Form 0920-0215 National Death Index - (NDI) Application Form
Application Form and Related Forms for the Operation of the National Death Index
Att A Updated NDI Application Form 021319
National Death Index - Application Form
OMB: 0920-0215
Attachment A: Updated National Death Index Application Form
FORM APPROVED OMB No. 0920-0215
Exp. Date 12/31/19

NATIONAL DEATH INDEX APPLICATION
As you complete this form, please call 301–458–4444
if you have any questions



![]()
CDC/NCHS-6205-1 (Rev. 12/2016)
NATIONAL DEATH INDEX
National Center for Health Statistics 3311 Toledo Road, Room 5292
Hyattsville, Maryland 20782
301-458-4444

CDC estimates the average public reporting burden for this collection of information as 4 hours per response, including the time for reviewing instructions, searching existing data/information sources, gathering and maintaining the data/information needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to CDC/ATSDR Information Collection Review Office, 1600 Clifton Road NE, MS D-74, Atlanta, Georgia 30333; ATTN: PRA (0920-0215).
Assurance of confidentiality- We take your privacy very seriously. All information that relates to or describes identifiable characteristics of individuals, a practice, or an establishment will be used only for statistical purposes. NCHS staff, contractors, and agents will not disclose or release responses in identifiable form without the consent of the individual or establishment in accordance with section 308(d) of the Public Health Service Act (42 U.S.C. 242m(d)).
Note: definition of “IDENTIFYING or IDENTIFIABLE death record information” — Any information on death certificates, other paper documents, or in computer files which by itself, or if linked with other records, would permit the identification of one or more individuals or establishments; for example, name(s), Social Security number, exact dates, addresses, and death certificate number. Even with the removal of direct identifiers and linkable study subject identification numbers, there is still a special concern that some combinations of the remaining variables could potentially be used to identify an individual. For example, a combination of date of birth, date of death, or cause of death is considered identifiable.
DEPARTMENT OF HEALTH AND HUMAN SERVICES |
Assigned NDI Application Number |
Public Health Service |
|
Centers for Disease Control and Prevention |
|
National Center for Health Statistics |
|
NATIONAL DEATH INDEX APPLICATION
1. |
Title of study or project (must match IRB)
|
|
2. |
Individual and organization requesting use of NDI |
|
|
Principal Investigator or Project Director: |
|
|
Title: |
|
|
Organization:
|
|
|
Address:
|
|
Phone no.: |
|
Ext: |
|
E-mail: |
|
Who should be contacted if more information is needed? |
|
Phone no.: |
|
Ext: |
|
E-mail: |
|

 3.
External funding sources? Yes No (Internal funding only)
3.
External funding sources? Yes No (Internal funding only)
List the names of all OTHER organizations providing funding for this project and indicate the type of support provided (i.e., grant, contract, cooperative agreement, interagency agreement, or other [specify]). NOTE: Except for a FEDERAL GRANT, each sponsor must complete and sign an NDI Supplemental Confidentiality Agreement at the end of this application.
Names of Organization(s) |
Type of Funding Support |
|
|
4. Data sources
List all organizations (including your own) that have collected (or will be collecting) data on the study subjects. Under each organization listed, describe the types of data collected. If any of the external organizations listed will be receiving IDENTIFYING or IDENTIFIABLE death record information, they must also be listed in item 5 below.
|


5. Will EXTERNAL organizations (other than the NDI applicant's organization) be receiving IDENTIFYING or IDENTIFIABLE death record information? Yes No
List
the names of all parties (organizations or outside consultants) that
will obtain IDENTIFYING
or IDENTIFIABLE death record information
or data derivatives from NDI.
Important:
Under
each organization (or consultant) listed below, specify that
organization's role and what project will be performed. Also specify
(1) what IDENTIFYING
or IDENTIFIABLE death record information
will be received, (2) in what form it will be received (e.g., death
certificates or computer files), and (3) how the information will
"flow" from one organization to another.
Parties employed by your organization must complete and sign the
Confidentiality Agreement. Parties in other organizations must
complete and sign an NDI Supplemental Confidentiality Agreement.
Name of Organization – Principal Investigator or Project Director |
Administrative Relationship |
Data Type |
|||
Organization's role and what project activities will be performed: |
|||||
6. Summary of study protocol or project activities
In responding to the following questions, please provide sufficient detail to describe your study or project and how data obtained via NDI will be used.

