Youth 1st, 2nd, 3rd and 4th (Male, Rural Smokeless) follow up questionnaire
Evaluation of the Food and Drug Administration's General Market Youth Tobacco Prevention Campaign
Attachment 02_R. Youth Follow up Instrument compressed (male rural smokeless) FU4
Youth 1st, 2nd, 3rd and 4th (Male, Rural Smokeless) follow up questionnaire
OMB: 0910-0753
Attachment 2_R: Youth FOLLOW-UP 4 INSTRUMENT
Form Approved
OMB No. 0910-0753
Exp. Date 9/30/2019
RIHSC No. 15-101CTP
Evaluation of the Rural Smokeless Tobacco Education Campaign (RuSTEC)
Programming conventions and specifications notes
Abbreviations used include ‘R’ for ‘respondent’ and ‘PNTA’ for ‘prefer not to answer.’
Prefer Not To Answer/Don’t Know/Refused/None of these are not allowed in combination with other responses.
Variable names and section headings are not displayed on screen.
Response options should not be labeled with numbers.
A back button will not be offered to respondents except during front-end administrative items.
Bolding conveys emphasis while capital letters convey instructions for programmers or interviewers.
Questionnaire will include a progress bar.
All items are required.
“Next” buttons will be displayed on every survey screen as appropriate.
All images should be arrange in such a way that focus on usability and layout. Images should be aligned and of similar sizes as one another.
Prefer Not to Answer should be visible only after a respondent attempts to skip the question without answering. A box will appear instructing them to select PNTA as their option if they mean to skip the item.
S0a/LOGIN PAGE. [IF WEB]
Thank you for logging in to the FDA Health and Media Study! Please enter your username and password. Your username is 8 characters such as XXX11111. If you can't find your username call (866) 214-2039.
Username: ___________________(Alpha numeric entry, 16 characters)
Password: ___________________(Alpha numeric entry, 16 characters)
The username and password are CASE SENSITIVE, so please type carefully.
PROGRAMMER: IF ENTRIES DON’T AGREE DISPLAY “Invalid username and/or password. Please verify your username and password and try again. Please remember passwords are case sensitive.”
ASK: All web respondents.
LAND. [IF R IS ON MOBILE DEVICE AND WEB]
It looks like you are viewing this survey on a mobile device. This survey works best in landscape mode. Taking the survey on a mobile device might take longer.

NEXT
ASK: All respondents who access the web survey via a mobile device.
Checkpoint: PROGRAMMER: USE MOST RECENTLY KNOWN DOB, WHETHER FROM WAVE4, WAVE 3, WAVE 2, OR BASELINE. IF WAVE4 DOB IS BLANK, THEN USE WAVE3 DOB. IF WAVE3 DOB IS BLANK, THEN USE WAVE2 DOB. IF WAVE2 DOB IS BLANK, THEN USE BASELINE DOB. CALCULATE DOB FROM MOST RECENTLY KNOWN DOB.
WID [IF WEB AND BLAGE<18]
Our records indicate that [YouthFname] previously participated in our study. Before we begin with the interview, we need the parent or guardian of [YouthFname] to review some information. Are you the parent or guardian of [YouthFname]?
Yes -> GO TO STATE
No -> GO TO WIDFP
ASK: Web respondents who are under the age of 18 or their parents.
DISPLAY: YouthFname is the respondent’s first name.
WIDFP [IF WEB AND WID=NO AND BLAGE<18]
Is [YouthFname]’s parent available to review this information? If not, please log back in to the website when the parent or guardian is available.
Yes, parent is available GO TO STATE
No, I will log back in when parent is available STAY ON THIS SCREEN
Back Next
ASK: Web respondents who indicate that the parent is not available in WID
DISPLAY: YouthFname is the respondent’s first name.
WID18 [IF WEB AND BLAGE>=18]
Our records indicate that [YouthFname] previously participated in our study. Are you [YouthFname]?
Yes -> GO TO STATE
No -> GO TO WIDFP18
ASK: All web respondents who are 18 years old or older
DISPLAY: YouthFname is the respondent’s first name.
WIDFP18 [IF WEB AND WID18=NO AND BLAGE>=18]
Is [YouthFname] available to begin the interview? If not, please log back in to the website when he is available.
Yes, he is available GO TO STATE
No, I will log back in when he is available STAY ON THIS SCREEN
Back Next
ASK: Web respondents who indicate that the respondent is not available in WID18
DISPLAY: YouthFname is the respondent’s first name.
STATE [IF WEB]
What state do you currently live in?
PROGRAMMER: DISPLAY AN ALPHABETIZED DROP DOWN OF ALL 50 STATES, WASHINGTON DC, AND AN OPTION FOR “I DON’T LIVE IN THE UNITED STATES”
ASK: Parents of web respondents who are under 18, or respondents who are 18 or older
COUNTY [IF WEB AND STATE NE I DON’T LIVE IN US]
What county do you currently live in?
PROGRAMMER: DISPLAY DROP DOWN LIST OF ALL COUNTIES WITHIN THE STATE SELECTED FROM STATE. HERE IS THE SOURCE FOR THE CONTENT.
PROGRAMMER: IF STATE=I DON’T LIVE IN US GO TO INELIG. COMPARE COUNTY TO BASELINE COUNTY FROM SAMPLE LIST. IF BASELINE COUNTY=COUNTY, GO TO PEMAIL. IF THE BASELINE COUNTY IS IN A INTERVENTION GROUP AND COUNTY IS IN CONTROL GO TO INELIG. IF THE BASELINE COUNTY IS IN A CONTROL COUNTY AND THE COUNTY IS IN INTERVENTION GO TO INELIG.
ELSE: IF BASELINE COUNTY NE COUNTY, BUT BASELINE COUNTY IS A C OR A D AND COUNTY IS A C OR D, GO TO PEMAIL. IF BASELINE COUNTY NE COUNTY BUT BASELINE COUNTY IS A B AND COUNTY IS A B, GO TO PEMAIL. IF BASELINE COUNTY NE COUNTY AND BASELINE COUNTY IS A C OR D AND COUNTY IS A B OR NOT IN LOOKUP TABLE, GO TO INELIG. IF BASELINE COUNTY NE COUNTY AND BASELINE COUNTY IS A B AND COUNTY IS A C OR D OR NOT IN LOOK UP TABLE, GO TO INELIG.
ASK: Parents of web respondents who are under 18, or respondents who are 18 or older who live in the United States
REMAIL [IF WEB AND BLAGE>=18 ]
In case we need to contact you about the study, please provide your email address.
Email Address:_________________ [ALLOW 50 CHARACTERS. FORMAT AS EMAIL ADDRESS]
Confirm Email Address:__________[MUST MATCH FIRST ENTRY]
Prefer not to answer
PROGRAMMER: VALIDATE FORMAT FOR EMAIL ADDRESS. IF FORMAT IS INCORRECT, PLEASE DISPLAY “Please enter a valid email address.” IF THE EMAIL ADDRESSES DON’T MATCH PLEASE DISPLAY “The email addresses do not match. Please try again. ”
ASK: All web respondents who are 18 or older
PEMAIL [IF WEB AND BLAGE<=18 ]
In case we need to contact you about the study, please provide your email address. Because your son is under 18, we will contact you about his participation in the study.
Parent Email Address:_________________ [ALLOW 50 CHARACTERS. FORMAT AS EMAIL ADDRESS]
Confirm Parent Email Address:__________[MUST MATCH FIRST ENTRY]
Prefer not to answer
PROGRAMMER: VALIDATE FORMAT FOR EMAIL ADDRESS. IF FORMAT IS INCORRECT, PLEASE DISPLAY “Please enter a valid email address.” IF THE EMAIL ADDRESSES DON’T MATCH PLEASE DISPLAY “The email addresses do not match. Please try again. ”
ASK: Parents of web respondents who are under 18
INELIG Thank you for your interest in this study. Unfortunately, you are no longer located in the study area.
NEXT
ASK: Respondents who do not live in the United States or who do not live in a county that is in the study area or respondents that have moved between treatment and control counties.
FINISH
Thank you for taking the survey. Please close this window.
PROGRAMMER: CODE ITEM AS 2244.
ASK: Respondents who are ineligible.
WPERMISS [IF WEB AND BLAGE<18]
(PARENT OR GUARDIAN PERMISSION FOR YOUTH INTERVIEW FOR WEB)
This text is to be read by the survey participant’s parent or guardian
The FDA Health and Media Study is designed to collect data from boys about their attitudes related to health, health behaviors, and advertisements they may have seen on TV, online, or heard on the radio.
If you recall, previously, your address was randomly chosen to take part in this study. [YOUTHFNAME] was selected to be in this study and has completed at least one survey to date. This study is being conducted again to measure what might have changed over time or what has stayed the same. We are asking your permission for your child’s participation in this next round of the survey.
Purpose of the Youth Survey
We want to interview your child about these topics again. The child’s answers, combined with the answers of other youth in the study, will improve our understanding of how public education campaigns affect youth. The answers may be shared with the FDA but not your child’s personal information.
The interview will last about 45 minutes, depending on responses.
Voluntary Participation
Your child’s participation in this study is completely voluntary. He can refuse to answer any or all questions. Your child has the right to stop the interview at any time.
Risks
There are no physical risks to your child from participating in this interview. It is possible that some questions might make your child mildly uncomfortable, depending on responses. No absolute guarantees can be made regarding the interception of data sent via the Internet. However, we are taking extensive precautions to protect the privacy of all data.
Future Contacts
There are no future contacts planned at this time.
Benefits
There are no direct benefits to your child from answering our questions. However, he will be contributing to important health research.
Compensation/Payment
Because your child’s contribution is important, we will offer your child a check for $25 if he completes the survey by [EARLY BIRD DATE] or a check for $20 after [EARLY BIRD DATE], as a token of appreciation for completing the survey. Your child will receive $20 in cash if he completes the survey in person.
Privacy
The survey answers will be entered into a computer and labeled with a case identification number. Your name and that of your child will not be reported with any information your child provides. Information your child provides will be combined with answers of many others and reported in a summary form. We will not share any information that your child gives us with you or with anyone outside the FDA and RTI research teams. All staff involved in this research are committed to privacy and have signed a Privacy Pledge.
Questions
If you have any questions about the study, you can call our project assistance line toll-free at (866) 214-2039, or email us at [email protected]. If you have any questions about your rights as a study participant, you may call the RTI Office of Research Protection at 1-866-214-2043 (a toll-free number).
If it is all right with you, please ask your child to answer the survey. Please give him privacy so he can answer the questions on his own. If you would rather he take the survey on a different device, he can log in on that device at the link on the study letter.
After you select your answer, please press “Next.”
