Safety Alert and Outbreak Advisory Templates Testing
Focus Groups as Used by the Food and Drug Administration
Appendix I - Message Templates
Safety Alert and Outbreak Advisory Templates Testing
OMB: 0910-0497
Appendix I
Message Templates
Safety Alert and Outbreak Advisory Focus Groups
OMB No: 0910-0497 Expiration Date: 10/31/2020
Paperwork Reduction Act Statement: According to the Paperwork Reduction Act of 1995, an agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a valid OMB control number. The valid OMB control number for this information collection is 0910-0497. The time required to complete this information collection is estimated to average 90 minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information.
Send comments regarding this burden estimate or any other aspects of this collection of information, including suggestions for reducing burden to [email protected].
Table of Contents
Safety Alert for Infant Formula 2
Template 1 2
Template 2 5
Template 3 7
Safety Alert for Tattoo Ink 9
Template 1 9
Template 2 11
Template 3 13
Outbreak Advisory for Romaine Lettuce 15
Template 1 15
Template 2 19
Template 3 22
Safety Alert for Infant Formula
Template 1

FDA Advises Consumers to Stop Using Infant Formula from Bobbie Baby Inc. Due to Insufficient Nutrients Levels
June 11, 2019
Recommendation
The FDA is advising parents and caregivers of infants who drink formula to stop using and throw away Bobbie Milk-Based Powder Companion Formula from Bobbie Baby Inc. due to insufficient nutrient content. Based on available information, this product was sold on the company’s website, hibobbie.com, and distributed in the San Francisco Bay Area of California.
Product Information:
Consumers should stop using and throw away all Bobbie Milk-Based Powder Companion Formula, Net wt. 14.1 oz (400g). This formula comes in a brown and green cardboard box (2, 4, or 8 units per box) with the Bobbie logo. Product of concern was shipped between the dates of May 12, 2019 and May 30, 2019.
Background:
An FDA inspection found that the firm violated regulations when making the formula. These FDA regulations are in place to ensure the health of developing infants who consumer infant formula.
Following discussion with the FDA, Bobbie Baby Inc. has agreed to remove the violative product from the market and is working with the FDA to alert consumers.

Product Issues:
The FDA is advising parents and caregivers to stop using and throw away Bobbie Milk-Based Powder Companion Formula. This product does not provide adequate nutrient levels for some infants, particularly:
infants born prematurely or with a low birth weight
infants born with low iron levels
infants at risk for iron deficiency.
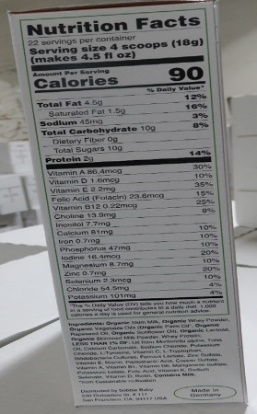
The product label does not tell parents and caregivers the amount of 12 vital nutrients the product contains. The nutrients are: linoleic acid, vitamin K, thiamine, riboflavin, vitamin B6, niacin, pantothenic acid, biotin, vitamin C, copper, iodine, and manganese. These nutrients are required and their absence may lead to poor growth, nutrient deficiencies, and/or serious health problems for developing infants.
General Safety Tips for Consumers:
Inadequate intake of iron during infancy may lead to iron deficiency anemia, which, if untreated, has irreversible cognitive and functional development outcomes.
Infant formula should include labeling that indicates the amounts of the following nutrients required in infant formula: linoleic acid, vitamin K, thiamine, riboflavin, vitamin B6, niacin, pantothenic acid, biotin, vitamin C, copper, iodine, and manganese. The absence of any of these key nutrients in infant formula may lead to poor growth, nutrient deficiencies, and/or serious health problems for developing infants.
Who to Contact
Consumers who have symptoms should contact their health care provider to report their symptoms and receive care.
To report a complaint or adverse event (illness or serious allergic reaction), you can
Call an FDA Consumer Complaint Coordinator if you wish to speak directly to a person about your problem.
Complete an electronic Voluntary MedWatch form online.
Complete a paper Voluntary MedWatch form that can be mailed to FDA.