 6a.
Will the information obtained via NDI be included in a registry
or any other type of study with long-term use or an indefinite end
date? Yes No
6a.
Will the information obtained via NDI be included in a registry
or any other type of study with long-term use or an indefinite end
date? Yes No
What type of study is this? (e.g., disease registry, longitudinal cohort study, cross-sectional study, case-control study)
|

 6b.
Are you getting causes of death? Yes No
6b.
Are you getting causes of death? Yes No
All applicants must complete item 6c. If your application involves a registry, be sure also to include the following information in item 6c. below: (1) the date the registry was founded, (2) the purpose of the registry, and (3) the eligibility criteria for including person in the registry. A registry should also refer to Attachment B for additional information to be included in this application.
6c. Purpose of study or project
|
Describe the health or medical problem(s) addressed by your study or project. Include some background information to support why the study or project is being done. What are the primary objectives? If appropriate, include a description of hypotheses to be tested.
7. Death record follow-back investigations
7a. Does this study or project plan to perform "death record follow-back" investigations? ("Follow-back investigations" means that once NDI identifies that certain study subjects are deceased, your staff plans to collect additional information on those subjects by going BACK to individuals or establishments that are mentioned in the subjects' actual death certificates.) NOTE: Follow-up refers to contacting the next-of-kin or health providers based on information already contained in researchers' file.

 Yes No
Yes No
If yes, refer to Attachment C for additional documentation needed.
7b. If yes, what type of respondents will be contacted? Check all that apply.
 Decedent’s
next-of-kin
Decedent’s
next-of-kin
 Physicians
Physicians
 Hospitals
Hospitals
 Other individuals or
establishments mentioned on death record
Other individuals or
establishments mentioned on death record
7c. What information will be obtained from EACH type of respondent?
|
7d. Name the organization(s) or consultant(s) who will be contacting EACH type of respondent:
|
7e. Name the methods to be used in conducting follow-back investigations, including how EACH type of contact will be made:
|
8. Institutional Review Board (IRB) for the Protection of Human Subjects
(Defined by the U.S. Department of Health and Human Services in the Code of Federal Regulations, Title 45, Part 46)
Evidence of a current IRB review is REQUIRED for all NDI applications (please ensure that NDI applicant's name is referenced in the IRB letter). If this study or project involves death record follow-back investigations as described in item 7, a special letter from the IRB is REQUIRED (as explained in Attachment C).


 8a.
IRB approval status Full Expedite
Exempt
8a.
IRB approval status Full Expedite
Exempt
8b. Include a copy of the IRB review and provide the following:
|
Name of IRB:
|
IRB's Multiple Project
Assurance (MPA) number or Federalwide Assurance (FWA) number:
(NOTE: If death record follow-back investigation will be performed as described in item 7, an explanation of why your organization does not require an IRB approval for such a study or project is not acceptable. If your organization does not have an IRB [that has been approved by the Office for Human Research Protections, Department of Health and Human Services], you may have the study reviewed by an approved IRB in another organization.)
9. Maintaining the Confidentiality of IDENTIFYING or IDENTIFIABLE death record information
9a. Name the organization(s), including your own, that will:
Submit records of study subjects for the NDI file search(es):
Receive the results of the NDI search directly:
|


Based on the results of the
NDI file search(es), will copies of death
certificates be
requested from state vital statistics offices? Yes No
Request copies of death certificates from the state vital statistics offices:
|
9b. Describe the following controls that would be used to store and maintain the confidentiality of the IDENTIFYING or IDENTIFIABLE death record information at your organization:
Physical controls—building guards, identification badges, key cards, closed circuit TV, and locked offices.
|
Technical controls—user identification, passwords, firewalls, encryption, virtual private network, intrusion detection system, and stand-alone desktop use only. Please be aware that the standard encryption requirement for sensitive federal information, like the NDI data, is FIPS-140-2 in accordance with NIST 800-53 (see: https://nvlpubs.nist.gov/nistpubs/FIPS/NIST.FIPS.140-2.pdf and https://nvlpubs.nist.gov/nistpubs/SpecialPublications/NIST.SP.800-53r4.pdf).
|
Administrative controls—frequency of backing up files, where backup files will be stored, methods to ensure least privilege access, methods for ensuring IDENTIFYING or IDENTIFIABLE death record information is not co-mingled with administrative records not part of this project, how use will be monitored to prevent use for purposes not approved for this project, how personnel using the system will be trained and made aware of their responsibilities for protecting the IDENTIFYING or IDENTIFIABLE death record information, methods for monitoring who has access to the data, and methods for ensuring return or destruction of data. Please include text indicating the number of persons who will have access to the backup files containing IDENTIFYING OR IDENTIFIABLE death record information.
|
NOTE: If multiple sites are involved in the above-mentioned study project, each site must describe its own controls that would be used to maintain the confidentiality of the IDENTIFYING or IDENTIFIABLE death record information.
10. Completion of study or project