1 Yes, I agree to allow my child to participate in this study. -> GO TO WASSENT
2 No, I do not want my child to participate in this study. -> GO TO PERMISREF.
DISPLAY: EARLY BIRD DATE is the date by which the respondents can receive a larger incentive for responding online. YOUTHFNAME is the respondent’s name.
ASK: Parents of minor respondents who are web respondents
PERMISREF [IF WPERMISS=2] Thank you for your time. Please close this window.
PROGRAMMER: CODE CASE AS REFUSAL.
EXIT
ASK: Parents who refuse to provide consent
WASSENT [IF WEB AND BLAGE<18] (Assent for Youth for WEB)
This screen is meant to be read by [YOUTHFNAME]
We are talking to boys in 30 cities across the United States. This study is sponsored by the U.S. Food and Drug Administration. You have previously taken part in this study by completing a survey. We are asking for you to participate again by completing this web survey.
The survey asks boys ages 11-19 about their attitudes related to health behaviors. The survey asks about advertisements they may have seen on TV or online. The advertisements may have also been heard on the radio. The survey will take about 45 minutes to complete. Up to 2,200 boys will take this survey. This survey is part of a research study being conducted by RTI International. There are no direct benefits for answering our questions but you will be helping with important health research.
Your parent or legal guardian has given permission for you to complete this survey.
Your name will be kept private. Your answers will be labeled with a number instead of your name. This makes it so only research staff will know these are your answers. Your answers may be shared with the FDA but not your personal information. We will not share any information you give us with your parents or anyone outside the FDA and RTI research teams. All of your answers will be kept private. It is not completely safe to send data through the Internet but we are doing everything we can to protect your data.
There are no physical risks but some questions might make you uncomfortable. If you don't want to take the survey, that is okay. If you don't want to answer a certain question, that is okay too. You may also choose to drop out of the survey at any time, for any reason and you may take a break at any time.
We will offer you a check for $25 if you take the survey before [EARLY BIRD DATE]. If you complete the survey after that we will offer you a check for $20. You will receive $20 in cash if you complete the survey in person.
You can call us if you have any questions about the study. The phone number is (866) 214-2039. You can also email us at [email protected]. You may also have questions about your rights as a study participant. For those questions call the RTI Office of Research Protection. Their phone number is (866) 214-2043. You can send them an email at [email protected].
After you select your answer, please press “Next.”
1 Yes, I agree to participate in this study. GO TO PRIV
2 No, I do not wish to participate in this study. GO TO ASSENTREF
DISPLAY: EARLY BIRD DATE is the date by which the respondents can receive a larger incentive for responding online. YOUTHFNAME is the respondent’s name.
ASK: Web respondents who are younger than 18 years old
ASSENTREF [IF WASSENT = 2] Thank you for your time. Please close this window.
PROGRAMMER: CODE CASE AS REFUSAL.
ASK: Respondents who refuse to provide consent
WCONSENT [IF WEB AND BLAGE>=18] (CONSENT FOR YOUTH 18 AND OVER FOR WEB)
This screen is meant to be read by [YOUTH FNAME]
We are talking to boys in 30 cities across the United States. This study is sponsored by the U.S. Food and Drug Administration. You have previously taken part in this study by completing at least one survey. In past surveys, your parent provided permission for you to participate. Now that you are 18 or older, we are asking for your consent to participate in this web survey.
The survey asks boys ages 11-19 about their attitudes related to health behaviors. The survey asks about advertisements they may have seen on TV or online. The advertisements may have also been heard on the radio. The survey will take about 45 minutes to complete. Up to 2,200 boys will take this survey. The survey is part of a research study being conducted by RTI International. There are no direct benefits for answering our questions but you will be helping with important health research.
Your name will be kept private. Your answers will be labeled with a number instead of your name. This makes it so only research staff will know these are your answers. Your answers may be shared with the FDA but not your personal information. We will not share any information you give us with your parents or anyone outside the FDA and RTI research teams. All of your answers will be kept private. It is not completely safe to send data through the Internet but we are doing everything we can to protect your data.
There are no physical risks but some questions might make you uncomfortable. If you don't want to take the survey, that is okay. If you don't want to answer a certain question, that is okay too. You may also choose to drop out of the survey at any time, for any reason and you may take a break at any time.
We will offer you a check for $25 if you complete the survey before [EARLY BIRD DATE]. If you complete the survey after that we will offer you a check for $20. You will receive $20 in cash if you complete the survey in person.
You can call us if you have any questions about the study. The phone number is (866) 214-2039. You can also email us at [email protected]. You may also have questions about your rights as a study participant. For those questions call the RTI Office of Research Protection. Their phone number is (866) 214-2043. You can send them an email at [email protected].
After you select your answer, please press “Next.”
1 Yes, I agree to participate in this study. – GO TO PRIV
2 No, I do not wish to participate in this study. – GO TO CONSENTREF
DISPLAY: EARLY BIRD DATE is the date by which the respondents can receive a larger incentive for responding online. YOUTHFNAME is the respondent’s name.
ASK: Web respondents who are 18 years old or older
CONSENTREF [IF WCONSENT = 2] Thank you for your time.
PROGRAMMER: CODE CASE AS REFUSAL.
ASK: Web respondents who are 18 years old and older and who refuse to provide consent
PRIV [IF WEB] Please make sure that you can answer the questions in private where no one can see your answers.
NEXT
ASK: Web respondents
DRIV [IF WEB] Do not answer the questions while driving.
NEXT
ASK: Web respondents
TUTOR3 [IF WEB] Please click on the answer to each survey question, using a mouse or a touchscreen. If you skip a question, an option for “Prefer not to answer” will appear. You can use that option if you don’t know the answer to a question, or if you choose to not answer a question. Remember that your answers will be kept private. We will not share the answers to your questions with your parents or anyone else outside the research team.
NEXT
ASK: Web respondents
FIID
[DISPLAY DATE OF INSTRUMENT RELEASE]
[IF CAPI] ENTER YOUR FIID. [6 NUMERIC DIGIT ENTRY]
Programmer: allow 6 digits in entry field.
ASK: Interviewers of CAPI surveys
DISPLAY: The date that the instrument version was released will be displayed on the screen to serve as a version number for the CAPI instrument.
CID [IF CAPI AND BLAGE<18]
Our records indicate that [YouthFname] previously participated in our study. Before we begin with the interview, we need the parent or guardian of [YouthFname] to review some information. Are you the parent or guardian of [YouthFname]?
Yes -> GO TO PERMISS
No -> GO TO CIDFP
ASK: All CAPI respondents.
DISPLAY: YouthFname is the respondent’s first name.
CIDFP [IF CAPI AND CID=NO AND BLAGE<18]
Is [YouthFname]’s parent available to review this information?
YES, PARENT IS AVAILABLE GO TO PERMISS
NO, THEY ARE NOT AVAILABLE STAY ON THIS SCREEN
INTERVIEWER: BREAK OFF THE INTERVIEWER AND SCHEDULE A TIME TO RETURN FOR WHEN PARENTS WILL BE AVAILABLE. REMIND HOUSEHOLD ABOUT WEB OPTION FOR COMPLETION.
PROGRAMMER: CODE AS A 1231
Previous
ASK: CAPI respondents who indicate that the parent is not available in CID
DISPLAY: YouthFname is the respondent’s first name.
CID18 [IF CAPI AND BLAGE>=18]
Our records indicate that [YouthFname] previously participated in our study. Are you [YouthFname]?
YES -> GO TO CONSENT
NO -> GO TO CIDFP18
ASK: All CAPI respondents who are 18 years old or older
DISPLAY: YouthFname is the respondent’s first name.
CIDFP18 [IF CAPI AND CID18=NO AND BLAGE>=18]
Is [YouthFname] available to begin the interview?
YES, HE IS AVAILABLE GO TO CONSENT
NO, HE IS NOT AVAILABLE STAY ON THIS SCREEN
INTERVIEWER: BREAK OFF THE INTERVIEWER AND SCHEDULE A TIME TO RETURN FOR WHEN HE IS AVAILABLE. REMIND HOUSEHOLD ABOUT WEB OPTION FOR COMPLETION.
Previous Next
ASK: CAPI respondents who indicate that the respondent is not available in CID18
DISPLAY: YouthFname is the respondent’s first name.
PERMISS [IF CAPI AND BLAGE<18]
(PARENT OR GUARDIAN PERMISSION FOR YOUTH INTERVIEW)
INTERVIEWER: HAND PINK PERMISSION FORM TO PARENT
The FDA Health and Media Study is designed to collect data from boys about their attitudes related to health, health behaviors, and advertisements they may have seen on TV, online, or heard on the radio.
If you recall, previously, your address was randomly chosen to take part in this study. [YOUTHFNAME] was selected to be in this study and has completed at least one survey to date. This study is being conducted again to measure what might have changed over time or what has stayed the same. We are asking your permission for your child’s participation in this next round of the survey.
Purpose of the Youth Survey
We want to interview your child about these topics again. The child’s answers, combined with the answers of other youth in the study, will improve our understanding of how public education campaigns affect youth. The answers may be shared with the FDA but not your child’s personal information.
Types of Questions for Youth
The interview will last about 45 minutes, depending on responses. The interviewer will ask the first few questions and then youth respondents will answer questions directly into a laptop. The interviews will be completed in a part of the household that allows your child to answer in private.
Voluntary Participation
Your child’s participation in this study is completely voluntary. He can refuse to answer any or all questions. Your child has the right to stop the interview at any time.
Risks
There are no physical risks to your child from participating in this interview. It is possible that some questions might make your child mildly uncomfortable, depending on responses. No absolute guarantees can be made regarding the interception of data sent via the Internet. However, we are taking extensive precautions to protect the privacy of all data.
Future Contacts
There are no future contacts planned at this time.
Benefits
There are no direct benefits to your child from answering our questions. However, he will be contributing to important health research.
Compensation/Payment
 Because
your child’s contribution is important, we will offer your
child $20 in cash as a token of appreciation for completing the
survey.
Because
your child’s contribution is important, we will offer your
child $20 in cash as a token of appreciation for completing the
survey.
Privacy
The survey answers will be entered into a computer and labeled with a case identification number. Your name and that of your child will not be reported with any information your child provides. Information your child provides will be combined with answers of many others and reported in a summary form. We will not share any information that your child gives us with you or with anyone outside the FDA and RTI research teams. All staff involved in this research are committed to privacy and have signed a Privacy Pledge.
Questions
If you have any questions about the study, you can call our project assistance line toll-free at (866) 214-2039, or email us at [email protected]. If you have any questions about your rights as a study participant, you may call the RTI Office of Research Protection at 1-866-214-2043 (a toll-free number).
You will be given a copy of this consent form to keep.
Do you give him permission to participate in the survey?
