboratory and analyzed in accordance with procedures and methods provided in Appendix D.
Outdoor Exposure - Air
Up to 3 samples of outdoor air will be taken in a centralized location within the EA sampling frame in both MA and DE. The same sampling apparatus used to collect the indoor air samples will be used to collect up to two 7-day outdoor air samples. Separate air samples will be collected at each time for the standard PFAS and for the FTOH PFAS. The use of the same sampling method will allow for comparison to the results of the indoor samples (4-5 liters/min pump). An additional outdoor sample will also be taken using a higher volume pump (10-20 liters/min) to achieve lower detection limits for PFAS. The sample and all appropriate QC samples will be sent to the laboratory for analysis for constituents provided in Appendix D.
Silicone Wristbands
In 20 households at each sampling location, one adult from each household will be asked to wear silicone wristbands to evaluate personal exposure to PFAS both within the home and outside the home. The person will wear either 2 or 3 wristbands, to allow for FTOH, TOF or QC samples, for a period of 7 days. The participant will remove the wristbands when showering, bathing or swimming. The sample and all appropriate QC samples will be sent to the laboratory for analysis for constituents provided in Appendix D.
Homegrown/local produce
Produce samples will be collected from markets throughout each EA sampling frames. Venues selling locally grown produce will be identified before the sampling campaign begins. Local contacts and various organizations can help to identify local food and farm resources (e.g., https://www.localfoodma.org/). The sampling team will identify “local” produce based on labeling in the markets or knowledge of suppliers to community-based farmers’ markets. To the extent possible, sampling will target produce grown in the community itself. The goal is to obtain 3 samples of 7 different types of produce at each EA location. The type of produce will be chosen based on availability. The sample and all appropriate QC samples will be sent to the laboratory for analysis for constituents provided in Appendix D.
Limitations of the Exposure Investigation
Although the environmental PFAS sampling may assist participants in better understanding their PFAS exposure, the results will not provide discrete information about all sources of exposure, such as PFAS-contaminated food. Additionally, it is not possible to identify every potential confounding exposure (e.g, age, sex, time of residence). CDC/ATSDR will take this limitation into account when drawing conclusions. The results of this investigation may generate new hypotheses about which potential PFAS exposure pathways may exist in these communities.
The results will not be generalizable to either EA participants in the community that were not included in the environmental sampling EI or to non-EA residents within the sampling frame. The EI is intended to allow a better understanding of PFAS exposure within each household tested.
Intended/Potential Use of Exposure Investigation Findings
Household environmental sampling results will be provided to each participant following laboratory analysis and quality assurance procedures. For the EAs, tap water was sampled, and the results were compared to federal and applicable state guidelines for PFAS in drinking water. Federal and state regulations or guidelines are not available for PFAS in any of the media that will be sampled. Therefore, the results of the sampling can only be used to evaluate the presence or absence, and if detected, to identify the amount of PFAS that may be present in each of the sampled media. CDC/ATSDR Minimal Risk Levels (MRLs) are available for four PFAS (i.e., PFOA, PFOS, PFNA, PFHxS) and may be used to screen data in soil. Data screening will allow ATSDR to evaluate whether the levels of the four PFAS in soil may be associated with potential health effects. Screening levels are only available for four PFAS, however, so there is uncertainty associated with potential health effects associated with other PFAS.
The results of the serum testing available from the EAs will be evaluated using the results of the environmental sampling EI and the questionnaire results from the EAs along with this EI. The results and conclusions from the environmental sampling EI will be released as a report for the general public as soon as possible, and findings will be submitted for publication in the peer-reviewed scientific literature.
Anticipated Risks and Benefits and Estimated Time Burden on Participants
Participating in the environmental sampling EI will provide participants with information about levels of PFAS in their home. The results of the environmental sampling and the questionnaire will be used to better interpret serum results from the EA. As with the EA, CDC/ATSDR will not be able to determine whether the levels of PFAS found in media in and outside the home may result in adverse effects.