 10a.
Is the study or project ongoing or open-ended? Yes No
10a.
Is the study or project ongoing or open-ended? Yes No
 If
no, indicate the scheduled termination date for the study:
If
no, indicate the scheduled termination date for the study:
10b. In what form (e.g., aggregate, statistical, report, etc.) and to whom (e.g., peer-reviewed scientific journals, monographs) will the results of your study or activities be released? (NDI would appreciate a courtesy copy of any publications that may result from the use of NDI data.)
|

 10c.
Will study subjects be notified of study results? Yes
No
10c.
Will study subjects be notified of study results? Yes
No
If yes, how will the subjects be notified?
|
11. Data disposition plan
Some state vital statistics offices have expressed concern about indefinite retention of IDENTIFYING or IDENTIFIABLE death record information that could be used in the future by other persons for other purposes.
Except for data stored in registries, or other approved long-term studies, all identifying or identifiable data received from NDI must be removed from all research records at the conclusion of the study or within 5 years after receipt of the NDI data – regardless of the data set in which the data are kept. This means that all identifiers or potentially identifiable data elements associated with cause-of-death codes must be removed from all analysis files unless there is no way to identify an individual decedent. This also means that any linked files (with crosswalks) must be destroyed. As long as there are no identifying or linkage variables remaining in the analytic or public-use file(s), cause(s) of death codes may remain in such file(s). (Note: Death certificates obtained directly from state offices may have to be shredded in less than 5 years depending on each state’s requirements.)
Based on the above requirements, when do you plan to dispose of all IDENTIFYING or IDENTIFIABLE death record information obtained from NDI? Give the proposed month and year of destruction, or enter UNKNOWN if this is an open-ended or ongoing study that has no specific disposition plan at this time.
Only complete item 2a. if the above date is UNKNOWN or if the date is more than 5 years after the month and year that you submitted this NDI application.
Please provide a strong justification for why the data need to be retained beyond this 5-year period.
Within 5 years of submitting your NDI application, you are responsible for either (1) requesting an extension or (2) certifying the NDI data have been returned to NCHS or destroyed (see Attachment A). The extension request or certification of data disposal must be submitted to NDI staff within 5 years—no later than the month and year stated in the box below.
National Death Index Confidentiality Agreement

Study
or project title:
The undersigned agrees to the following terms and conditions associated with this National Death Index (NDI) application and to the use of the information obtained from (1) NDI, (2) state death records, and (3) death record follow-back investigations:
Except for persons or organizations specified in the approved NDI application, no data will be published or released in any form to any party if a particular individual or establishment is identifiable. ALL REQUESTS FOR IDENTIFIABLE
DATA OBTAINED VIA NDI WILL BE REFERRED IMMEDIATELY TO THE NATIONAL CENTER FOR HEALTH STATISTICS (NCHS). In accordance with Section 308(d) of the Public Health Service Act, such identifiable data will specifically not be provided in response to a direct order from an official of any government agency, the Administration or Congress, or in response to an order from a court of justice.
The identifying information will be used ONLY for statistical purposes in medical and health research.
The identifying information will not be used as a basis for legal, administrative, or other actions that may directly affect those particular individuals or establishments as a result of their specific identification in this project.
The identifying information will be used only for the study or project proposed and the purpose described in the approved NDI application. The information cannot be used for a research project other than the one described in the application until a separate NDI application for that project has been submitted to, and approved by, NCHS.
NCHS obtains death record information via contracts with the state vital statistics offices. These contracts contain specific restrictions on the use of the information by NDI and by the NDI Plus service (gives NDI users cause-of-death codes). By providing NCHS with these assurances, I understand that I am also providing the same assurances to the state vital statistics offices. Violation of the terms and conditions of this agreement may result in immediate annulment of the agreement by NCHS, the requirement to return all NDI data and related materials, and denial of future use of NDI. Violation of the terms of the agreement may also be a violation of federal criminal law under 18 U.S.C. Section 1001. NCHS will pursue all legal remedies in the event of unauthorized disclosure of identifiable information from NDI data. Violation of the terms of the agreement are also subject to state legal remedies.
The original version of the NDI data must be retained at a single location. One active copy of the NDI data can reside on a secure server where only those persons identified in the agreement and who have signed a non-disclosure statement can access the NDI data. An additional backup copy can be made if the NDI data are secured in the same manner as the active copy of the NDI data. The NDI data may not be re-released to others except as specified in item 5 of the NDI application.
Servers housing identifiable NDI data must be protected by a firewall and not directly accessible from the Internet. Access to NDI data must be controlled by active directory and restricted to only those persons identified in the agreement and who have signed a non-disclosure statement to ensure that identifiable NDI data cannot be used or taken by unauthorized individuals. NDI data cannot be stored or accessed on personal computers, laptops. All persons must have completed computer security training required by their institution. All printouts, or other physical products containing identifiable information derived from NDI must be kept in locked cabinets, file drawers, or other secure locations when not in use. Printouts, tabulations, reports, and other materials must be edited for any possible disclosures of NDI identifiable data prior to making the information available to anyone other than those persons identified in this agreement.
Except for data stored in registries or approved long-term studies, all identifying or identifiable data received from NDI must be removed from all research records at the conclusion of the study or within 5 years after receipt of the NDI data—regardless of the data set in which the data are kept—unless an extension has been granted by NDI. The original version of the NDI data must be returned to NCHS or destroyed. Files—including backup files and derived files—with NDI identifying or identifiable data must be both deleted and overwritten to prevent recovery of the data (see Attachment A).
Organization/researcher agrees to report any confirmed or suspected losses, including theft and unauthorized disclosure/access, of personally identifiable information (PII) from the NCHS data file(s) to the CDC Computer Security Incident Response Team’s (CSIRT) 24 x 7 Emergency Number (1-866-655-2245) within one hour. After notifying CSIRT, Organization/researcher will notify the NCHS Division of Vital Statistics Director, Steven Schwartz (email: [email protected] or phone: 301-458-4210), with the incident number issued by CDC CSIRT. Organization/researcher will not communicate PII details via email.
National Death Index Confidentiality Agreement (continued)
Authorized NCHS staff or agents may, upon request, be granted access to {name of user} facilities, where confidential NDI data are kept or used, for the purpose of inspecting the data security arrangements.
I understand that while state vital statistics offices may receive copies of this application, states may require additional information or assurances before responding to requests for copies of death certificates or for death record information. Some states may not be able to honor certain requests because of the proposed uses of the state data. Furthermore, once data from a particular state are received, I understand that I and other users of the data are subject to that state’s laws and regulations relating to disclosure of information on individuals or establishments.