YES - GO TO ASSENT
NO -> GO TO CAPIREF
ASK: Parents of minor CAPI respondents
CAPIREF [IF PERMISS=REFUSE] Thank you for your time.
PROGRAMMER: CODE AS A REFUSAL.
ASSENT [IF CAPI AND BLAGE<18]
INTERVIEWER: HAND BLUE ASSENT FORM TO RESPONDENT
We are talking to boys in 30 cities across the United States. The study is sponsored by the U.S. Food and Drug Administration. You have previously taken part in this study by completing a survey. We are asking for you to participate again by completing this survey.
The survey asks boys ages 11-19 about their attitudes related to health behaviors. The survey asks about advertisements they may have seen on TV or online. The advertisements may have also been heard on the radio. The survey will take about 45 minutes to complete. Up to 2,200 boys will take this survey. The survey is part of a research study being conducted by RTI International. There are no direct benefits for answering our questions but you will be helping with important health research.
Your parent or guardian has given permission for you to complete this survey.
Your name will be kept private. Your answers will be labeled with a number instead of your name. This makes it so only research staff will know these are your answers. Your answers may be shared with the FDA but not your personal information. We will not share any information you give us with your parents or anyone outside the FDA or RTI research teams. All of your answers will be kept private. It is not completely safe to send data through the Internet but we are doing everything we can to protect your data.
There are no physical risks but some questions might make you uncomfortable. If you don't want to take the survey, that is okay. If you don't want to answer a certain question, that is okay too. You may also choose to drop out of the survey at any time, for any reason and you may take a break at any time.
We will offer you $20 in cash to thank you for taking time to complete the survey.
You can call us if you have questions about the study. The phone number is (866) 214-2039. You can also email us at [email protected]. You may also have questions about your rights as a study participant. For those questions call the RTI Office of Research Protection. Their phone number is (866) 214-2043. You can send them an email at [email protected].
Do you agree to take this survey?
YES -> GO TO OMB
NO -> GO TO CAPIREF2
[PROGRAMMER: IF ITEM IS LEFT BLANK, HARD PROMPT “Interviewer, this is a required item. Please do your best to fill out the item.”]
ASK: Minor CAPI respondents
CAPIREF2 [IF ASSENT=NO] Thank you for your time.
PROGRAMMER: CODE AS REFUSAL
ASK: CAPI minor respondents who refuse assent
CONSENT [IF CAPI AND BLAGE>=18]
(CONSENT FOR YOUTH 18 AND OVER FOR CAPI)
INTERVIEWER: HAND YELLOW CONSENT FORM TO RESPONDENT
We are talking to boys in 30 cities across the United States. The study is sponsored by the U.S. Food and Drug Administration. You have previously taken part in this study by completing at least one survey. In past surveys, your parent provided permission for you to participate. Now that you are 18 or older, we are asking for your consent to participate in this survey.
The survey asks boys ages 11-19 about their attitudes related to health behaviors. The survey asks about advertisements they may have seen on TV or online. The advertisements may have also been heard on the radio. The survey will take about 45 minutes to complete. Up to 2,200 boys will take this survey. The survey is part of a research study being conducted by RTI International. There are no direct benefits for answering our questions but you will be helping with important health research.
Your name will be kept private. Your answers will be labeled with a number instead of your name. This makes it so only research staff will know these are your answers. Your answers may be shared with the FDA but not your personal information. We will not share any information you give us with your parents or anyone outside the FDA and RTI research teams. All of your answers will be kept private. It is not completely safe to send data through the Internet but we are doing everything we can to protect your data.
There are no physical risks but some questions might make you uncomfortable. If you don't want to take the survey, that is okay. If you don't want to answer a certain question, that is okay too. You may also choose to drop out of the survey at any time, for any reason and you may take a break at any time.
We will offer you $20 in cash to thank you for taking time to complete the survey.
You can call us if you have questions about the study. The phone number is (866) 214-2039. You can also email us at [email protected]. You may also have questions about your rights as a study participant. For those questions call the RTI Office of Research Protection. Their phone number is (866) 214-2043. You can send them an email at [email protected].
Do you agree to take this survey?
YES -> GO TO OMB
NO -> GO TO CAPIREF3
[PROGRAMMER: IF ITEM IS LEFT BLANK, HARD PROMPT “INTERVIEWER, THIS IS A REQUIRED ITEM. PLEASE DO YOUR BEST TO FILL OUT THE ITEM.”]
ASK: CAPI Respondents who are 18 years old or older
CAPIREF3 [IF CONSENT=NO] Thank you for your time.
PROGRAMMER: CODE AS REFUSAL
ASK: CAPI respondents 18 years old and older who refuse consent
OMB [IF CAPI] [OPTIONAL. READ TEXT ONLY AS NECESSARY]
OMB No: 0910-0753 Expiration Date: 09/30/2019
Paperwork Reduction Act Statement: The public reporting burden for this collection of information has been estimated to average 45 minutes per response. Send comments regarding this burden estimate or any other aspects of this collection of information, including suggestions for reducing burden to [email protected].
NEXT
ASK: Optional question for CAPI respondents
Y_ACINTRO [IF CAPI]
Now I'd like you to read the questions and enter your answers into the laptop yourself. This will allow you to answer the questions in complete privacy. I will not be able to see the answers you type into the computer. If you skip a question, an option for “Prefer not to answer” will appear. You can use that option if you don’t know the answer to a question, or if you choose to not answer a question. Let me explain how to use the laptop.
MOVE COMPUTER SO YOUTH CAN USE IT AND POINT OUT THE FOLLOWING:
- NUMBER KEYS
- MOUSE TO CLICK RESPONSES AND NEXT
CAUTION RESPONDENT ABOUT ON/OFF SWITCH.
WHEN RESPONDENT IS READY, SELECT THE CONTINUE RESPONSE OPTION AND CLICK THE “NEXT” BUTTON.
INTERVIEWER: HAND LAPTOP TO RESPONDENT
Continue
Next
ASK: CAPI Respondents
Y_AC1 [IF CAPI]
The next questions are for practice. You will answer these questions on your own. Select the “Continue” response option and click the “Next ” button to see the first question.
Continue
Next
ASK: CAPI Respondents
Y_AC2a [IF CAPI]
In which state do you currently live?
1 Alabama
2 Alaska
3 Arizona
4 Arkansas
5 California
6 Colorado
7 Connecticut
8 Delaware
9 Florida
10 Georgia
11 Hawaii
12 Idaho
13 Illinois
14 Indiana
15 Iowa
16 Kansas
17 Kentucky
18 Louisiana
19 Maine
20 Maryland
21 Massachusetts
22 Michigan
23 Minnesota
24 Mississippi
25 Missouri
26 Montana
27 Nebraska
28 Nevada
29 New Hampshire
30 New Jersey
31 New Mexico
32 New York
33 North Carolina
34 North Dakota
35 Ohio
36 Pennsylvania
37 Rhode Island
38 South Carolina
39 Rhode Island
40 South Carolina
41 South Dakota
42 Tennessee
43 Texas
44 Utah
45 Vermont
46 Virginia
47 Washington
48 West Virginia
49 Wisconsin
50 Wyoming
51 District of Columbia
ASK: CAPI Respondents
Y_AC3a [IF CAPI]
You have recorded that you live in [Y_AC2a]. Is this correct?
1 Yes -> GO TO Y_AC4a
2 No -> GO TO Y_C2a
9 Prefer not to answer
PROGRAMMER: IF Y_AC3a=2 OR PNTA DISPLAY A MESSAGE: ‘PLEASE RE-ENTER YOUR ANSWER’ AND RETURN TO Y_C2A
ASK: CAPI Respondents
DISPLAY: Answer from Y-AC2a
Y_AC4a [IF CAPI]
How many times in the past 6 months did you go to the movies?
1 1 time
2 2 times
3 3 times
4 4 times
5 5 or more times
8 I have not done this in the past 6 months
9 Prefer not to answer
ASK: CAPI Respondents
Y_AC5a [IF CAPI]
In the last 30 days, on how many days did you eat ice cream?
_______________ (Range: 0-30)
Prefer not to answer
ASK: CAPI Respondents
Y_AC6 [IF CAPI]
Thank you. If you have any questions about how to use the laptop, please feel free to ask your interviewer.
Please select the “Continue” response option and click the “Next >” button to begin the next portion of the interview.
Continue
NEXT
ASK: CAPI Respondents
Y_video [IF WEB] Please try to view this video to make sure you can see it.
[DISPLAY TEST VIDEO]
Are you able to view this video?
Yes -> GO TO DOB
No - > GO TO EXIT
IF Y_video IS NO (=2), display this message:
Viewing the videos in this survey is important. Try logging into the survey using a different computer or browser. If that doesn’t work, you will not be able to take the survey online.
EXIT
PROGRAMMER: CODE AS BREAKOFF
PROGRAMMER: IF NO, need to begin with the viewing of the Y_VIDEO when the R comes back to the survey from a different device.
ASK: Web respondents
Section A: Demographic Items
DOB: THIS QUESTION SHOULD BE COMPLETED BY [YOUTHFNAME]
What is your date of birth?
Month: __________________ Day: ______________ Year: ___________
Confirm your date of birth.
Month: __________________ Day: ______________ Year: ___________
PROGRAMMER:
PROGRAM DROP DOWN LISTS WITH MONTH, DAY AND YEAR. YEAR SHOULD RANGE FROM 1995 – 2007. SPELL OUT MONTHS IN FULL.
Please make sure that no invalid dates appear. That is Feb 30, Nov 31, etc. cannot be valid. Do not allow future dates. If the date is not valid, please display a hard error, “Please enter a valid date.” THIS ITEM SHOULD BE A REQUIRED ITEM. Use double entry verification. Both dates must match. If dates do not match, display a hard error, “Entries do not match. Please try again.”
DO NOT ALLOW MISSING DATA FOR THIS ITEM.
CALCULATE FILLAGE= (DATE TODAY)- DOB
ASK: All respondents
CHECKPOINT: PROGRAMMER: CHECK DOB AGAINST FU2DOB. IF DOB MATCHES FU2DOB, GO TO BINTRO. IF THE DOBS DO NOT MATCH, CHECK AGAINST FU1_DOB. IF DOB AND FU1_DOB MATCH,GO TO BINTRO. IF THE DOBS DO NOT MATCH, CHECK AGAINST BLDOB. IF DOB MATCHES BLDOB, GO TO BINTRO. IF BLDOB AND FU1_DOB AND FU2DOB ARE BLANK GO TO BINTRO. IF THOSE DOBS DO NOT MATCH, GO TO DOB2.
DOB2 [IF (BLDOB OR FU1_DOB OR FU2 DOB NE DOB) AND (BLDOB AND FU1_DOB AND FU2 DOB ARE NOT BLANK)] So that we can ask you the right questions, we need your correct age. Again, what is your date of birth?