Visit www.fda.gov/fcic for additional consumer and industry assistance.
Submit Questions Electronically
Safety Alert for Infant Formula
Template 2

FDA Advises Consumers to Stop Using Infant Formula from Bobbie Baby Inc.
June 7, 2019
Audience
Parents and other caregivers of infants who consume infant formula
Product
Product Name: Bobbie Milk-Based Powder Companion Formula, Net wt. 14.1 oz (400g)
Packaging: Brown and green cardboard boxes (2, 4, or 8 units per box) with the bobbie logo
Lot numbers: Codes on the product of concern are; L6236501Z001; Use By: 9.15.2020; L6236501Z002; Use By: 9.15.2020; L6236501Z003; Use By: 9.16.2020; L6236501Z004; Use By: 9.16.2020
Dates: Product of concern was shipped between the dates of May 12, 2019 and May 30, 2019
Purpose
Parents and other caregivers of infants should stop using bobbie Milk-Based Powder Companion Formula from Bobbie Baby Inc. This product was manufactured in Germany and imported into the United States. The formula was sold online by Bobbie Baby Inc. through the firm website. Based on the current information provided to the FDA by the firm, products were sold and distributed only to consumers in the San Francisco Bay Area of California. The FDA advises parents and caregivers to stop using and buying this formula because these products do not provide adequate nutrient levels for some infants, particularly for infants born prematurely or with a low birth weight, having low iron levels at birth or at risk for becoming iron deficient due to illness. Inadequate intake of iron during infancy may lead to iron deficiency anemia, which, if untreated, has irreversible cognitive and functional development outcomes.
This product also lacks the labeling for amounts of the following nutrients required in infant formula: linoleic acid, vitamin K, thiamine, riboflavin, vitamin B6, niacin, pantothenic acid, biotin, vitamin C, copper, iodine, and manganese. The absence of any of these key nutrients in infant formula may lead to poor growth, nutrient deficiencies, and/or serious health problems for developing infants.
Summary of Problem and Scope
Recently, the FDA conducted an inspection of Bobbie Baby Inc., and determined that the firm is not in compliance with FDA regulations instituted to ensure the health of developing infants who consume infant formula. The firm was inspected under the Infant Formula Requirements (21 Part 106 and Part 107) included in Title 21 of the Code of Federal Regulations.
Review of the firm’s product labeling during the inspection revealed that the product listed above was being marketed as an infant formula.
These products have not been manufactured in compliance with infant formula regulations.
FDA Actions
Following discussion with the FDA, Bobbie Baby Inc. has agreed to remove the violative product from the market and is working with FDA to alert consumers.
Recommendations for Parents and Caregivers of Infants
Parents and other caregivers of infants who have recently purchased this product should discontinue use and throw it away.
Parents and other caregivers of infants who have recently used this product and are concerned about the health of their child should contact their health care provider.
To report a complaint or adverse event (illness or serious allergic reaction), you can
Call an FDA Consumer Complaint Coordinator if you wish to speak directly to a person about your problem.
Complete an electronic Voluntary MedWatch form online.
Complete a paper Voluntary MedWatch form that can be mailed to FDA.
Visit www.fda.gov/fcic for additional consumer and industry assistance.
Reporting Problems to the FDA
Consumers who have experienced an adverse event (illness or injury) after using this formula should consult their healthcare professional. Consumers should also consider reporting their adverse event to MedWatch: FDA’s Safety Information and Adverse Event Reporting Program. The FDA encourages consumers with questions about product safety to submit an inquiry, or to visit www.fda.gov/fcic for additional information.

Safety Alert for Infant Formula
Template 3

FDA Advises Consumers to Stop Using Infant Formula from Bobbie Baby Inc. due to Inadequate Nutrient Levels (June 2017)
Product
Parents and other caregivers of infants should stop using bobbie Milk-Based Powder Companion Formula from Bobbie Baby Inc.
