20171121 LDC questions from OMB SRTR responses
20171121 LDC questions from OMB SRTR responses.docx
Scientific Registry of Transplant Recipients Information Collection Effort for Potential Donors for Living Organ Donation (SRTR)
20171121 LDC questions from OMB SRTR responses.docx
OMB: 0906-0034
General Questions:
Why is the pilot limited to kidney and liver donations?
The majority of living organ donations are livers and kidneys. The Organ Procurement and Transplantation Network (OPTN) policy for follow-up also focuses only on the liver and kidney living donors.
How were the pilot sites selected?
Pilot site were selected on a volunteer basis. The Scientific Registry of Transplant Recipients (SRTR) chose sites that would also reflect the diversity of sites and organ donation candidates throughout the OPTN network.
What outcomes are of interest to the program in deciding whether to continue the registry? What does a “successful” pilot look like?
A successful pilot would demonstrate that collecting initial screening data on donor candidates does not impose an excessive burden to the transplant centers, and candidates are willing to be contacted for follow up. Additionally, a successful pilot would show the benefit of collecting data on candidates who do not donate by allowing for accurate risk analysis of living donation.
If successful, does HRSA have plans to expand the scope of the registry to include additional organs?
Since the majority of living donations are liver or kidney, there are no plans to expand the scope of the registry beyond these two organs.
What types of data analysis are planned?
SRTR will analyze the characteristics of donors and controls at the initial screening. Specifically, continuous variables (e.g., age at screening) will be summarized by a mean and standard deviation, whereas categorical variables (e.g., sex) will be summarized by frequencies and percentages. The characteristics of the donors who do not donate will be stratified by the reasons for non-donation. Finally, the descriptive statistics for both donors and potential donors who do not donate will be stratified by year of initial screening to determine how the characteristics of donors and potential donors who do not donate change over time.
In addition, Living Donor Collective (LDC) data will be linked to available registries to provide potential and past donors and caregivers with the most up-to-date information on risks that may be attributable to donation, such as lifetime risk of end-stage renal disease (ESRD) and mortality. Links to registry data may include Centers for Medicare and Medicaid Services data to determine which donors develop ESRD or to the National Center for Health Statistics and National Death Index to obtain data on deaths and causes of death among donors. SRTR will also link LDC data to the Pharmaceutical Claims Data clearinghouse. These records will allow us to examine comorbid conditions related to specific medication usage, e.g., hypertension, diabetes, gout, and depression.
Is the program using the following SORN for the pilot: 09-15-0055? If so, did/does it need to be modified to cover the pilot?
No, system of record notice 09-15-0055 will not be used for this pilot program. The program is developing a separate secure online data collection system to allow transplant programs to submit data collected at the time of initial in-person evaluation, and at the time of donation or at the time of reporting reasons for not donating.
Related to the question above, please check the following two RISC and OIRA Consolidated Information System fields for accuracy (this is how they were submitted in the system):
Does this Information Collection Request (ICR) request any personally identifiable information (see OMB Circular No. A-130 for an explanation of this term)? Please consult with your agency's privacy program when making this determination.
Yes, personally identifiable information will be collected.
Does this ICR include a form that requires a Privacy Act Statement (see 5 U.S.C. §552a(e)(3))? Please consult with your agency's privacy program when making this determination.
No, SRTR is recognized as a public health authority under the HIPAA Privacy Rule (42 CFR 164.512(b)).
The supporting statement mentions an electronic system – please provide screenshots of the system.