__________________MM/ DD/ YYYY
Confirm your date of birth.
__________________MM/ DD/ YYYY
PROGRAMMER: USE DROP DOWN MENU. Only allow 1-12 in MM, 1-31 in DD. Please make sure that no invalid dates appear. That is Feb 30, Nov 31, etc. cannot be valid. Do not allow future dates. If the date is not valid, please display a hard error, “Please enter a valid date.” THIS ITEM SHOULD BE A REQUIRED ITEM. Use double entry verification. Both dates must match. If dates do not match, display a hard error, “Entries do not match. Please try again.”
DO NOT ALLOW MISSING DATA FOR THIS ITEM.
CALCULATE FILLAGE= (DATE TODAY)- DOB2
ASK: Respondents who enter a birthday in DOB that doesn’t match their entry from either baseline or follow up 1
CHECKPOINT
CHECKPOINT: PROGRAMMER: CHECK DOB2 AGAINST FU2_DOB. IF THE TWO DOBS MATCH,GO TO BINTRO. IF THE DOBS DO NOT MATCH, CHECK AGAINST FU1_DOB. IF DOB2 AND FU1_DOB MATCH,GO TO BINTRO. IF THOSE DOBS DO NOT MATCH, CHECK AGAINST BASELINE DOB. IF THOSE DOBS DO NOT MATCH AND WEB, GO TO DOBINELIG
_______________________________________________________________
DOBINELIG [IF WEB AND (DOB2 NE BLDOB OR FU1_DOB OR FU2_DOB) CHECKPOINT We’re sorry, but we are not able to locate your file in our records. For this reason, you will not be able to take this survey online at this time. Please call 866-214-2039 with any questions.
Thank you for your time.
PROGRAMMER: EXIT PROGRAM AND CODE AS INELIGIBLE
[IF CAPI AGE IS INCONSISTENT, ALLOW RESPONSES AND CONTINUE]
ASK: Respondents who entered a birthday that doesn’t match any of the birthdays from prior waves
Section B: Tobacco Use Behavior
Cigarette Use
PROGRAMMER: PLEASE RANDOMIZE THE ORDER IN WHICH THE TOBACCO QUESTIONS ARE PRESENTED IN EACH SECTION (E.G., CIGARETTE QUESTIONS IN SECTION B WOULD COME FIRST SOMETIMES WHILE OTHER TIMES, SMOKELESS TOBACCO QUESTIONS WOULD COME FIRST).
THE FOLLOWING ARE ITEMS THAT GO TOGETHER:
B1- B4 == cigarettes
B5- B9 == smokeless tobacco products
B10- B11== cigars
B12- B13 == hookah
B14- B16 == e-cigs
Please randomize without breaking the blocks of items that go together sequentially.
B_INTRO. The next section asks about your experiences with tobacco products.
NEXT
ASK: All respondents
Checkpoint: If BLB1 = 1 OR FU1B1 = 1 OR FU2B1=1 OR FU3B1=1 then go to B2. Else Ask B1.
B1. Have you ever tried cigarette smoking, even one or two puffs?
1 Yes -> GO TO B2
2 No -> GO TO NEXT RANDOM BLOCK IN SECTION B, OR IF THE LAST RANDOM BLOCK GO TO SECTION C
9 Prefer not to answer -> GO TO B2
PROGRAMMER: STARTING WITH THIS ITEM DISPLAY “Prefer not to answer” AFTER RESPONDENT TRIES TO SKIP QUESTION.
ASK: Respondents who did not report ever trying cigarette smoking at the follow-up3 or follow-up 2 or follow-up 1 or baseline interviews.
CHECKPOINT: If B1 = 2 then go to the next random block in section B, or if the last random block go to section C. Else Ask B2 through B4.
B2. [IF BLB1=1 OR FU1B1 = 1 OR FU2B1=1 OR FU3B1=1] Previously, you reported that you have tried cigarette smoking.
[IF B1=1 or 9 OR BLB1=1 OR FU1B1 = 1 OR FU2B1=1 OR FU3B1=1] During the past 30 days, on how many days did you smoke cigarettes?
1 0 days
2 1 or 2 days
3 3 to 5 days
4 6 to 9 days
5 10 to 19 days
6 20 to 29 days
7 All 30 days
9 Prefer not to answer
ASK: Respondents who reported trying cigarette smoking or did not answer the question about trying cigarette smoking in B1 today or who reported smoking in previous interviews. Respondents who report smoking at the follow-up3 or follow-up 2 or follow-up 1 or baseline interviews receive an additional introductory sentence for flow.
B3. [IF (BLB1=1 OR FU1B1=1 OR FU2B1=1 OR FU3B1=1 OR B1=1 OR 9) AND B2 NE 1] During the past 30 days, on the days you smoked, how many cigarettes did you smoke per day?
1 Less than 1 cigarette per day
2 1 cigarette per day
3 2 to 5 cigarettes per day
4 6 to 10 cigarettes per day
5 11 to 20 cigarettes per day
6 More than 20 cigarettes per day
9 Prefer not to answer
ASK: Respondents who reported trying cigarette smoking or did not answer the question about trying cigarette smoking in B1 today or who reported smoking in previous interviews and who reported smoking 1 or more of the past 30 days or did not answer the question about how many of the past 30 days they had smoked in B2 today.
B4. [IF (BLB1=1 OR FU1B1=1 OR FU2B1=1 OR FU3B1=1 OR B1=1 OR 9)] About how many cigarettes have you smoked in your entire life? Your best guess is fine.
1 0 cigarettes
2 1 or more puffs but never a whole cigarette
3 1 cigarette
4 2 to 5 cigarettes
5 6 to 15 cigarettes (about 1/2 a pack total)
6 16 to 25 cigarettes (about 1 pack total)
7 26 to 99 cigarettes (more than 1 pack, but less than 5 packs)
8 100 or more cigarettes (5 or more packs)
9 Prefer not to answer
ASK: Respondents who reported trying cigarette smoking or did not answer the question about trying cigarette smoking in B1 today or who report trying cigarettes at the follow-up 3 or follow-up 2 or follow-up 1 or baseline interviews.
Other Tobacco Product Use
Checkpoint: If BLB5 = 1 OR FU1B5 = 1 OR FU2B5=1 OR FU3B5=1 then go to B6. Else Ask B5.
B5. The next questions are about smokeless tobacco, such as dip, chewing tobacco, snuff, or snus. Common brands include Copenhagen, Grizzly, Skoal, Camel Snus, Kodiak, and Longhorn.

Have you ever used smokeless tobacco even just a small amount?
1 Yes -> GO TO B6
2 No -> GO TO NEXT RANDOM BLOCK IN SECTION B, OR IF THE LAST RANDOM BLOCK GO TO SECTION C
9 Prefer not to answer -> GO TO B6
ASK: Respondents who reported never having used smokeless tobacco or did not answer the question about using smokeless tobacco in B5 at the previous interviews.
CHECKPOINT: If B5 = 2 go to the next random block in section B, or if the last random block go to section C. Else Ask B6.
B6. [IF BLB5=1 OR FU1B5=1 OR FU2B5=1 OR FU3B5=1 OR (B5=1 OR 9)] During the past 30 days, on how many days did you use smokeless tobacco?
1 0 days
2 1 or 2 days
3 3 to 5 days
4 6 to 9 days
5 10 to 19 days
6 20 to 29 days or
7 All 30 days
9 Prefer not to answer
ASK: Respondents who reported having used smokeless tobacco in any of the previous interviews or did not answer the question about using smokeless tobacco in B5 today.
B7. [IF BLB5=1 OR FU1B5=1 OR FU2B5=1 OR FU3B5=1 OR (B5=1 OR 9)] How many times have you used smokeless tobacco in your entire life?
1 1 time
2 2 to 10 times
3 11 to 20 times
4 21 to 50 times
5 51 to 99 times
6 100 or more times
9 Prefer not to answer
ASK: Respondents who reported having used smokeless tobacco in any of the previous interviews or did not answer the question about using smokeless tobacco in B5 today.
B8. [IF BLB5=1 OR FU1B5=1 OR FU2B5=1 OR FU3B5=1 OR (B5=1 OR 9)] How often do you swallow smokeless tobacco juices?
1 Always
2 Sometimes
3 Rarely
4 Never
9 Prefer not to answer
ASK: Respondents who reported having used smokeless tobacco in any of the previous interviews or did not answer the question about using smokeless tobacco in B5 today.
B9. [IF BLB5=1 OR FU1B5=1 OR FU2B5=1 OR FU3B5=1 OR (B5=1 OR 9)] How soon after you wake up do you use smokeless tobacco?
1 Within 5 minutes
2 6 to 30 minutes
3 31 to 60 minutes
4 More than 60 minutes
9 Prefer not to answer
ASK: Respondents who reported having used smokeless tobacco in any of the previous interviews or did not answer the question about using smokeless tobacco in B5 today.
Checkpoint: If BLB10 = 1 OR FU1B10 = 1 OR FU2B10=1 OR FU3B10=1 then go to the next random block in section B, or if the last random block go to section C. Else Ask B10.
B10. The next questions are about cigars, cigarillos, or little cigars such as Black & Mild, Swisher Sweets, Dutch Masters, Phillies Blunts, Prime Time, and Winchester.

Have you ever smoked cigars, cigarillos, or little cigars even one time?
1 Yes -> GO TO B11
2 No -> GO TO NEXT RANDOM BLOCK IN SECTION B, OR IF THE LAST RANDOM BLOCK GO TO SECTION C
9 Prefer not to answer –> GO TO B11
ASK: Respondents who reported never smoking cigars, cigarillos, or little cigars or did not answer the question about smoking cigars, cigarillos, or little cigars in B10 at the previous interviews.
CHECKPOINT: If B10 = 2 then go to the next random block in section B, or if the last random block go to section C. Else Ask B11.
B11. [IF BLB10=1 OR FU1B10=1 OR FU2B10=1 OR FU3B10=1 OR (B10=1 OR 9)] During the past 30 days, on how many days did you smoke cigars, cigarillos, or little cigars?
1 0 days
2 1 or 2 days
3 3 to 5 days
4 6 to 9 days
5 10 to 19 days
6 20 to 29 days or
7 All 30 days
9 Prefer not to answer
ASK: Respondents who reported smoking cigars, cigarillos, or little cigars in any of the three interviews or did not answer the question about smoking cigars, cigarillos, or little cigars in B10 today.
Checkpoint: If BLB12 = 1 OR FU1B12 = 1 OR FU2B12=1 OR FU3B12=1 then go to the next random block in section B, or if the last random block go to section C. Else Ask B12.
B12. Have you ever tried smoking tobacco out of a water pipe (also called “hookah”), even one time?