The Data Steward for this project is: Title:
Name
Organization:
Work phone number: E-mail address:
As Data Steward, I affirm I will act as the custodian of the NDI files and will be responsible for the observance of conditions
of use.
I will notify the NDI Director, Dr. Lillian Ingster (301–458–4286; [email protected]):
When access to the NDI data is no longer needed, (see Attachment A)
If a change in site access is contemplated
Of the intent to modify the project’s purpose
If these responsibilities are to be transferred
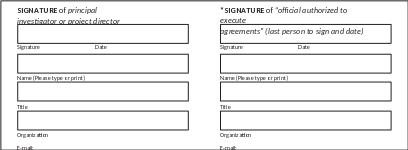
Signature of Data Steward: Date:
* NOTE: The “official authorized to execute agreements” will vary among organizations. Whenever possible, NDI prefers that this official be someone at a higher level of authority than the principal investigator or other persons responsible for the study or project; for example, a university official authorized to sign grant proposals, a company vice president, a government division, or bureau director. By signing this agreement as the authorized official, you are declaring that you have the authority to make the above assurances on behalf of the university, company, agency, or other organization and to bind the organization to the terms of this agreement, and you take responsibility for the confidentiality assurances of all organizations or individuals who are participating in this study.
For those individuals planning to sign digitally, note that not all types of electronic signatures are acceptable. For more information see Attachment D.
I have reviewed this NDI application. All the statements made in this application and in any confidentiality assurances related to this application are true, complete, and correct to the best of my knowledge and belief. My signature below indicates my agreement to comply with the stated statutorily based requirements with the knowledge that deliberately making a false statement in any matter within the jurisdiction of any department or agency of the federal government violates 18 USC 1001 and is punishable by a fine of up to $10,000 or up to 5 years in prison.

National
Death Index Supplemental Confidentiality Agreement
A separate Supplemental Confidentiality Agreement must be completed and signed by each EXTERNAL organization or consultant funding or participating in this study, as listed in items 3 and 5 of the NDI application. The Supplemental Confidentiality Agreement(s) must then be submitted as an attachment to the application. THIS REQUIREMENT IS WAIVED ONLY FOR A FEDERAL GRANT, AND THEN ONLY WHEN THE NDI APPLICANT (GRANTEE) CAN GIVE ASSURANCES THAT THE IDENTIFYING INFORMATION OBTAINED DIRECTLY OR INDIRECTLY FROM NDI WILL UNDER NO CIRCUMSTANCES BE PROVIDED TO THE GRANTOR.