Participating in this investigation does not have anticipated risk other than the time it will take people to participate. The total time it will take participants to read through and sign the consent form, respond to the questionnaire, and complete the sampling will vary based on the number of samples that will be collected at each household. It is expected that the burden on the homeowner will vary between 1 hour at a single appointment for those homes where only a dust sample is collected, to 4 hours over 2 appointments (2 hours each) for those homes with the more robust sampling collection.
At the time of sampling, all appropriate COVID-19 precautions will be taken as indicated by current CDC guidance https://www.cdc.gov/coronavirus/2019-ncov/index.html. All CDC/ATSDR and EPA personnel that will be conducting the sampling in participant’s homes will be fully vaccinated for COVID-19. . At a minimum, all EI team members will be monitored twice daily for elevated temperature and a symptom check and will wear a surgical mask and wear gloves when sampling inside the home of an EA participant. Additional PPE will be identified in the field, as appropriate, based on current CDC guidance at the time of sampling.
Given that the analysis of PFAS in the collected environmental samples is still exploratory, only the presence or absence of PFAS, and, if detected, the nature of the PFAS in samples will result from the sampling. There are no regulatory or health standards available in any of the sampled media that would allow for a comparison. CDC/ATSDR Minimal Risk Levels (MRLs) are available for four PFAS (i.e., PFOA, PFOS, PFNA, PFHxS) and may be used to screen data in soil. Participants will be informed that their participation in this EI will help advance the understanding of PFAS exposure.
All sample collection and analysis are provided at no cost to participants.
Personal privacy will be protected to the fullest extent possible by applicable federal and state laws and regulations. For the data sets collected at both sites, the HIPAA Safe Harbor de-identification method will be applied to extract specific personally identifiable information (PII) and store them separately from other information. All documents with PII (i.e., consent forms, collection logs, etc.) will be kept in locked cabinets, and all electronic data will be stored on password-protected network servers behind firewalls, accessible only to those staff working directly with raw data. Coded environmental samples will be sent to the laboratories—no PII will be included. Any reports produced from this information will not identify specific individuals.
Records will be retained and disposed of in accordance with the CDC/ATSDR Records Control Schedule. Physical copies of assessment materials and reports will be maintained at CDC/ATSDR until no longer needed by program officials and will be kept in accordance with the corresponding retention schedules. Computer documents will be disposed of when no longer needed by program officials. Personal identifiers will be deleted from records when no longer needed and will be retained no longer than five years. Disposal methods will include erasing computer files, shredding paper materials, or transferring records to the Federal Records Center when no longer needed for evaluation and analysis. Records are retained for 20 years.
In compliance with federal and state privacy protection laws and regulations, and as indicated in the consent form, the limited data set may be shared with other federal, state and/or local public health and environmental agencies via data use agreements for research purposes to advance the scientific understanding of human exposures to PFAS. These agencies must also protect this private information. Each state health department will act in compliance with their respective Sunshine Laws, which may impact the potential for information sharing.
Environmental Data Handling and Evaluation
All environmental samples, data quality assurance and data quality control will be performed by the identified laboratory in accordance with laboratory methods capable of quantifying PFAS in all sampled media since no EPA methods are available in the sampled media (see the SAP in Appendix D).
Questionnaire data will be collected using the Epi Info software tool and will be kept in a secure and encrypted electronic database.
All data will be transmitted via secure connections and methods to CDC/ATSDR for incorporation into a centralized data management repository protected by CDC/ATSDR network firewall and additional security access controls. All results will be electronically transmitted in spreadsheet format using a secured and password-protected network. Deidentified data will be transmitted to the US EPA Office of Research and Development (ORD) through a secured directory to allow for access by EPA.