1 Yes -> GO TO B13
2 No - > GO TO NEXT RANDOM BLOCK IN SECTION B, OR IF THE LAST RANDOM BLOCK GO TO SECTION C
9 Prefer not to answer ->GO TO B13
ASK: Respondents who reported never having tried smoking tobacco out of a water pipe or did not answer the question about trying smoking tobacco out of a water pipe in B12 at the previous interviews.
CHECKPOINT: If B12 = 2 then go to the next random block in section B, or if the last random block go to section C. Else Ask B13.
B13. [IF BLB12=1 OR FU1B12=1 OR FU2B12=1 OR FU3B12=1 OR (B12= 1 OR 9)]During the past 30 days, on how many days did you smoke tobacco out of a water pipe (also called “hookah”)?
1 0 days
2 1 or 2 days
3 3 to 5 days
4 6 to 9 days
5 10 to 19 days
6 20 to 29 days or
7 All 30 days
9 Prefer not to answer
ASK: Respondents who reported having tried smoking tobacco out of a water pipe in any of the three interviews or did not answer the question about using smokeless tobacco in B12 today.
Checkpoint: If BLB14 = 1 OR FU1B14 = 1 OR FU2B14=1 OR FU3B14=1 then go to the next random block in section B, or if the last random block go to section C. Else Ask B14.
B14. The next questions are about e-cigarettes (e-cigs), e-hookahs, vape pens, hookah pens and personal vaporizers.
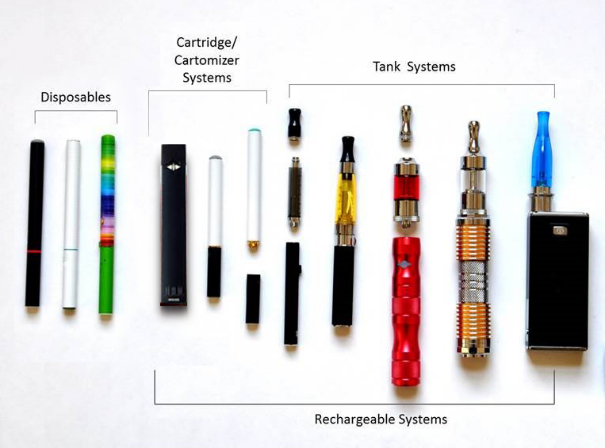
Have you ever tried any e-cigs or vape pens, even one time?
1 Yes –> GO TO B15
2 No -> GO TO NEXT RANDOM BLOCK IN SECTION B, OR IF THE LAST RANDOM BLOCK GO TO SECTION C
9 Prefer not to answer –> GO TO B15
ASK: Respondents who reported never having tried any e-cigs or vape pens or did not answer the question about trying any e-cigs or vape pens at the follow-up 1 or baseline interviews.
CHECKPOINT: If B14 = 2 then go to the next random block in section B, or if the last random block go to section C. Else Ask B15 and B16.
B15. [IF BLB14=1 OR FU1B14=1 OR FU2B14=1 OR FU3B14=1 OR (B14= 1 OR 9)]During the past 30 days, on how many days did you use e-cigarettes?
1 0 days
2 1 or 2 days
3 3 to 5 days
4 6 to 9 days
5 10 to 19 days
6 20 to 29 days or
7 All 30 days
9 Prefer not to answer
ASK: Respondents who reported having ever tried any e-cigs or vape pens in any of the three interviews or did not answer the question about ever trying any e-cigs or vape pens in B14 today
B16. [IF BLB14=1 OR FU1B14=1 OR FU2B14=1 OR FU3B14=1 OR (B14= 1 OR 9)] Does the e-cig you usually use contain nicotine?
1 Yes
2 No
3 Don’t Know
9 Prefer not to answer
ASK: Respondents who reported having ever tried any e-cigs or vape pens in any of the three interviews or did not answer the question about ever trying any e-cigs or vape pens in B14 today
Section C: Tobacco Use Intentions and Self-Efficacy
PROGRAMMER: RANDOMIZE THE ORDERING OF THE SETS C1, C2, AND C3.
C1. Thinking about the future…
|
|
Definitely Yes |
Probably Yes |
Probably Not |
Definitely Not |
Prefer Not to Answer |
C1_1. |
Do you think that you will smoke a cigarette soon? |
1 |
2 |
3 |
4 |
9 |
C1_2. |
Do you think you will smoke a cigarette at any time in the next year? |
1 |
2 |
3 |
4 |
9 |
C1_3. |
If one of your best friends were to offer you a cigarette, would you smoke it? |
1 |
2 |
3 |
4 |
9 |
C1_4. |
Have you ever been curious about smoking cigarettes? |
1 |
2 |
3 |
4 |
9 |
ASK: All respondents
C2. Thinking about the future…
|
|
Definitely Yes |
Probably Yes |
Probably Not |
Definitely Not |
Prefer Not to Answer |
C2_1. |
Do you think that you will use smokeless tobacco soon? |
1 |
2 |
3 |
4 |
9 |
C2_2. |
Do you think you will use smokeless tobacco at any time in the next year? |
1 |
2 |
3 |
4 |
9 |
C2_3. |
If one of your best friends were to offer you smokeless tobacco would you use it? |
1 |
2 |
3 |
4 |
9 |
C2_4 |
Have you ever been curious about using smokeless tobacco? |
1 |
2 |
3 |
4 |
9 |
ASK: All respondents
C3. Thinking about the future…
|
|
Definitely Yes |
Probably Yes |
Probably Not |
Definitely Not |
Prefer Not to Answer |
C3_1. |
Do you think that you will use an e-cig soon? |
1 |
2 |
3 |
4 |
9 |
C3_2. |
Do you think you will use an e-cig at any time in the next year? |
1 |
2 |
3 |
4 |
9 |
C3_3. |
If one of your best friends were to offer you an e-cig, would you use it? |
1 |
2 |
3 |
4 |
9 |
C3_4. |
Have you ever been curious about using e-cigs? |
1 |
2 |
3 |
4 |
9 |
ASK: All respondents
C4. How sure are you that, if you really wanted to, you could say no to smokeless tobacco, if…
PROGRAMMER: RANDOMIZE C4_1-C4_3
|
Not at all sure |
Slightly sure |
Somewhat sure |
Mostly sure |
Completely sure |
Prefer Not to Answer |
C4_1. You are hanging out where most people are using it? |
1 |
2 |
3 |
4 |
5 |
9 |
C4_2. A friend offers it? |
1 |
2 |
3 |
4 |
5 |
9 |
C4_3. A family member offers it? |
1 |
2 |
3 |
4 |
5 |
9 |
ASK: All respondents
Section D: Cessation (Intention, Behavior, Motivation)
PROGRAMMER: RANDOMIZE THE SET:
D1-D2 ==CIGARETTES
D3-D4 == SMOKELESS TOBACCO
Cigarette Use
D1. [IF B2=2-7 OR 9]
During the past 3 months, did you stop smoking cigarettes for one day or longer because you were trying to quit smoking cigarettes for good?
1 Yes
2 No
9 Prefer not to answer
ASK: Respondents who report smoking more than 0 days in the past 30 days in B2, or who report that they don’t know how many days they smoked in the past 30 days.
D2. [IF D1 NE BLANK]
How much do you want to stop smoking?
1 Not at all
2 A little
3 Somewhat
4 A lot
9 Prefer not to answer
ASK: Respondents who report smoking more than 0 days in the past 30 days in B2, or who report that they don’t know how many days they smoked in the past 30 days.
Other Tobacco Use
D3. [IF B6=2-7 OR 9]
During the past 3 months, did you stop using smokeless tobacco for one day or longer because you were trying to quit using smokeless tobacco for good?
1 Yes
2 No
9 Prefer not to answer
ASK: Respondents who report using smokeless tobacco more than 0 days in the past 30 days in B6, or who report that they don’t know how many days they used smokeless tobacco in the past 30.
D4. [IF D3 NE BLANK]
How much do you want to stop using smokeless tobacco?
1 Not at all
2 A little
3 Somewhat
4 A lot
9 Prefer not to answer
ASK: Respondents who report using smokeless tobacco more than 0 days in the past 30 days in B6, or who report that they don’t know how many days they used smokeless tobacco in the past 30.
Section E: Attitudes, Beliefs & Risk Perceptions, Social Norm
EINTRO. The next set of questions asks for your opinions on cigarette use and other tobacco products.
NEXT
ASK: All respondents
E1. Smoking cigarettes is…
PROGRAMMER: RANDOMIZE E1_1-E1_3
PROGRAMMER: DISPLAY ITEM LIKE THAT BELOW. R WILL CLICK BUTTON TO ENTER RESPONSE. FIND A WAY TO DISPLAY A PREFER NOT TO ANSWER.
E1_1. |
Bad |
|
|
|
|
|
|
|
Good |
E1_2. |
Unenjoyable |
|
|
|
|
|
|
|
Enjoyable |
E1_3. |
Harmful |
|
|
|
|
|
|
|
Not Harmful |
ASK: All respondents
E2. Using smokeless tobacco is…
PROGRAMMER: RANDOMIZE E2_1-E2_3
PROGRAMMER: DISPLAY ITEM LIKE THAT BELOW. R WILL CLICK BUTTON TO ENTER RESPONSE. FIND A WAY TO DISPLAY A PREFER NOT TO ANSWER.
E2_1. |
Bad |
|
|
|
|
|
|
|
Good |
E2_2. |
Unenjoyable |
|
|
|
|
|
|
|
Enjoyable |
E2_3. |
Harmful |
|
|
|
|
|
|
|
Not Harmful |
ASK: All respondents
E3. Using e-cigs or vape pens is…
PROGRAMMER: RANDOMIZE E3_1-E3_3
DISPLAY ITEM LIKE THAT BELOW. R WILL CLICK BUTTON TO ENTER RESPONSE. FIND A WAY TO DISPLAY A PREFER NOT TO ANSWER.