Product Name: bobbie Milk-Based Powder Companion Formula, Net wt. 14.1 oz (400g)
Packaging: Brown and green cardboard boxes (2, 4, or 8 units per box) with the “bobbie” logo
Lot numbers: Codes on the product of concern are; L6236501Z001; Use By: 9.15.2020; L6236501Z002; Use By: 9.15.2020; L6236501Z003; Use By: 9.16.2020; L6236501Z004; Use By: 9.16.2020
Dates: Product of concern was shipped between the dates of May 12, 2019 and May 30, 2019
Sold at: This product was manufactured in Germany and imported into the United States. It is sold online at https://www.hibobbie.com/. Based on the current information provided to the FDA by the firm, products were sold and distributed only to consumers in the San Francisco Bay Area of California.
Problem
These products do not provide adequate nutrient levels for some infants, particularly for infants born prematurely or with a low birth weight, having low iron levels at birth or at risk for becoming iron deficient due to illness. Inadequate intake of iron during infancy may lead to iron deficiency anemia, which, if untreated, has irreversible cognitive and functional development outcomes.
This product also lacks the labeling for amounts of the following nutrients required in infant formula: linoleic acid, vitamin K, thiamine, riboflavin, vitamin B6, niacin, pantothenic acid, biotin, vitamin C, copper, iodine, and manganese. The absence of any of these key nutrients in infant formula may lead to poor growth, nutrient deficiencies, and/or serious health problems for developing infants.
Recommendations
Parents and other caregivers of infants who have recently purchased this product should discontinue use and throw it away.
Parents and other caregivers of infants who have recently used this product and are concerned about the health of their child should contact their health care provider.
FDA Actions
The FDA conducted an inspection of Bobbie Baby Inc., and determined that the firm is not in compliance with FDA regulations instituted to ensure the health of developing infants who consume infant formula. The firm was inspected under the Infant Formula Requirements (21 Part 106 and Part 107) included in Title 21 of the Code of Federal Regulations.
Review of the firm’s product labeling during the inspection revealed that the product listed above was being marketed as an infant formula.
These products have not been manufactured in compliance with infant formula regulations.
Following discussion with the FDA, Bobbie Baby Inc. has agreed to remove the violative product from the market and is working with FDA to alert consumers.
Safety Alert for Tattoo Ink
Template 1

Certain Tattoo Inks Contaminated with Microorganisms; April 2019
FDA warns public to avoid recalled tattoo inks
April 01, 2019
Recommendation
The FDA has become aware of contaminated tattoo inks through its FY2018-2019 inspections of distributors and manufacturers, and subsequent microbiological analysis of sampled tattoo inks.
The FDA is alerting consumers, tattoo artists, and retailers to avoid the tattoo inks that have been recalled due to contamination with bacteria on the list below.
Recommendations for Consumers
Ask the tattoo artist or studio about the tattoo ink they use and avoid the tattoo inks listed below, due to risk of infection and injury.
Recommendations for Tattoo Artists, and Retailers
Avoid using or selling the tattoo inks mentioned below, due to risk of infection and injury
Background
The FDA has identified 7 tattoo inks contaminated with pathogens harmful to human health. The tattoo inks were manufactured or distributed by 4 out of 12 inspected firms. In addition to the 7 contaminated inks, we found low counts of bacteria in about 50% of 60 analyzed inks, which may also present a health hazard. Tattoo inks were analyzed using methods described in the Bacteriological Analytical Manual Chapter 23: Microbiological Methods for Cosmetics, which is the general method used to determine bacterial contamination of cosmetics.
The FDA will continue to work with manufacturers and retailers to remove the contaminated product from the market.
What Products are recalled?
The following tattoo inks have been recalled because they are contaminated with microorganisms:
Scalpaink SC, Scalpaink PA, and Scalpaink AL tattoo inks manufactured by Scalp Aesthetics (all lots)
Dynamic Color - Black tattoo ink manufactured by Dynamic Color Inc and distributed by Salt & Light Tattoo Supply and Teen Bean Ventures LLC (dba Antone's Ink) (lots 12024090 and 12026090)
Solid Ink-Diablo tattoo ink manufactured by Color Art Inc. (dba Solid Ink) and distributed by Teen Bean Ventures LLC (dba Antone's Ink) (lot 10.19.18)
*Intenze-True Black tattoo ink manufactured by Intenze Products Inc and distributed by Kingpin Tattoo Supply (lot SS278)
What is microorganism contamination?