Descriptive statistics (mean or geometric mean, median, interquartile range, percentiles, minimum, maximum, % detected, etc.) will be prepared for each medium-analyte combination, as appropriate. Consistent with the procedures used to analyze the CDC/ATSDR PFAS Exposure Assessments, categorical responses with <10 values will be collapsed with other categorical responses. To meet the investigation objectives, we will perform several statistical analysis procedures (Table 3).
Table 3: Investigation Objective Statistical Methodology
Objective |
Statistical Methodology |
Identify the presence/concentrations of PFAS in indoor and outdoor residential environmental media and estimate media and route specific intake doses, as appropriate. |
We will generally follow procedures consistent with CDC/ATSDR’s exposure point concentration guidance document (ATSDR 2019) that describes the agency’s approach to handling of censoring and calculating summary values (in this case, median and interquartile range values), and confidence limits on these values. We will modify the procedures in the guidance to calculate two – sided, 95% confidence limits using percentile bootstraps. If censoring is present in the data, we will be following the CDC/ATSDR guidance and Helsel, 2011. If multiple observations are collected per household (e.g. dust microvacuum samples) they will be averaged by household prior to calculating summary statistics.
|
Evaluate association between sources of PFAS, their presence in environmental media, and exposure. |
We have prepared a questionnaire (Appendix C) that relates several sources of PFAS to either environmental concentrations of PFAS in differing environmental media. Categorical questions with dichotomous responses will be evaluated for the effect on PFAS environmental media concentrations using two-sample Wilcoxon; Mann-Whitney (WRS) test. For instance, for question 16 in Appendix C1 (If you have carpet or rugs in your home, have you ever treated that carpet/rug with stain-resistant products?) , we will perform WRS to test the null hypothesis that the PFAS dust levels are the same in these homes. Source questions with multiple categories will be evaluated with Kruskal-Wallis one-way analysis of variance, followed by pairwise comparisons using Dunn’s multiple comparisons with Benjamini and Hochberg as outlined in Helsel, Hirsch, Ryberge, Archfield and Gilroy 2020. The Dunn’s multiple contrast may be performed as a many – to – one comparison or all – pairs comparisons. For instance, question 23 in Appendix C1 (How often do you dust or wipe down surfaces in your home, including windowsills? ), we will test the null hypothesis that PFAS in dust in households with higher cleaning categories are lower than the lowest category (“Never”) using the Kruskal Wallis followed by Dunn’s one – to – many multiple contrast test. For questions such as Appendix C1 question 25, “Have you or do you currently use or have any of the following products in your home?” we will evaluate the null hypothesis of PFAS concentrations in household dust is the same across all usage levels for each product using the Dunn’s one – to – many multiple contrast test. Since there may be additive combinations of consumer product usage, we will use hierarchical clustering to look at combinations of consumer product usage and their effect on PFAS media levels. We will compare PFAS environmental media concentrations across these groups using Kruskal Wallis followed by Dunn’s test with all – pairs comparisons to identify if a cluster of consumer product usage is higher or lower than the others.
|
Use media specific concentration measurements to estimate media and route specific intakes; and compare estimated intake doses to measured serum levels.
|
We will estimate route specific and media specific intake rates of PFAS. The total and route and media specific intake rates will be compared with serum measurements of PFAS from the Exposure Assessments. Because there is a high probability of censoring in both the dependent and the independent measurements, we will test the null hypothesis that there is no monotonic relationship between estimated intakes and serum PFAS using Kendall’s tau and the Akritas-Theil-Sen (ATS) line (Akritas, 1995, Helsel 2011).
For univariate and multiple regression analysis of the relationship of PFAS serum to the demographics, survey questions, and estimated exposure intake, we will use the R package “survey” (Lumley 2010) to account for household clustering (since there may be multiple observations of serum PFAS per household). For multivariate analysis, variable selection will follow backward stepwise procedures. To be consistent with the CDC/ATSDR Exposure Assessment, we will substitute nondetect values with the square root of two and use the log transformation to reduce the skew of the data. We will regress geometric means for PFAS with 60% or more detection rates.
|
Household sampling results will be provided electronically or by mail to the participants (Appendix F). Following dissemination of individual results, an environmental sampling EI team member will be available to discuss individual questions by phone or email.