Name and title of Principal Investigator, Project Director, Project Officer, or other responsible official:

 Organization
name and complete mailing address:
Organization
name and complete mailing address:

 Telephone
number: E-mail:
Telephone
number: E-mail:
Will this organization (or individual) receive any of the identifying or identifiable death record information obtained from the NDI, state death records, or death record follow-back investigations? “Identifying or identifiable death record information” refers to any information on death certificates, other paper documents, or in computer files which by themselves, or if linked with other records, would permit the identification of one or more individuals or establishments. For example, a combination of date of birth, date of death, or cause of death is considered identifiable.


 Yes No Maybe
Yes No Maybe
Does this organization (or individual) have any contractual or other rights to the identifying information referred to above?


 Yes No Maybe
Yes No Maybe
If you
answered "No"
to
both questions 1 and 2, skip questions 3 and 4 below and just
provide the two requested signatures below. If you answered "Yes"
or
"Maybe"
to
either questions 1 or 2, please complete questions 3 and 4 below and
provide three signatures.
National Death Index Supplemental Confidentiality Agreement (continued)
In the box below, describe how your organization will store and maintain the confidentiality of the identifying or identifiable death record information obtained from (1) NDI, (2) state death records, and (3) death record follow-back investigations.
“IDENTIFYING or IDENTIFIABLE death record information” — Any information on death certificates, other paper documents, or in computer files which by itself, or if linked with other records, would permit the identification of one or more individuals or establishments; for example, name(s), Social Security number, exact dates, addresses, and death certificate number. Even with the removal of direct identifiers and linkable study subject identification numbers, there is still a special concern that some combinations of the remaining variables could potentially be used to identify an individual. For example, a combination of date of birth, date of death, or cause of death is considered identifiable.
Describe the following controls that would be used to maintain the confidentiality of the NDI data:
Physical controls–building guards, identification badges, key cards, closed circuit TV, and locked offices.
Technical controls–user identification, passwords, firewalls, encryption, virtual private network, intrusion
detection system, and stand-alone desktop use only.
Please be aware that the standard encryption requirement for sensitive federal information, like the NDI data, is FIPS-140-2 in accordance with NIST 800-53 (see: https://nvlpubs.nist.gov/nistpubs/FIPS/NIST.FIPS.140-2.pdf and https://nvlpubs.nist.gov/nistpubs/SpecialPublications/NIST.SP.800-53r4.pdf).
Administrative controls–frequency of backing up files, where backup files will be stored, methods
to ensure least privilege access, methods for ensuring IDENTIFYING OR IDENTIFIABLE death record information is not co-mingled with administrative records not part of this project, how use will be monitored to prevent use for purposes not approved for this project, how personnel using the system will be trained and made aware of their responsibilities for protecting the IDENTIFYING OR IDENTIFIABLE death record information, methods for monitoring who has access to the data, and methods for ensuring return or destruction of data. Please include text indicating the number of persons who will have access to the backup files containing IDENTIFYING OR IDENTIFIABLE death record information.

NOTE:
If multiple sites are involved in the above-mentioned study project,
each site must describe its own controls that would be used to
maintain the confidentiality of the NDI data.
How and when will your organization dispose of identifying or identifiable death record data? If your organization has
no plans to dispose of some or all of the identifying or identifiable death record data, please explain why.

National Death Index Supplemental Confidentiality Agreement (continued)
Title of study or project:

The undersigned hereby agrees to the following terms and conditions associated with this National Death Index (NDI) application and to the use of the information obtained from (1) NDI, (2) state death records, and (3) death record follow-back investigations:
Except for persons or organizations specified in the approved NDI application, no data will be published or released in any form to any party if a particular individual or establishment is identifiable. ALL REQUESTS FOR IDENTIFIABLE DATA OBTAINED VIA NDI WILL BE REFERRED IMMEDIATELY TO THE NATIONAL CENTER FOR HEALTH STATISTICS (NCHS). In accordance with Section 308(d) of the Public Health Service Act, such identifiable data will specifically not be provided in response to a direct order from an official of any government agency, the Administration or Congress, or in response to an order from a court of justice.
The identifying information will be used ONLY for statistical purposes in medical and health research.
The identifying information will not be used as a basis for legal, administrative, or other actions that may directly affect those particular individuals or establishments as a result of their specific identification in this project.
The identifying information will be used only for the study or project proposed and the purpose described in the approved NDI application. The information cannot be used for a research project other than the one described in the application until a separate NDI application for that project has been submitted to, and approved by, NCHS.
NCHS obtains death record information via contracts with the state vital statistics offices. These contracts contain specific restrictions on the use of the information by NDI and by the NDI Plus service (gives NDI users cause-of-death codes). By providing NCHS with these assurances, I understand that I am also providing the same assurances to the state vital statistics offices. Violation of the terms and conditions of this agreement may result in immediate annulment of the agreement by NCHS, the requirement to return all NDI data and related materials, and denial of future use of NDI. Violation of the terms of the agreement may also be a violation of federal criminal law under 18 U.S.C. Section 1001. NCHS will pursue all legal remedies in the event of unauthorized disclosure of identifiable information from NDI data. Violation of the terms of the agreement are also subject to state legal remedies.
The original version of the NDI data must be retained at a single location. One active copy of the NDI data can reside on a secure server where only those persons identified in the agreement and who have signed a non-disclosure statement can access the NDI data. An additional backup copy can be made if the NDI data are secured in the same manner as the active copy of the NDI data. The NDI data may not be re-released to others except as specified in item 5 of the NDI application.
Servers housing identifiable NDI data must be protected by a firewall and not directly accessible from the Internet. Access to NDI data must be controlled by active directory and restricted to only those persons identified in the agreement and who have signed a non-disclosure statement to ensure that identifiable NDI data cannot be used or taken by unauthorized individuals. NDI data cannot be stored or accessed on personal computers, laptops. All persons must have completed computer security training required by their institution. All printouts, or other physical products containing identifiable information derived from NDI must be kept in locked cabinets, file drawers, or other secure locations when not in use. Printouts, tabulations, reports, and other materials must be edited for any possible disclosures of NDI identifiable data prior to making the information available to anyone other than those persons identified in this agreement.
Except for data stored in registries or approved long-term studies, all identifying or identifiable data received from NDI must be removed from all research records at the conclusion of the study or within 5 years after receipt of the NDI data—regardless of the data set in which the data are kept—unless an extension has been granted by NDI. The original version of the NDI data must be returned to NCHS or destroyed. Files—including backup files and derived files—with NDI identifying or identifiable data must be both deleted and overwritten to prevent recovery of the data (see Attachment A).
Organization/researcher agrees to report any confirmed or suspected losses, including theft and unauthorized disclosure/access, of personally identifiable information (PII) from the NCHS data file(s) to the CDC Computer Security Incident Response Team’s (CSIRT) 24 x 7 Emergency Number (1-866-655-2245) within one hour. After notifying CSIRT, Organization/researcher will notify the NCHS Division of Vital Statistics Director, Steven Schwartz (email: [email protected] or phone: 301-458-4210), with the incident number issued by CDC CSIRT. Organization/researcher will not communicate PII details via email.
 National
Death Index Supplemental Confidentiality Agreement (continued)
National
Death Index Supplemental Confidentiality Agreement (continued)
Authorized NCHS staff or agents may, upon request, be granted access to {name of user} facilities, where confidential NDI data are kept or used, for the purpose of inspecting the data security arrangements.
I understand that while state vital statistics offices may receive copies of this application, states may require additional information or assurances before responding to requests for copies of death certificates or for death record information. Some states may not be able to honor certain requests because of the proposed uses of the state data. Furthermore, once data from a particular state are received, I understand that I and other users of the data are subject to that state’s laws and regulations relating to disclosure of information on individuals or establishments.
NOTE:
If your response to both items 1 and 2 above was "No", you
must still sign this form below; HOWEVER,
it is understood that the terms specified in item 5 above do not
apply to you or to your organization. And, because you will not be
receiving identifiable NDI data, you would not need a Data Steward’s
signature.
* NOTE: The “official authorized to execute agreements” will vary among organizations. Whenever possible, the NDI prefers that this official be someone at a higher level of authority than the principal investigator or other persons responsible for the study or project; for example, a university official authorized to sign grant proposals, a company vice president, a government division, or bureau director. By signing this agreement as the authorized official, you are declaring that you have the authority to make the above assurances on behalf of the university, company, agency or other organization and to bind the organization to the terms of this agreement and you take responsibility for the confidentiality assurances of all organizations or individuals who are participating in this study.
For those individuals planning to sign digitally, please keep in mind that not all types of electronic signatures are acceptable. For more information see Attachment D.
The
Data Steward for this
project is: Title:
Name Organization:
Work
phone number: E-mail
address:
As
Data Steward, I affirm I will act as the custodian of the NDI files
and will be responsible for the observance of conditions of
use.
I will notify the NDI Director, Dr. Lillian
Ingster (301–458–4286;
[email protected]). When
access to the NDI data is no longer needed, (see
Attachment
A) If
a change in site access is contemplated Of
the intent to modify the project’s purpose If
these responsibilities are to be transferred Signature
of
Data Steward: Date:

Use the multi-purpose form on the next page to notify the NDI program of one of the following events:
When you have disposed of ALL the identifying or identifiable death record information obtained
from NDI.
If your initial NDI application was submitted more than 5 years ago and you are now submitting a Repeat NDI Request (and have never completed this form).
To request an extension for the retention of your identifying or identifiable death record
information beyond 5 years from when your initial NDI application was submitted.
If you have already been approved for a 1 to 5 year extension, to request another extension beyond your previously approved extension period.