Tattoo inks contaminated with microorganisms even at low numbers can cause infections and lead to serious health injuries, when injected into the skin during tattooing procedure, since there is an increased risk of infection any time the skin barrier is broken.
Commonly reported symptoms of tattoo ink associated infections include the appearance of rashes or lesions consisting of red papules in areas where the contaminated ink has been applied. Some tattoo infections can result in permanent scarring. Indications of an infection can be difficult to recognize as other conditions (e.g., allergic reactions) may initially have similar signs and symptoms, leading to misdiagnosis and ineffective treatments.
Who to Contact
Consumers who have symptoms should contact their health care provider to report their symptoms and receive care.
To report a complaint or adverse event (illness or serious allergic reaction), you can
Call an FDA Consumer Complaint Coordinator if you wish to speak directly to a person about your problem.
Complete an electronic Voluntary MedWatch form online.
Complete a paper Voluntary MedWatch form that can be mailed to FDA.
Visit www.fda.gov/fcic for additional consumer and industry assistance.
Submit Questions Electronically
Safety Alert for Tattoo Ink
Template 2

FDA Advises Consumers, Tattoo Artists, and Retailers to Avoid Using or Selling Certain Tattoo Inks Contaminated with Microorganisms
Date Issued
April 01, 2019
Audience
Consumers who are considering a new tattoo
Tattoo artists
Retailers of tattoo inks
Product
The following tattoo inks have been recalled because they are contaminated with microorganisms:
Scalpaink SC, Scalpaink PA, and Scalpaink AL tattoo inks manufactured by Scalp Aesthetics (all lots)
Dynamic Color - Black tattoo ink manufactured by Dynamic Color Inc and distributed by Salt & Light Tattoo Supply and Teen Bean Ventures LLC (dba Antone's Ink) (lots 12024090 and 12026090)
Solid Ink-Diablo tattoo ink manufactured by Color Art Inc. (dba Solid Ink) and distributed by Teen Bean Ventures LLC (dba Antone's Ink) (lot 10.19.18)
*Intenze-True Black tattoo ink manufactured by Intenze Products Inc and distributed by Kingpin Tattoo Supply (lot SS278)
Purpose
The FDA is alerting consumers, tattoo artists, and retailers of the potential for serious injury from use of tattoo inks that are contaminated with bacteria. Tattoo inks contaminated with microorganisms even at low numbers can cause infections and lead to serious health injuries, when injected into the skin during tattooing procedure, since there is an increased risk of infection any time the skin barrier is broken.
Commonly reported symptoms of tattoo ink associated infections include the appearance of rashes or lesions consisting of red papules in areas where the contaminated ink has been applied. Some tattoo infections can result in permanent scarring. Indications of an infection can be difficult to recognize as other conditions (e.g., allergic reactions) may initially have similar signs and symptoms, leading to misdiagnosis and ineffective treatments.
Summary of Problem and Scope
The FDA has become aware of contaminated tattoo inks through its FY2018-2019 inspections of distributors and manufacturers, and subsequent microbiological analysis of sampled tattoo inks. The FDA has identified 7 tattoo inks contaminated with pathogens harmful to human health. The tattoo inks were manufactured or distributed by 4 out of 12 inspected firms. In addition to the 7 contaminated inks, we found low counts of bacteria in about 50% of 60 analyzed inks, which may also present a health hazard. Tattoo inks were analyzed using methods described in the Bacteriological Analytical Manual Chapter 23: Microbiological Methods for Cosmetics, which is the general method used to determine bacterial contamination of cosmetics.
Recommendations for Consumers
Ask the tattoo artist or studio about the tattoo ink they use and avoid the tattoo inks listed above, due to risk of infection and injury.
Recommendations for Tattoo Artists, and Retailers
Avoid using or selling the tattoo inks mentioned above, due to risk of infection and injury
FDA Actions
The FDA will continue to work with manufacturers and retailers to remove the contaminated product from the market.