At the conclusion of the environmental sampling EI, a report will summarize the overall aggregate findings and conclusions of the investigation but will not reveal personal identifiers. If warranted, recommendations for additional actions such as continued monitoring, educational activities, or interventions to reduce exposure will be made. Findings from the environmental sampling EIs will be summarized in a final report and in manuscript(s) submitted for publication in the peer-reviewed scientific literature.
References are cited in the Sampling and Analysis Plan in Appendix D
Akritas, M.G., Murphy, S.A., LaValley, M.P., 1995. The Theil-Sen Estimator With Doubly Censored Data and Applications to Astronomy. Journal of the American Statistical Association 90, 170–177. https://doi.org/10.2307/2291140
Agency for Toxic Substances and Disease Registry [ATSDR]. 2019. Exposure Point Concentration Guidance for Discrete Sampling. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service, July 12.
Agency for Toxic Substances and Disease Registry [ATSDR]. 2021. PFAS Exposure Assessment Site Locations [PFAS Exposure Assessment Site Locations | ATSDR (cdc.gov)].
Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B, 57, 289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. https://www.jstor.org/stable/2346101.
Bizkarguenaga E. Zabeleta I, Mijangos L, Iparraguirre A, Ferdandez LA, Prieto A, Zuloaga O. 2016. Uptake of perfluorooctanoic acid, perfluorooctane sulfonate and perfluorooctane sulfonamide by carrot and lettuce from compost amended soil. Science of the Total Environment. 571: 444-451.
Blaine AC, Rich CD, Sedlacko EM, Hundal LS, Kumar K, Lau C, Mills MA, Harris KM, Higgins CP. 2014. Perfluoroalkyl acid distribution in various plant compartments of edible crops grown in biosolids-amended soils. Environmental Science and Technology. 48(14): 7858-7865.
Centers for Disease Control and Prevention [CDC]. 2021. National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data.Per- and Polyfluorinated Substances (PFAS) Factsheet | National Biomonitoring Program | CDC.
Felizeter S, McLachlan MS, deVoogt P. 2012. Uptake of perfluorinated alkyl acids by hydroponically grown lettuce (Lactuca sativa). Environmental Science and Technology. 46(21): 11735-11743
Genualdi, Susan & Beekman, Jessica & Carlos, Katherine & Fisher, Christine & Young, Wendy & Dejager, Lowri & Begley, Timothy. (2021). Analysis of per- and poly-fluoroalkyl substances (PFAS) in processed foods from FDA’s Total Diet Study. Analytical and Bioanalytical Chemistry. 414. 10.1007/s00216-021-03610-2.
Ghisi R, Vamerali T, Manzetti S. 2019. Accumulation of perfluorinated alkyl substances (PFAS) in agricultural plants: A review. Environ Res. 169: 326-341
Hawaii Department of Health. 2016. Use of Multi Incremental Samples to Characterize DU’s. http://www.hawaiidoh.org/tgm.aspx?p=0402a.aspx
Helsel D. 2011. Statistics for Censored Environmental Data using Minitab and R, second Edition. Hoboken, NJ: John Wiley and Sons.
Helsel, D.R., Hirsch, R.M., Ryberg, K.R., Archfield, S.A., and Gilroy, E.J., 2020, Statistical methods in water resources: U.S. Geological Survey Techniques and Methods, book 4, chapter A3, 458 p., https://doi.org/10.3133/tm4a3. [Supersedes USGS Techniques of Water-Resources Investigations, book 4, chapter A3, version 1.1.]