E3_1. |
Bad |
|
|
|
|
|
|
|
Good |
E3_2. |
Unenjoyable |
|
|
|
|
|
|
|
Enjoyable |
E3_3. |
Harmful |
|
|
|
|
|
|
|
Not Harmful |
ASK: All respondents
E4. How much do you agree or disagree with the following statements? If I use smokeless tobacco, I will…
PROGRAMMER: RANDOMIZE E4_1- E4_9
|
|
Strongly Disagree |
Disagree |
Neither Agree or Disagree
|
Agree |
Strongly Agree |
Prefer Not to Answer |
E4_1. |
Damage my body |
1 |
2 |
3 |
4 |
5 |
9 |
E4_2. |
Be controlled by smokeless tobacco |
1 |
2 |
3 |
4 |
5 |
9 |
E4_3. |
Be more attractive |
1 |
2 |
3 |
4 |
5 |
9 |
E4_4. |
Develop cancer of the lip, mouth, tongue or throat |
1 |
2 |
3 |
4 |
5 |
9 |
E4_5. |
Develop sexual and/or fertility problems |
1 |
2 |
3 |
4 |
5 |
9 |
E4_6. |
Fit in |
1 |
2 |
3 |
4 |
5 |
9 |
E4_7. |
Be unable to stop when I want to |
1 |
2 |
3 |
4 |
5 |
9 |
E4_8. |
Lose my teeth |
1 |
2 |
3 |
4 |
5 |
9 |
E4_9. |
Shorten my life |
1 |
2 |
3 |
4 |
5 |
9 |
ASK: All respondents
E4_10a. How much do you agree or disagree with the following statements? If I use smokeless tobacco, I will…
PROGRAMMER: RANDOMIZE E4_10- E4_19
|
|
|
|
|
|
|
|
E4_10 . |
Get sick more often |
1 |
2 |
3 |
4 |
5 |
9 |
E4_11. |
End up wasting money on smokeless tobacco |
1 |
2 |
3 |
4 |
5 |
9 |
E4_12. |
Feel more relaxed |
1 |
2 |
3 |
4 |
5 |
9 |
E4_13. |
Miss out on things I enjoy doing |
1 |
2 |
3 |
4 |
5 |
9 |
E4_14. |
Gross out people I want to date |
1 |
2 |
3 |
4 |
5 |
9 |
E4_16. |
Develop gum disease |
1 |
2 |
3 |
4 |
5 |
9 |
E4_17. |
Develop red or white patches in the mouth |
1 |
2 |
3 |
4 |
5 |
9 |
E4_18. |
Consume harmful chemicals |
1 |
2 |
3 |
4 |
5 |
9 |
E4_19. |
Lose my jaw |
1 |
2 |
3 |
4 |
5 |
9 |
ASK: All respondents
E5. How much do you agree or disagree with the following statements? If I smoke cigarettes I will…
PROGRAMMER: RANDOMIZE E5_1- E5_11
|
|
Strongly Disagree |
Disagree |
Neither Agree or Disagree
|
Agree |
Strongly Agree |
Prefer Not to Answer |
E5_1. |
Be controlled by smoking |
1 |
2 |
3 |
4 |
5 |
9 |
E5_2. |
Be more attractive |
1 |
2 |
3 |
4 |
5 |
9 |
E5_3. |
Develop cancer of the lip, mouth, tongue or throat |
1 |
2 |
3 |
4 |
5 |
9 |
E5_4. |
Develop sexual and/or fertility problems |
1 |
2 |
3 |
4 |
5 |
9 |
E5_5. |
Be unable to stop when I want to |
1 |
2 |
3 |
4 |
5 |
9 |
E5_6. |
Develop skin problems |
1 |
2 |
3 |
4 |
5 |
9 |
E5_7. |
Lose my teeth |
1 |
2 |
3 |
4 |
5 |
9 |
E5_8. |
Feel more relaxed |
1 |
2 |
3 |
4 |
5 |
9 |
E5_9. |
Shorten my life |
1 |
2 |
3 |
4 |
5 |
9 |
E5_10. |
End up wasting money on cigarettes |
1 |
2 |
3 |
4 |
5 |
9 |
E5_11. |
Be more popular |
1 |
2 |
3 |
4 |
5 |
9 |
ASK: All respondents
E6. How much do you agree or disagree with the following statements about smoking cigarettes?
PROGRAMMER: RANDOMIZE E6_1- E6_5. Keep E6_5 as the last item.
|
|
Strongly Disagree |
Disagree |
Neither Agree or Disagree
|
Agree |
Strongly Agree |
Prefer Not to Answer |
E6_1. |
Smoking can cause immediate damage to my body. |
1 |
2 |
3 |
4 |
5 |
9 |
E6_2. |
Smoking cigarettes helps people relieve stress. |
1 |
2 |
3 |
4 |
5 |
9 |
E6_3. |
Cigarette ingredients are disgusting. |
1 |
2 |
3 |
4 |
5 |
9 |
E6_4. |
Smoking cigarettes is a manly thing to do. |
1 |
2 |
3 |
4 |
5 |
9 |
E6_5 |
Please select the option labeled ‘Disagree’ as your answer. |
1 |
2 |
3 |
4 |
5 |
9 |
ASK: All respondents
E7. How much do you agree or disagree with the following statements about using smokeless tobacco?
PROGRAMMER: RANDOMIZE E7_1- E7_10
|
|
Strongly Disagree |
Disagree |
Neither Agree or Disagree |
Agree |
Strongly Agree |
Prefer Not to Answer |
E7_1. |
Using smokeless tobacco can cause immediate damage to my body. |
1 |
2 |
3 |
4 |
5 |
9 |
E7_2. |
It is safe for me to use smokeless tobacco for only a year or two, as long as I quit after that. |
1 |
2 |
3 |
4 |
5 |
9 |
E7_3. |
If I used smokeless tobacco occasionally I would not become addicted. |
1 |
2 |
3 |
4 |
5 |
9 |
E7_4. |
Using smokeless tobacco helps people relieve stress. |
1 |
2 |
3 |
4 |
5 |
9 |
E7_5. |
Using smokeless tobacco is disgusting. |
1 |
2 |
3 |
4 |
5 |
9 |
E7_7. |
Using smokeless tobacco is a way to show others you’re not afraid to take risks. |
1 |
2 |
3 |
4 |
5 |
9 |
E7_9 |
Using smokeless tobacco is a manly thing to do. |
1 |
2 |
3 |
4 |
5 |
9 |
E7_10. |
Using smokeless tobacco can cause red patches which can lead to mouth cancer. |
1 |
2 |
3 |
4 |
5 |
9 |
ASK: All respondents
E8_4. Does smokeless tobacco such as dip, chewing tobacco, or snuff contain Formaldehyde, a chemical used to preserve dead animals?
Definitely yes
Probably yes
Probably not
Definitely not
Don’t Know
Prefer not to answer
ASK: All respondents
E9. How many of your four closest friends…
PROGRAMMER: RANDOMIZE E9_1 – E9_3
|
|
None |
One |
Two |
Three |
Four |
Prefer Not to Answer |
E9_1. |
Smoke cigarettes? |
0 |
1 |
2 |
3 |
4 |
9 |
E9_2. |
Use smokeless tobacco? |
0 |
1 |
2 |
3 |
4 |
9 |
E9_3. |
Use e-cigs? |
0 |
1 |
2 |
3 |
4 |
9 |
ASK: All respondents
E10. How many others your age…
PROGRAMMER: RANDOMIZE E10_1 – E10_3
|
|
None |
A few |
Some |
Most |
All |
Prefer Not to Answer |
E10_1. |
Smoke cigarettes? |
0 |
1 |
2 |
3 |
4 |
9 |
E10_2. |
Use smokeless tobacco? |
0 |
1 |
2 |
3 |
4 |
9 |
E10_3. |
Use e-cigs? |
0 |
1 |
2 |
3 |
4 |
9 |
ASK: All respondents
Section F: Media Use and Awareness
F1. Next, we’d like to ask you about your use of TV and other media.
How often do you…
PROGRAMMER: RANDOMIZE F1_1 – F1_8
|
Several times a day |
About once a day |
3-5 days a week |
1-2 days a week |
Every few weeks |
Less often |
Never |
Prefer Not to Answer |
F1_1. Watch television? |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
9 |
F1_2. Watch videos on YouTube/Twitch? |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
9 |
F1_3. Listen to radio? |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
9 |
F1_4. Listen to streaming radio? |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
9 |
F1_5. Play games on any electronic devices including cell phones/ smartphones, computers, laptops, tablets, consoles (Xbox, Wii, PS) and handheld players (Nintendo DS, Sony PSP, iPod)? |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
9 |
F1_7. Watch Netflix, Hulu or Amazon Prime video? |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
9 |
ASK: All respondents
F2. Thinking about the social networking sites you use, about how often do you visit or use the following…
PROGRAMMER: RANDOMIZE F2_1 – F2_7
|
Several times a day |
About once a day |
3-5 days a week |
1-2 days a week |
Every few weeks |
Less often |
Never |
Prefer Not to Answer |
F2_1. |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
9 |
F2_2. |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
9 |
F2_3. |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
9 |
F2_5. Snapchat |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
9 |
F2_7. Skype |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
9 |
ASK: All respondents
F3_V2. Thinking about the following websites, about how often do you visit or use the following…
PROGRAMMER: RANDOMIZE ALL
|
Several times a day |
About once a day |
3-5 days a week |
1-2 days a week |
Every few weeks |
Less often |
Never |
Prefer Not to Answer |
F3_1. YouTube www.youtube.com
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
9 |
F3_4. Spotify www.spotify.com |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
9 |
F3_5. Bleacher Report www.bleacherreport.com |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
9 |
F3_6. Major League Gaming www.majorleaguegaming.com |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
9 |
F3_7. SoundCloud www.soundcloud.com |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
9 |
F3_8. Oovoo www.oovoo.com |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
9 |
ASK: All respondents
F4. We want to ask you about some slogans or themes that might or might not have appeared in the media around here, as part of ads about tobacco.
NEXT
PROGRAMMER: RANDOMIZE F4_1 – F4_6
ASK: All respondents
F4_1. Since [FILL DATE], have you seen or heard the following slogan or theme?
truth
1 Yes
2 No
3 Not sure
9 Prefer not to answer
ASK: All respondents
F4_2. Since [FILL DATE], have you seen or heard the following slogan or theme?
D own
and Dirty
own
and Dirty
1 Yes
2 No
3 Not sure
9 Prefer not to answer
ASK: All respondents
F4_3. Since [FILL DATE], have you seen or heard the following slogan or theme?
D igital
Youth Against Tobacco (DYAT)
igital
Youth Against Tobacco (DYAT)
1 Yes
2 No
3 Not sure
9 Prefer not to answer
ASK: All respondents
F4_4. Since [FILL DATE], have you seen or heard the following slogan or theme?
T he
Real Cost
he
Real Cost
1 Yes
2 No
3 Not sure
9 Prefer not to answer
ASK: All respondents
F4_4a. Since [FILL DATE], have you seen or heard the following slogan or theme?
The Real Cost, Smokeless Doesn’t Mean Harmless
1 Yes
Yes
2 No
3 Not sure
9 Prefer not to answer
ASK: All respondents
F4_5. Since [FILL DATE], have you seen or heard the following slogan or theme?
Tips from Former Smokers (Tips)

1 Yes
2 No
3 Not sure
9 Prefer not to answer
ASK: All respondents
F4_6. Since [FILL DATE], have you seen or heard the following slogan or theme?