Reporting Problems to the FDA
Consumers who have experienced symptoms of infection or an injury after administration of a tattoo, should consult their healthcare professional and inform their tattoo artist. Consumers should also consider reporting their injury to MedWatch: FDA’s Safety Information and Adverse Event Reporting Program. The FDA encourages consumers with questions about food safety to Submit an Inquiry, or to visit www.fda.gov/fcic for additional information.
*Pending recall as of 4/9/2019
Drafted: GPeriz: HFS-125: 4/2/2019
Reviewed: JHuang: HFS-125: 4/2/2019
Reviewed/ Edited: KDewan: HFS: 125: 4/3/2019
Reviewed/Edited: KScarborough: HFS: 125: 4/3/2019
Reviewed/edited: LMKatz: HFS-100: 4/8/2019
Safety Alert for Tattoo Ink
Template 3

FDA Advises Consumers, Tattoo Artists, and Retailers to Avoid Using or Selling Certain Tattoo Inks Contaminated with Microorganisms
Products
The following tattoo inks have been recalled because they are contaminated with microorganisms:
Scalpaink SC, Scalpaink PA, and Scalpaink AL tattoo inks manufactured by Scalp Aesthetics (all lots)
Dynamic Color - Black tattoo ink manufactured by Dynamic Color Inc and distributed by Salt & Light Tattoo Supply and Teen Bean Ventures LLC (dba Antone's Ink) (lots 12024090 and 12026090)
Solid Ink-Diablo tattoo ink manufactured by Color Art Inc. (dba Solid Ink) and distributed by Teen Bean Ventures LLC (dba Antone's Ink) (lot 10.19.18)
*Intenze-True Black tattoo ink manufactured by Intenze Products Inc and distributed by Kingpin Tattoo Supply (lot SS278)
Sold at
Tattoo Art Studios
Problem
Tattoo inks contaminated with microorganisms
FDA Actions
The FDA will continue to work with manufacturers and retailers to remove the contaminated product from the market.
Recommendations
The FDA is alerting consumers, tattoo artists, and retailers of the potential for serious injury from use of tattoo inks that are contaminated with bacteria. Tattoo inks contaminated with microorganisms even at low numbers can cause infections and lead to serious health injuries, when injected into the skin during tattooing procedure, since there is an increased risk of infection any time the skin barrier is broken.
Commonly reported symptoms of tattoo ink associated infections include the appearance of rashes or lesions consisting of red papules in areas where the contaminated ink has been applied. Some tattoo infections can result in permanent scarring. Indications of an infection can be difficult to recognize as other conditions (e.g., allergic reactions) may initially have similar signs and symptoms, leading to misdiagnosis and ineffective treatments.
Most Recent Update
Outbreak Advisory for Romaine Lettuce
Template 1

Outbreak Investigation of lllnesses caused by E. coli O157:H7 November 2019
FDA
working to determine the source of contamination found in Ready Pac
Bistro Chicken Caesar Salad bought in Maryland
November 20, 2019
Recommendation
Consumers are advised not to eat Ready Pac Bistro® Chicken Caesar Salad, lot #255406963, UPC 0 77745 27249 8, “Best By” date Oct. 31, 2019, purchased from Sam’s Club stores in Maryland. State testing of unopened salad purchased by an ill person identified the presence of E. coli O157 in the romaine lettuce. It should be noted that the “Best By” date was almost 3 weeks ago, so this product is not likely in stores. Consumers should not eat this specific product, regardless of where it was purchased.
Background
FDA, CDC and state health authorities are investigating an outbreak of illnesses caused by E. coli O157:H7 in the U.S.
According to the CDC, as of November 18, 2019, 17 people infected with the outbreak strain of E. coli O157:H7 have been reported from eight states. The case patients report that illnesses started on dates ranging from September 24, 2019 to November 8, 2019.
Two cases reported from Maryland have been linked to this outbreak by Whole Genome Sequencing (WGS), through analysis of clinical samples taken from those patients. The Maryland Department of Health identified E. coli O157 in an unopened package of Ready Pac Bistro® Chicken Caesar Salad collected from an ill person’s home in Maryland which was purchased from a Sam’s Club in that state. Preliminary information indicates that romaine lettuce used in the product that tested positive was harvested in mid-October and is no longer within current expiration dates. To date, the food sample has not yet been definitively linked to the Maryland cases or other E. coli O157 illnesses in the multi-state outbreak. WGS analysis is currently underway for this sample to determine if it is closely related genetically to the E. coli found in people in this outbreak.