Some state vital statistics offices have expressed concern about indefinite retention of “IDENTIFYING or IDENTIFIABLE death record information” that could be used in the future by other persons for other purposes.
[Definition of IDENTIFYING or IDENTIFIABLE death record information—Any information on death certificates, other paper documents, or in computer files that by itself, or if linked with other records, would permit the identification of one or more individuals or establishments. Such information includes name(s), Social Security number, exact dates, addresses, and death certificate number. Even with the removal of direct identifiers and linkable study subject
identification numbers, there is still a special concern that some combinations of the remaining variables could potentially be used to identify an individual (e.g., a combination of date of birth, date of death, or cause of death is considered identifiable).
Except for data stored in registries or other approved long-term studies, all IDENTIFYING or IDENTIFIABLE death record information derived or received from NDI must be removed from all research records at the conclusion of the study or within 5 years after receipt of your initial NDI application—regardless of the data set in which the data are kept. This means that all identifiers or potentially identifiable data elements associated with cause-of-death codes must be removed from all analysis files unless there is no way to identify an individual decedent. This also means that any linked files (with crosswalks) are to be destroyed. As long as there are no identifying or linking variables remaining in the analytic or public-use file(s), cause(s)-of-death codes may remain in such file(s).
(NOTE: Death certificates obtained directly from state offices may have to be shredded in less than 5 years depending on each state’s requirements.)
National Death Index Data Disposition Form (continued)
Attachment A






 Title
of study or project:
Title
of study or project:
Phone
no.: E-mail:
or Project Director:
Title:
Organization:
Address:
 As
the Data Custodian for the above-listed study or project, I affirm
that
all electronic and paper files containing identifiable NDI data
have been destroyed on:
As
the Data Custodian for the above-listed study or project, I affirm
that
all electronic and paper files containing identifiable NDI data
have been destroyed on:
(If not destroyed, put NA and answer items 3–5 below.)
 I
also affirm
that
all derivative and back-up copies
have
I
also affirm
that
all derivative and back-up copies
have
been destroyed on:
(If not destroyed yet, put NA and answer items 3–5 below.)
 When
will the identifiable death record information be destroyed? (Enter
UNKNOWN if this is an open-ended or ongoing study that has no
specific disposition plan at this
time.)
When
will the identifiable death record information be destroyed? (Enter
UNKNOWN if this is an open-ended or ongoing study that has no
specific disposition plan at this
time.)
If the answer to item 3 is: (1) unknown, (2) more than 5 years after you submitted your NDI application, or (3) more than 5 years after you last requested an extension for the retention of your data, please provide a strong justification for why the data need to be retained beyond the 5-year period.
5. If it has been more than 5 years since your initial NDI application (or since your last request for an extension), are you requesting an extension for the retention of identifiable NDI data? |
|
YES |
|
|
NO |
6. If your extension is approved, you are responsible for submitting |
|
|
|
|
|
this form when your data have been destroyed OR within 5 years |
|
|
|
|
|
from now, but no later than the date you indicate in the box to the right. |
|
|
|
|
|
Data Steward (print name and title) |
Signature |
|
|
|
Date |
Principal Investigator or Project Director (print name and title) |
Signature |
|
|
|
Date |