F resh
Empire
resh
Empire
1 Yes
2 No
3 Not sure
9 Prefer not to answer
ASK: All respondents
F4_7. To show us that you are paying attention, please select Never as your response to this item.
1 Never
2 Rarely
3 Sometimes
4 Often
5 Very Often
9 Prefer not to answer
ASK: All respondents
F5_3. [IF F4_4a=1 or 3] Where have you seen or heard about The Real Cost, Smokeless Doesn’t Mean Harmless Campaign?
PROGRAMMER: RANDOMIZE OPTIONS
|
|
Yes |
No |
Prefer not to answer |
F5_3a. |
On TV or the Internet/online |
|
|
|
F5_3b. |
On the radio |
|
|
|
F5_3c. |
Billboards or other outdoor ads |
|
|
|
F5_3d. |
At the movie theater |
|
|
|
ASK: Respondents who report having seen or heard The Real Cost theme in F4_4a, or who report they are not sure if they have seen or heard The Real Cost theme.
F29. How many celebrities, athletes, musicians, or artists do you follow on social media (e.g., Joe Rogan, Dude Perfect, Hannah Barron, Jess DeLorenzo, Eva Shockey, Travis Pastrana, Jason Aldean)?
0
1-2
3-4
5 or more
Prefer not to answer
ASK: All respondents
F7_x. Now we would like to show you some advertisements that have been shown in the U.S.
PROGRAMMER: RANDOMIZE BATTERIES OF QUESTIONS ABOUT VIDEOS. DON’T ALLOW A RESPONSE UNTIL THE VIDEO HAS PLAYED FOR 15 SECONDS. REPEAT F8_X – F13f_X FOR EACH ADDITIONAL VIDEO. THE LIST OF VIDEOS IS IN ATTACHMENT 12a.
Next
ASK: All respondents
PROGRAMMER: EMBED [TO BE DETERMINED] VIDEO
[SCREENSHOT OF TBD VIDEO]
F8_X. Apart from this survey, how frequently have you seen this ad since [FILL DATE]?
1 Never
2 Rarely
3 Sometimes
4 Often
5 Very Often
9 Prefer not to answer
ASK: All respondents
[SCREENSHOT OF TBD VIDEO]
F11_X. Please tell us if you strongly disagree, disagree, neither agree or disagree, agree, or strongly agree with the following statements.
PROGRAMMER: RANDOMIZE ALL
|
|
Strongly Disagree |
Disagree |
Neither Agree or Disagree |
Agree |
Strongly Agree |
Prefer Not to Answer |
F11_X1 |
This ad is worth remembering |
1 |
2 |
3 |
4 |
5 |
9 |
F11_X2 |
This ad grabbed my attention |
1 |
2 |
3 |
4 |
5 |
9 |
F11_X3 |
This ad is powerful |
1 |
2 |
3 |
4 |
5 |
9 |
F11_X4 |
This ad is informative |
1 |
2 |
3 |
4 |
5 |
9 |
F11_X5 |
This ad is meaningful to me |
1 |
2 |
3 |
4 |
5 |
9 |
F11_X6 |
This ad is convincing |
1 |
2 |
3 |
4 |
5 |
9 |
F11_X7 |
This ad is terrible |
1 |
2 |
3 |
4 |
5 |
9 |
F11_X10 |
This ad told me things I never knew before about tobacco |
1 |
2 |
3 |
4 |
5 |
9 |
F11_X12 |
This ad gave me good reasons not to use tobacco |
1 |
2 |
3 |
4 |
5 |
9 |
ASK: All respondents
[SCREENSHOT OF TBD VIDEO]
F13_X. On scale of 1 to 5, where 1 means “not at all” and 5 means “very”, please indicate how much this ad made you feel…
PROGRAMMER: RANDOMIZE ORDER
|
|
1 Not at all |
2 |
3 |
4 |
5 Very |
Prefer not to answer |
F13a_X.
|
Afraid |
1 |
2 |
3 |
4 |
5 |
9 |
F13b_X.
|
Hopeful |
1 |
2 |
3 |
4 |
5 |
9 |
F13c_X.
|
Motivated
|
1 |
2 |
3 |
4 |
5 |
9 |
F13d_X.
|
Worried |
1 |
2 |
3 |
4 |
5 |
9 |
F13e_X. |
Understood
|
1 |
2 |
3 |
4 |
5 |
9 |
F13f_X. |
Surprised
|
1 |
2 |
3 |
4 |
5 |
9 |
ASK: All respondents
F7_INTRO. The next few questions are about other ads.
ASK: All respondents
F7_1. Apart from this survey, how frequently have you seen these ads since [FILL DATE]?





1 Never
2 Rarely
3 Sometimes
4 Often
5 Very Often
9 Prefer not to answer
ASK: All respondents
F7_2. Apart from this survey, how frequently have you seen these ads since [FILL DATE]?

1 Never
2 Rarely
3 Sometimes
4 Often
5 Very Often
9 Prefer not to answer
ASK: All respondents
F7_3. Apart from this survey, how frequently have you seen these ads since [FILL DATE]?

1 Never
2 Rarely
3 Sometimes
4 Often
5 Very Often
9 Prefer not to answer
ASK: All respondents
F7_4. Apart from this survey, how frequently have you seen these ads since [FILL DATE]?

1 Never
2 Rarely
3 Sometimes
4 Often
5 Very Often
9 Prefer not to answer
ASK: All respondents
STREAMING RADIO AWARENESS
F24. Since [FILL DATE], have you heard about The Real Cost, Smokeless Doesn’t Mean Harmless Campaign on streaming radio?

1 Yes
2 No
3 Not Sure
9 Prefer not to answer
ASK: All respondents
F24a. Now we would like to play you some radio clips that have aired in the U.S. Once you have listened to the clip, please click on the Next arrow below to continue with the survey.
NEXT
PROGRAMMER: RANDOMIZE RADIO CLIPS. DON’T ALLOW A RESPONSE UNTIL THE AD HAS PLAYED FOR 10 SECONDS. REPEAT F25_X – F26_X FOR EACH RADIO AD. THE LIST OF RADIO ADS IS IN ATTACHMENT 12a.
ASK: All respondents
PROGRAMMER: EMBED RADIO CLIP [TBD]
F25_X. Apart from this survey, how frequently have you heard this on the radio since [FILL DATE]?
1 Never
2 Rarely
3 Sometimes
4 Often
5 Very Often
9 Prefer not to answer
ASK: All respondents
F26_X. How much do you agree or disagree with the following statement?
|
|
Strongly Disagree |
Disagree |
Neither Agree or Disagree |
Agree |
Strongly Agree |
Prefer Not to Answer |
|
This radio ad is convincing |
1 |
2 |
3 |
4 |
5 |
9 |
ASK: All respondents
F28. Apart from this survey, how frequently have you seen any of these advertisements on websites you visited since [FILL DATE]?
[INSERT BANNER AD COLLAGE]
1 Never
2 Rarely
3 Sometimes
4 Often
5 Very Often
9 Prefer not to answer
ASK: All respondents
F18a. Have you visited www.therealcost.gov/dip since [FILL DATE]?
1 Yes
2 No
9 Prefer not to answer
ASK: All respondents
F14_x. [F8_1 NE 1 OR F8_2 NE 1 OR F8_3 NE 1OR F8_5 NE 1 OR F8_6 NE 1 OR F25_1 NE 1 OR F25_9 NE 1 OR F25_10 NE 1 OR F25_11 NE 1 OR F25_12 NE 1] Did you talk to anyone in person or online about these ads?
1 Yes
2 No
9 Prefer not to answer
ASK: Respondents who did NOT report having never seen or heard Face of Dip in F8_1, Football in F8_2, Jeans in F8_3, Pounds in F8_5, Don’t Search It in F8_6, Come On in F25_1, Warning Signs in F25_9, The Ring in F25_10, Don’t Search It in F25_11, or Can’t get addiction out of my head in F25_12.
F5. Do your parents have rules about what you are allowed to do on the computer, which video games you are allowed to play, or what music you’re allowed to listen to?
1 Yes, my parents have lots of rules about it.
2 Yes, my parents have a few rules about it.
3 No, my parents don’t have any rules about it.
9 Prefer not to answer
ASK: All respondents
F6. How often do your parents let you watch movies or videos that are rated R?
1 Never
2 Once in a while
3 Sometimes
4 All the time
9 Prefer not to answer
ASK: All respondents
F7. Please tell us if you strongly agree, agree, disagree, or strongly disagree with the following statements.
I try to do what my parents want me to do.
1 Strongly agree
2 Agree
3 Disagree
4 Strongly disagree
9 Prefer not to answer
ASK: All respondents
F8. What my parents think of me is important.
1 Strongly agree
2 Agree
3 Disagree
4 Strongly disagree
9 Prefer not to answer
ASK: All respondents
F9. I do what my friends want me to do, even if I don’t want to.
1 Strongly agree
2 Agree
3 Disagree
4 Strongly disagree
9 Prefer not to answer
ASK: All respondents
F10. To keep my friends, I’d even do things I don’t want to do.
1 Strongly agree
2 Agree
3 Disagree
4 Strongly disagree
9 Prefer not to answer
ASK: All respondents
Section G: Environment
G1. The next section asks some questions about your household and peers.
Other than you, has anyone who lives with you used any of the following during the past 30 days? Select all that apply.
1 Cigarettes
2 Smokeless tobacco
3 Cigars, cigarillos, or little cigars
4 Tobacco out of a water pipe (also called “hookah”)
5 e-cigarettes, vape pens, and other electronic vapor products
6 Any other form of tobacco
7 No, no one who lives with me has used any form of tobacco during the past 30 days
9 Prefer not to answer
PROGRAMMER: ALLOW RESPONDENTS TO SELECT MORE THAN ONE RESPONSE ON 1-6.
IF RESPONSE 7 WAS CHOSEN WITH ANY OTHER RESPONSE OPTIONS, “You indicated that no one who lives with you used any form of tobacco during the past 30 days and also said that in the past 30 days someone has used a form of tobacco. Please choose either someone or no one has smoked any form of tobacco as your response.”
ASK: All respondents
G2. Do you have any brother(s) and/or sister(s) who have used smokeless tobacco during the past 30 days?
1 Yes
2 No
3 I don’t know
4 I don’t have any brothers or sisters
9 Prefer not to answer
ASK: All respondents
G3. Which statement best describes the rules about smoking in your home? Would you say…
1 Smoking is not allowed anywhere inside your home
2 Smoking is allowed in some places or at some times
3 Smoking is allowed anywhere inside the home
4 There are no rules about smoking inside the home
9 Prefer not to answer
ASK: All respondents
G4. Which statement best describes the rules about using smokeless tobacco in your home? Would you say…
1 Smokeless tobacco is not allowed anywhere inside your home
2 Smokeless tobacco is allowed in some places or at some times
3 Smokeless tobacco is allowed anywhere inside the home
4 There are no rules about using smokeless tobacco inside the home
9 Prefer not to answer
ASK: All respondents
G5. How well would you say you have done in school? Would you say…
1 Much better than average
2 Better than average
3 Average
4 Below average
5 Much worse than average
9 Prefer not to answer
ASK: All respondents
G6. Please tell us if you strongly disagree, disagree, neither agree nor disagree, agree, or strongly agree with the following statements.