HUD. n.d. Wipe Sample of Settled Dust for Lead Determination. https://www.hud.gov/sites/documents/LBPH-40.PDF
Interstate Technology Regulatory Council [ITRC]. 2018. Site Characterization Considerations, Sampling Precautions, and Laboratory Analytical Methods for Per- and Polyfluoroalkyl Substances (PFAS).
Interstate Technology Regulatory Council [ITRC]. 2020. Incremental Sampling Methodology Update. https://ism-2.itrcweb.org/
Lechner M, Knapp H. 2011. Carryover of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) from soil to plant and distribution to the different plant compartments studied in cultures of carrots (Daucus carota ssp. Sativus), potatoes (Solanum tuberosum), and cucumbers (Cucumis Sativus). Journal of Agricultural and Food Chemistry. 59(20): 11011-11018.
Levasseur JL, Hammel SC, Hoffman K, Phillips AL, Zhang S, Ye X, Calafat AC, Webster TF, Stapleton HM. 2021. Young children’s exposure to phenols in the home: Associations between house dust, hand wipes, silicone wristbands, and urinary biomarkers. Environment International (147). https://doi.org/10.1016/j.envint.2020.106317
Liu Z, Lu Y, Song X, Jones K, Sweetman AJ. Johnson AC, Zhang M, Lu X, Su C. 2019. Multiple crop bioaccumulation and human exposure of perfluoroalkyl substances around a mega fluorochemical industrial park, China: Implication for planting optimization and food safety. Environ Int. 127: 671-684
Minnesota Department of Health [MDH]. 2010. House Dust Field Sampling Protocol for Perfluorochemical (PFC) Analysis.
National Children’s Study. n.d. Environmental Vacuum Bag Dust Technician Collect SOP V1.0.
National Institute of Environmental Health Sciences. 2016. Perfluorinated Chemicals (PFCs). National Institutes of Health, Research Triangle Park, NC.
Navarro I, de la Torre A, Sanz P, Porcel MÁ, Pro J, Carbonell G, de los Angeles Martínez M. 2017. Uptake of perfluoroalkyl substances and halogenated flame retardants by crop plants grown in biosolids-amended soils. Environmental Research. 152: 199-206.
Roth J, Abusallout I, Hill T, Holton C, Thapa U, Hanigan D. 2020. Release of volatile per- and polyfluoroalkyl substances from aqueous film-forming foam. Environ. Sci. Technol. Lett. 7:164−170. https://pubs.acs.org/doi/suppl/10.1021/acs.estlett.0c00052/suppl_file/ez0c00052_si_001.pdf
Scher, D.P., Kelly, J.E., Huset, C.A., Barry, K.M., Hoffbeck, R.W., Yingling, V.L., and Messing, R.B. (2018) Occurrence of perfluoroalkyl substances (PFAS) in garden produce at homes with a history of PFAS-contaminated drinking water. Chemosphere. 196: 548-555
T. Lumley (2010) Complex Surveys: A Guide to Analysis Using R. John Wiley and Sons. Hoboken, NJ: John Wiley and Sons.
U.S. Environmental Protection Agency [EPA]. 2017. Field Collection Standard Operating Procedures (SOPs) for an EPA Pilot Study Evaluating Personal, Housing, and Community Factors Influencing Children’s Potential Exposures to Indoor Contaminants at Various Lifestages (EPA Pilot Study Add-On to the Green Housing Study). [SOP for Collection of Indoor Air Samples using Active Samplers]. Office of Research and Development. https://cfpub.epa.gov/si/si_public_record_report.cfm?dirEntryId=335764&Lab=NERL
Wen, B., Wu, Y., Zhang, H., Liu, Y., Hu, X., Huang, H., and Zhang, S. (2016) The roles of protein and lipid in the accumulation and distribution of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in plants grown in biosolids-amended soils. Environmental Pollution. 216: 682-688
Protocol, PFAS EPA IAA
Last
Revised:
Page
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Modified | 0000-00-00 |
| File Created | 2022-03-18 |
© 2026 OMB.report | Privacy Policy