As analysis is underway, FDA is tracing back the supply of the romaine lettuce in the Caesar salad. FDA has identified possible farms in Salinas, California. FDA is deploying investigators to the farms in question to determine the source and extent of the contamination. More information will be forthcoming as the investigation proceeds.
Although the ill people interviewed in Maryland reported eating Ready Pac Bistro® Chicken Caesar Salad, at this time, ill people in other states have not reported eating this particular salad. Therefore, exposure to this product alone does not fully explain other cases in the outbreak.
State and local public health officials are interviewing ill people to determine what they ate and other exposures of interest in the week before their illness started.

Case Counts
Total
Illnesses: 17
Hospitalizations: 7
Deaths: 0
Last
illness onset: November 8, 2019
States with Cases: AZ (1), CA
(2), CO (1), ID (3), MD (2), MT (1), WA (1), WI (6)
What is E. coli?
E. coli are mostly harmless bacteria that live in the intestines of people and animals and contribute to intestinal health. However, eating or drinking food or water contaminated with certain types of E. coli can cause mild to severe gastrointestinal illness. Some types of pathogenic (illness-causing) E. coli, such as Shiga toxin-producing E. coli (STEC), can be life-threatening.
People infected with pathogenic E. coli can start to notice symptoms anywhere from a few days after consuming contaminated food or as much as nine days later. Generally, the symptoms include severe stomach cramps, diarrhea, fever, nausea, and/or vomiting.
The severity or presence of certain symptoms may depend on the type of pathogenic E. coli causing the infection. Some infections can cause severe bloody diarrhea and lead to life-threatening conditions, such as a type of kidney failure called hemolytic uremic syndrome (HUS), or the development of high blood pressure, chronic kidney disease, and neurologic problems. Other infections may have no symptoms or may resolve without medical treatment within five to seven days.
Due to the range in severity of illness, people should consult their health care provider if they suspect that they have developed symptoms that resemble an E. coli infection, including HUS, but even healthy older children and young adults can become seriously ill.
People of any age can become infected with pathogenic E. coli. Children under the age of 5 years, adults older than 65, and people with weakened immune systems are more likely to develop severe illness as a result of an E. coli infection. However, even healthy older children and young adults can become seriously ill.
General Food Safety Tips for Retailers
Restaurants and retailers should always practice safe food handling and preparation measures. It is recommended that employees wash hands, utensils, and surfaces with hot, soapy water before and after handling food.
Regular frequent cleaning and sanitizing of food contact surfaces and utensils used in food preparation may help to minimize the likelihood of cross-contamination.
Wash and sanitize display cases and refrigerators regularly.
Wash and sanitize cutting boards, surfaces, and utensils used to prepare, serve, or store food.
Wash hands with hot water and soap following the cleaning and sanitation process.
General Food Safety Tips for Consumers
People should consult their health care provider if they suspect that they have developed symptoms that resemble an E. coli infection.
Consumers should follow these steps for preventing foodborne illness:
Wash the inside walls and shelves of the refrigerator, cutting boards and countertops, and utensils that may have contacted contaminated foods; then sanitize them with a solution of one tablespoon of chlorine bleach to one gallon of hot water; dry with a clean cloth or paper towel that has not been previously used.
Wash and sanitize surfaces used to serve or store potentially contaminated products.
Wash hands with warm water and soap following the cleaning and sanitation process.
Consumers can also submit a voluntarily report, a complaint, or adverse event (illness or serious allergic reaction) related to a food product.
Who to Contact
Consumers who have symptoms should contact their health care provider to report their symptoms and receive care.
To report a complaint or adverse event (illness or serious allergic reaction), you can
Call an FDA Consumer Complaint Coordinator if you wish to speak directly to a person about your problem.
Complete an electronic Voluntary MedWatch form online.