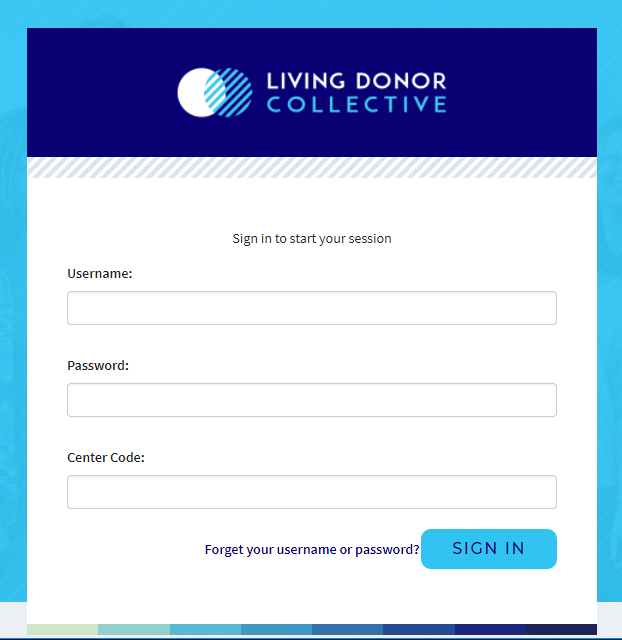
Log in page:
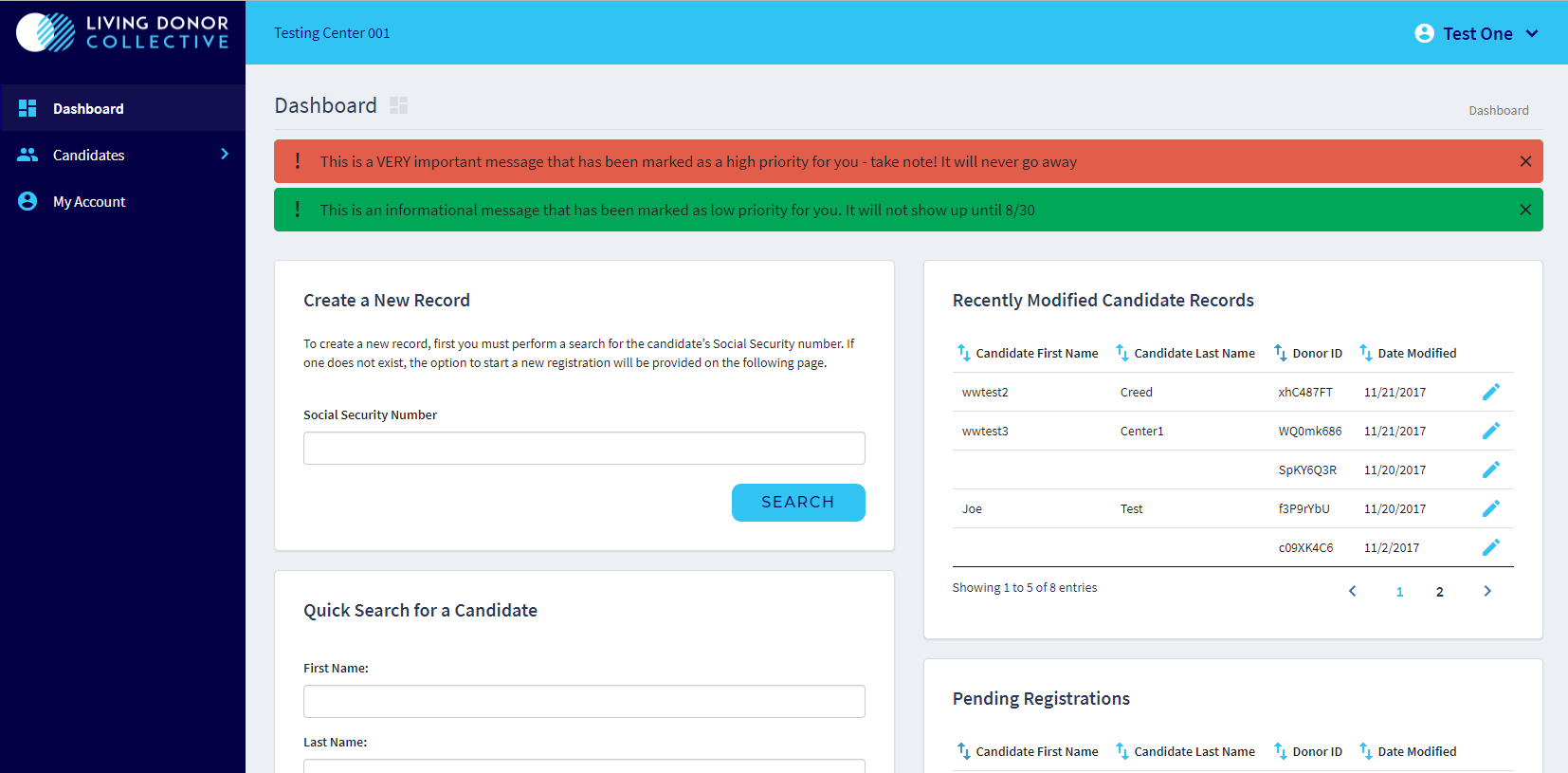
Dashboard (test environment):
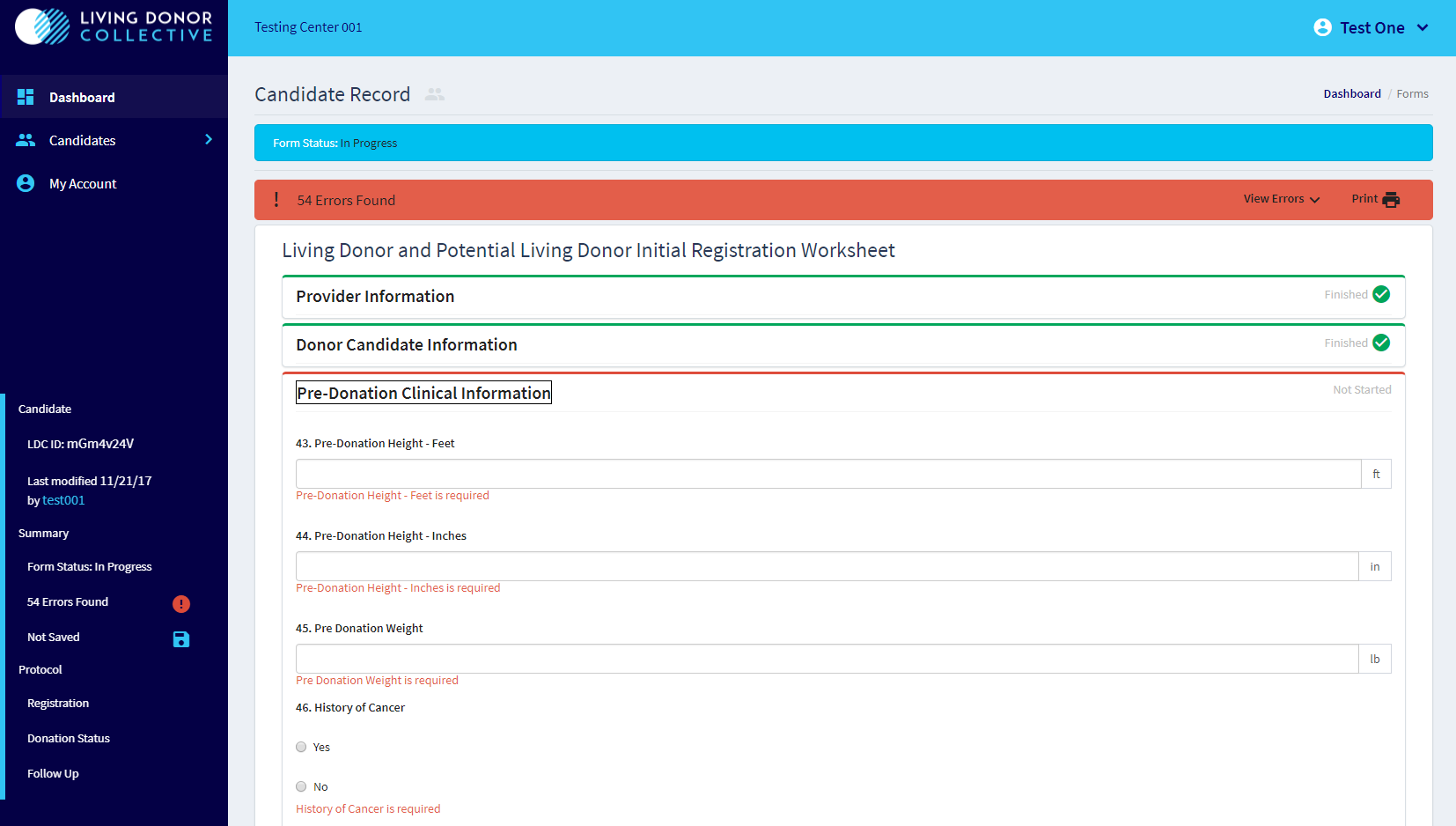
Data Entry Screen (dummy data):
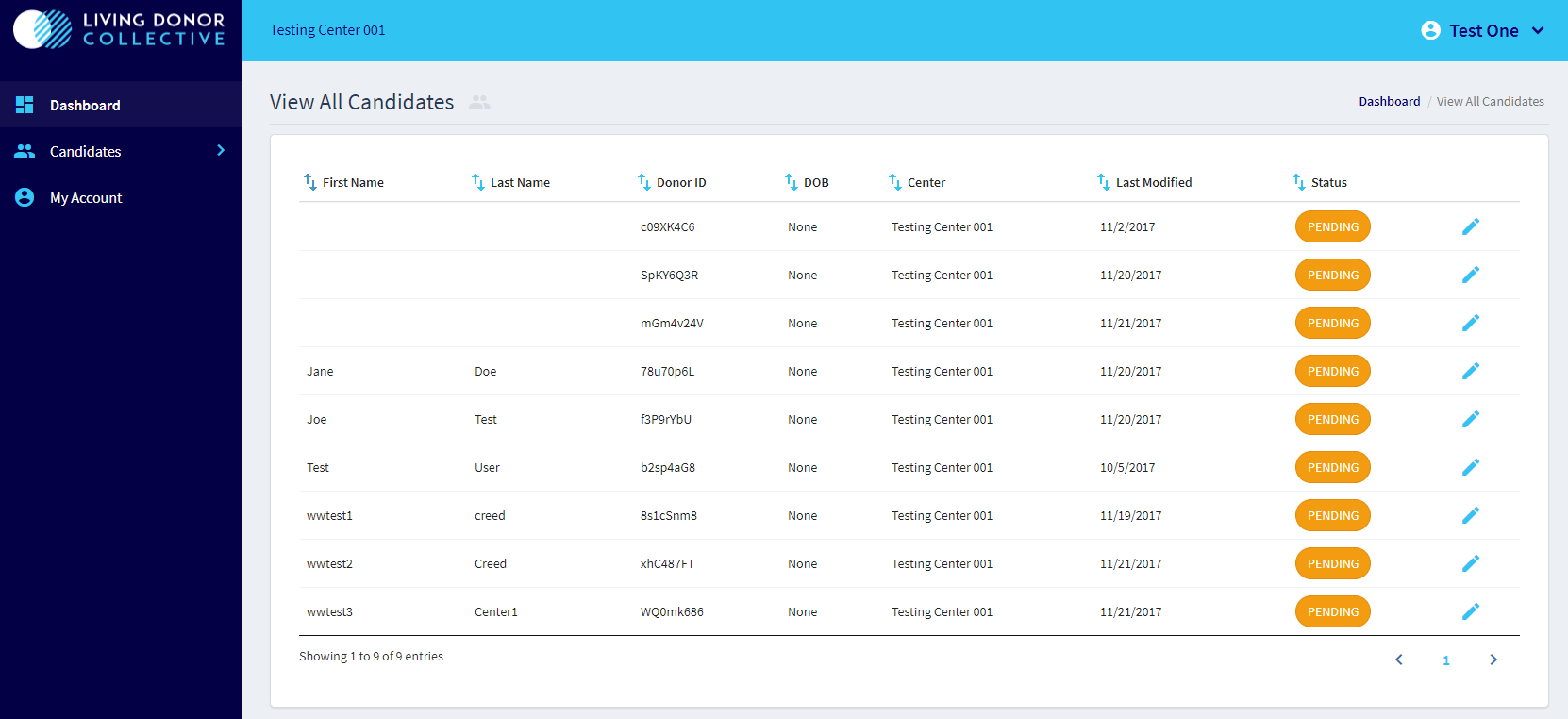
All Candidate Overview (dummy data):
Living Donor and Potential Living Donor Initial Registration Worksheet
Will potential donors be filling this form out or will the provider be asking questions and recording responses?
The transplant center will be collecting this information from potential living donors who are evaluated at their centers and will be recording them in electronic data collection system.
What is the rationale for requesting SSN? We generally discourage the collection of this identifier.
The SRTR’s responsibilities of providing analytic support to the OPTN also include linkages of the OPTN data set with other databases such as the Social Security Death Master File for purposes of validation and enhancement. There are currently no viable alternatives to using SSN. The major obstacle is the OPTN/SRTR needs to identify patients across the nation in independent data systems with no other common identifiers.
If asking for race/ethnicity, the questions should be asked separately – ethnicity (Hispanic/Latino) first, followed by race.
Where possible, questions align with the OPTN data collection formats. The race/ethnicity question corresponds with how OPTN asks this question.
What is the rationale for asking about citizenship?
This question also corresponds with the OPTN data collection forms for living donors.
Potential Living Donor Follow-up Form
Who will be conducting the follow-up call? Suggest making the affiliation clear in the introduction.
Follow-up information will be collected by SRTR and not by the transplant programs. SRTR will establish procedures for maintaining contact with participants by using a brief survey instrument. Participants will be contacted via postal mail, email, social media, or phone approximately 1 year after donation or 1 year after determination not to donate and approximately every 1 to 2 years thereafter.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Owner |
| File Modified | 0000-00-00 |
| File Created | 2021-01-15 |
© 2026 OMB.report | Privacy Policy