I feel close to people at my school. Would you say you…
1 Strongly disagree
2 Disagree
3 Neither agree nor disagree
4 Agree
5 Strongly agree
9 Prefer not to answer
ASK: All respondents
G7. I am happy to be at my school. Would you say you…
1 Strongly disagree
2 Disagree
3 Neither agree nor disagree
4 Agree
5 Strongly agree
9 Prefer not to answer
ASK: All respondents
G8. I feel like I am a part of my school. Would you say you…
1 Strongly disagree
2 Disagree
3 Neither agree nor disagree
4 Agree
5 Strongly agree
9 Prefer not to answer
ASK: All respondents
G9. How far do you think you will go in school?
1 I don’t plan to go to school anymore
2 9th grade
3 10th grade
4 11th grade
5 12th grade or GED
6 Some college or technical school but no degree
7 Technical school degree
8 College degree
9 Graduate school, medical school, or law school
99 Prefer not to answer
ASK: All respondents
G10. How many close friends do you have? Close friends include people whom you feel at ease with, can talk to about private matters, and can call on for help.
__________ (Range: 0-7)
PROGRAMMER: NUMERIC STRING. ALLOW A MINIMUM OF 0 AND MAXIMUM OF 7. IF ANYTHING ELSE IS TYPED IN ERROR MESSAGE SHOULD SAY, “You have entered a number outside the allowed range. Please enter an answer between 0 and 7.”
9 Prefer not to answer
ASK: All respondents
G11. How often do you attend church or religious services? Would you say…
1 Never
2 Less than once a month
3 About once a month
4 About 2 or 3 times a month
5 Once a week
6 More than once a week
9 Prefer not to answer
ASK: All respondents
G12. Please tell us if you strongly disagree, disagree, neither agree nor disagree, agree, or strongly agree with the following statements.
I would like to explore strange places. Would you say you…
1 Strongly disagree
2 Disagree
3 Neither agree nor disagree
4 Agree
5 Strongly agree
9 Prefer not to answer
ASK: All respondents
G13. I like to do frightening things. Would you say you…
1 Strongly disagree
2 Disagree
3 Neither agree nor disagree
4 Agree
5 Strongly agree
9 Prefer not to answer
ASK: All respondents
G14. I like new and exciting experiences, even if I have to break the rules. Would you say you…
1 Strongly disagree
2 Disagree
3 Neither agree nor disagree
4 Agree
5 Strongly agree
9 Prefer not to answer
ASK: All respondents
G15. I prefer friends who are exciting and unpredictable. Would you say you…
1 Strongly disagree
2 Disagree
3 Neither agree nor disagree
4 Agree
5 Strongly agree
9 Prefer not to answer
ASK: All respondents
G16. Thinking about your mental health, which includes stress, depression, and problems with emotions, for how many days during the past 30 days was your mental health not good?
_____ Number of days
33 Don’t know
99 Prefer not to answer
PROGRAMMER: NUMERIC STRING. ALLOW A MINIMUM OF 0 AND MAXIMUM OF 30.
IF ANYTHING ELSE IS TYPED IN, ERROR MESSAGE SHOULD SAY, “You have entered a number outside the allowed range. Please enter a number between 0 and 30.”
RESPONDENTS CAN ONLY RESPOND WITH THE OPTION DON’T KNOW, OR TYPE IN A NUMERIC RESPONSE. IF RESPONDENTS TRY TO DO MULTIPLE THINGS, ERROR MESSAGE SHOULD SAY “You have entered a number and selected Don’t Know. Please choose one or the other as your response.”
ASK: All respondents
G17. The next section asks some questions about how you feel about your current relationship with your parents or guardians.
Thinking about the adult or adults you live with, would you say you are satisfied with the way you communicate with each other?
1 Strongly disagree
2 Disagree
3 Neither agree nor disagree
4 Agree
5 Strongly agree
9 Prefer not to answer
ASK: All respondents
G18. How close do you feel to the adult or adults you live with?
1 Not at all close
2 Not very close
3 Somewhat close
4 Quite close
5 Very close
9 Prefer not to answer
ASK: All respondents
G19. How often has a parent or other adult caregiver said things that really hurt your feelings or made you feel like you were not wanted or loved?
1 One time
2 Two times
3 Three to five times
4 Six to ten times
5 More than ten times
6 This has never happened
7 Don’t know
9 Prefer not to answer
ASK: All respondents
G20. Has a parent or other adult caregiver ever talked to you about reasons for not using smokeless tobacco?
1 Yes
2 No
9 Prefer not to answer
ASK: All respondents
G21. During the past 7 days, on how many days did you and one or both of your parents or other adult caregivers do something together just for fun?
__________
9 Prefer not to answer
PROGRAMMER: NUMERIC STRING. ALLOW A MINIMUM OF 0 AND MAXIMUM OF 7. IF ANYTHING ELSE IS TYPED IN ERROR MESSAGE SHOULD SAY, “You have entered a number outside the allowed range. Please enter a number between 0 and 7.”
ASK: All respondents
FINAL [IF CAPI] That was the last question. Once you move past this screen, your responses will be locked. They cannot be seen by your interviewer. Please tell your interviewer that you are finished. Thank you for taking the time to complete the survey.
Next
CODE [IF CAPI] INTERVIEWER - ENTER 3 DIGIT CODE TO LOCK RESPONSES.
Programmer: Code is RTI
ASK: CAPI respondents
Section H: Closing Contact Items
H4. [IF CAPI AND FILLAGE <18] INTERVIEWER: ASK ITEM OF PARENTS
[IF CAPI] Thank you for your responses! We may need to reach you again to ask questions about this interview. Please provide your phone number including area code in case we are unable to reach you by e-mail.
OPEN END NUM
VALIDATION: MIN 0 MAX 9999999999
(__ __ __) __ __ __ - __ __ __ __ Phone Number [ALLOW 10 CHARACTERS]
Prefer not to answer
PROGRAMMER: VALIDATE FORMAT FOR PHONE NUMBER. DO NOT ALLOW 0 OR 1 AS THE BEGINNING DIGIT OF AN AREA CODE OR PREFIX. IF FORMAT IS INCORRECT, PLEASE DISPLAY “Please enter a valid phone number. The phone number must be a 10-digit number. Do not include dashes or other special characters.”
ASK: CAPI Respondents
INCENT01 [IF CAPI]
PROGRAMMER: DISPLAY CASE ID FROM IFMS ON SCREEN.
INTERVIEWER: SIGN COMPENSATION RECEIPT AND HAND $20 TO R
DID YOU GIVE THE RESPONDENT THEIR INCENTIVE?
1 YES
2 NO
PROGRAMMER: HARD PROMPT “INTERVIEWER, THIS IS A REQUIRED ITEM. PLEASE DO YOUR BEST TO FILL OUT THE ITEM.”]
ASK: CAPI respondents
INCENT02
[IF INCENT01=1 AND CAPI] I have signed this form to indicate that I have given you $20 cash for completing this interview. You may have received an invitation to take this survey on the web. Since we completed this survey today, there is no need for you to take the web survey. Thanks again!
NEXT
ASK: CAPI respondents who have been given the $20 cash incentive by interviewer in INCENT01
INCENT03 [IF INCENT01=2 AND CAPI] INTERVIEWER, PLEASE INDICATE WHY YOU ARE NOT ABLE TO GIVE THE INCENTIVE TO THE RESPONDENT.
_________________ (1000 Characters)
[PROGRAMMER: HARD PROMPT “INTERVIEWER, THIS IS A REQUIRED ITEM. PLEASE DO YOUR BEST TO FILL OUT THE ITEM.”]
ASK: CAPI respondents who have NOT been given the $20 cash incentive by interviewer in INCENT01
FIDBF1 [IF CAPI]
INTERVIEWER: DO NOT READ TO THE RESPONDENT.
DID YOU HAVE TO READ ANY OF THE QUESTIONS OUT LOUD TO THE RESPONDENT?
Yes
No
ASK: Interviewers of CAPI surveys
FIDBF2 [IF CAPI]
PLEASE DESCRIBE THE ASSISTANCE THAT YOU PROVIDED TO THE RESPONDENTS. WHAT WERE HIS READING ABILITIES? HOW MUCH HELP DID HE REQUIRE? OTHERWISE, ENTER ANY NOTES YOU HAVE ABOUT THIS CASE
PROGRAMMER: OPEN ENDED. CHARACTER LIMIT: 5000
END
ASK: Interviewers of CAPI surveys
VERIFY [IF WEB]
Including this one, how many surveys have you completed on this topic?
__________ (Range: 1-10)
ASK: Web respondents
COMMNT [IF WEB]
Thank you for completing the survey. Please enter any comments that you have about the interview.
______________________ PROGRAMMER: PROGRAM OPEN ENDED ITEM WITH 2000 CHARACTER LIMIT. MAKE ITEM OPTIONAL.
Next
ASK: Web respondents
WEBTH [IF WEB]
To thank you for completing the survey, you will receive a check for [$20/$25 IF BEFORE OR AFTER EARLY BIRD DATE]. We will need to collect some information from you so that we can mail you a check. This information will be kept completely confidential in secure and protected data files and will be separate from the responses provided in the survey. If you would like to decline receiving this payment, you can leave the information blank and then simply select “Prefer not to answer” to continue to the next screen.
First name: YFNAME
Last name: YLNAME
What is the best address where we should mail the check?
Street: YSTREET
City: YCITY
State: YSTATE
Zip Code: YZIP
PROGRAMMER: PLEASE HAVE YFNAME, YLNAME, YCITY YSTATE ONLY ALLOW ALPHA CHARACTERS AND HAVE YZIP ONLY ALLOW NUMERIC CHARACTERS
ASK: Web respondents
THANKS.
Thank you again for your participation.
EXIT
OMB No: 0910-0753 Expiration Date: 09/30/2019
Paperwork Reduction Act Statement: The public reporting burden for this collection of information has been estimated to average 45 minutes per response. Send comments regarding this burden estimate or any other aspects of this collection of information, including suggestions for reducing burden to [email protected].
MIEND. You may now close your browser or navigate away from this page.
Page
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Rao, Pamela * |
| File Modified | 0000-00-00 |
| File Created | 2021-01-15 |
© 2026 OMB.report | Privacy Policy