 Mail
form to: National Death Index, NCHS, 3311 Toledo Road, Room 5292,
Hyattsville, MD 20782
Mail
form to: National Death Index, NCHS, 3311 Toledo Road, Room 5292,
Hyattsville, MD 20782
Registries and Long-term Use and Indefinite End-date Studies: Additional Information Required for NDI Application
In addition to the information requested of all NDI applicants, the NDI application submitted must also include the following information in item 6 of the application:
Brief descriptions of specific studies that are being performed now or are being planned. After describing such studies, the applicant should state the following:
“Should there be any significant deviations from such studies, we fully understand that an amended NDI application must first be submitted to and approved by NCHS.”
(The purpose of the above requirements is to provide evidence that the organization in fact will be using the registry mortality data base solely for “statistical purposes in medical and health research.”)
If the applicant indicates that no death record follow-back investigations will be implemented, the applicant must make the following statement:
“Should follow-back investigations become necessary, and involve death records obtained via NDI, it is understood that first I must (1) submit an amended application describing the follow-back investigations, (2) obtain and submit an approval from an Institutional Review Board for the Protection of Human Subjects, and (3) wait for the amended application to be reviewed by the NDI advisers and approved by the NCHS Director.
A specific statement that all hard-copy death record information obtained via NDI, including copies of death certificates, will be flagged and stored separately from any administrative records or from statistical records that could be used in the future for purposes not described in the application. Computer records containing death record information obtained via NDI must also be flagged so that they will not be used in the future for purposes not described in the application.
National Death Index (NDI) Requirements for Approval by an Institutional Review Board (IRB) for the Protection of Human Subjects
General NDI requirements for IRB approvals:
The IRB approval be granted by (a) an institution which has a Multiple Project Assurance (MPA) or a Federalwide Assurance (FWA) approved by the Department of Health and Human Services (DHHS) or (b) by an independent IRB registered with DHHS.
If the NDI applicant’s institution has an IRB (or its equivalent) that is not approved by DHHS, the applicant must submit additional documentation describing the IRB and listing how its membership is constituted.
An “expedited” IRB review and approval is acceptable if performed by an institution having an MPA and if the research meets the conditions for “expedited” IRB review described in 45 CFR 46.110(a) or (b).
If an applicant’s study or project does not require an IRB approval, the applicant must at least submit documentation from an IRB that the study or project is EXEMPT from the IRB approval requirements.
The review and approval by an IRB must occur prior to approval of the NDI application.
Specific NDI requirements for studies involving death record follow-back investigations:
The applicant must obtain a letter from the IRB indicating specifically that the study’s death record follow-back methodology has been reviewed and approved and that the review of the study also included an assessment of any potential emotional harm and undue respondent burden that may be caused by the proposed follow-back activities. (Of concern are any contacts made to next-of-kin, physicians, hospitals, or other establishments based on information appearing on death certificates obtained via use of NDI.)
The letter must include language similar to the following statement (but tailored specifically to the study that was reviewed):
“We have reviewed this study in conjunction with your application to use NDI. We are satisfied that the procedure to be used to obtain additional information on deceased study subjects (from next-of-kin, physicians, hospitals, or others) provides appropriate protection to the respondents with respect to minimizing respondent burden, maintaining confidentiality, protecting their privacy, and avoiding or minimizing any emotional or other harm that may affect respondents. Our review included an assessment of all existing or proposed contact letters, telephone techniques, questionnaires, and consent forms used in the death record follow-back investigations. These were all deemed to be satisfactory.”
If the applicant is unable to obtain such a letter from the IRB, the study’s IRB approval document must include attachments that clearly show that the IRB’s review included the death record follow-back methodology.
Attachment C (Continued)
Rationale:
It is understood that most studies using NDI do not involve diagnostic, therapeutic, or any other forms of physical contacts with human subjects, and consequently, do not receive or need to receive IRB approvals based on requirements set forth by their own institution or by the regulations for the protection of human subjects implemented by DHHS (45 CFR 46). However, the National Center for Health Statistics and many state vital statistics offices are concerned about the invasion of privacy, potential emotional harm, and undue respondent burden that can result (from contacts made to next-of-kin, physicians, hospitals, and others) as part of death record follow-back investigations that are determined to be essential components of some studies. Because of this concern, an IRB should review the follow-back methodology to be used in such studies, including review of all contact letters or telephone techniques, questionnaires, and consent forms (for release of medical records), as well as procedures for insuring that the information obtained remains confidential.
Therefore, IRB approvals are required for NDI approvals for studies involving death record follow-back investigations. We are hopeful that IRB committees will be both supportive and responsive to this requirement, even though reviews of such studies are neither customary nor required for other purposes, and may even be “exempt” as defined by DHHS regulations 45 CFR 46.101(b)
NDI APPLICANTS AND IRB COMMITTEES REQUIRING ADDITIONAL INFORMATION ON THE ABOVE REQUIREMENTS SHOULD CONTACT NDI STAFF ON AT: 301–458–4444.
Attachment D
Digital Signatures
The Centers for Disease Control and Prevention (CDC) accepts digital signatures from any federal agency that employs a PIV or CAC card under the “interoperability requirement” of HSPD-12, as long as revocation information is available from that PIV or CAC card at the time we receive the form.
For persons who do not have a U.S. government-issued PIV or CAC card, CDC currently has no way of verifying that the signatures are authentic. As technology changes, digital signatures may become an option.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | National Death Index Application Form |
| Subject | filliable form |
| Author | National Center for Health Statistics |
| File Modified | 0000-00-00 |
| File Created | 2021-01-20 |
© 2026 OMB.report | Privacy Policy