Complete a paper Voluntary MedWatch form that can be mailed to FDA.
Visit www.fda.gov/fcic for additional consumer and industry assistance.
Submit Questions Electronically
Additional Information
Outbreak Advisory for Romaine Lettuce
Template 2

FDA Advises Consumers to not eat certain Ready Pac Bistro® Chicken Caesar Salads purchased from Sam’s Club stores in Maryland
November 20, 2019
Audience
Consumers
Product
Product Name: Ready Pac Bistro® Chicken Caesar Salad, lot, purchased from Sam’s Club stores in Maryland.
Packaging: Green and white packaging with a Bistro label on the front
Lot numbers: Codes on the product of concern are; #255406963, UPC 0 77745 27249 8
Dates: Products of concern with Best Buy date: Oct. 31, 2019
Purpose
Consumers are advised not to eat Ready Pac Bistro® Chicken Caesar Salad, lot #255406963, UPC 0 77745 27249 8, “Best By” date Oct. 31, 2019, purchased from Sam’s Club stores in Maryland. State testing of unopened salad purchased by an ill person identified the presence of E. coli O157 in the romaine lettuce. It should be noted that the “Best By” date was almost 3 weeks ago, so this product is not likely in stores. Consumers should not eat this specific product, regardless of where it was purchased.
E. coli are mostly harmless bacteria that live in the intestines of people and animals and contribute to intestinal health. However, eating or drinking food or water contaminated with certain types of E. coli can cause mild to severe gastrointestinal illness. Some types of pathogenic (illness-causing) E. coli, such as Shiga toxin-producing E. coli (STEC), can be life-threatening.
People infected with pathogenic E. coli can start to notice symptoms anywhere from a few days after consuming contaminated food or as much as nine days later. Generally, the symptoms include severe stomach cramps, diarrhea, fever, nausea, and/or vomiting.
The severity or presence of certain symptoms may depend on the type of pathogenic E. coli causing the infection. Some infections can cause severe bloody diarrhea and lead to life-threatening conditions, such as a type of kidney failure called hemolytic uremic syndrome (HUS), or the development of high blood pressure, chronic kidney disease, and neurologic problems. Other infections may have no symptoms or may resolve without medical treatment within five to seven days.
Due to the range in severity of illness, people should consult their health care provider if they suspect that they have developed symptoms that resemble an E. coli infection, including HUS, but even healthy older children and young adults can become seriously ill.
People of any age can become infected with pathogenic E. coli. Children under the age of 5 years, adults older than 65, and people with weakened immune systems are more likely to develop severe illness as a result of an E. coli infection. However, even healthy older children and young adults can become seriously ill.
Summary of Problem and Scope
FDA, CDC and state health authorities are investigating an outbreak of illnesses caused by E. coli O157:H7 in the U.S.
According to the CDC, as of November 18, 2019, 17 people infected with the outbreak strain of E. coli O157:H7 have been reported from eight states. The case patients report that illnesses started on dates ranging from September 24, 2019 to November 8, 2019.
Two cases reported from Maryland have been linked to this outbreak by Whole Genome Sequencing (WGS), through analysis of clinical samples taken from those patients. The Maryland Department of Health identified E. coli O157 in an unopened package of Ready Pac Bistro® Chicken Caesar Salad collected from an ill person’s home in Maryland which was purchased from a Sam’s Club in that state. Preliminary information indicates that romaine lettuce used in the product that tested positive was harvested in mid-October and is no longer within current expiration dates. To date, the food sample has not yet been definitively linked to the Maryland cases or other E. coli O157 illnesses in the multi-state outbreak. WGS analysis is currently underway for this sample to determine if it is closely related genetically to the E. coli found in people in this outbreak.
Although the ill people interviewed in Maryland reported eating Ready Pac Bistro® Chicken Caesar Salad, at this time, ill people in other states have not reported eating this particular salad. Therefore, exposure to this product alone does not fully explain other cases in the outbreak.
State and local public health officials are interviewing ill people to determine what they ate and other exposures of interest in the week before their illness started.
FDA Actions
FDA is tracing back the supply of the romaine lettuce in the Caesar salad. FDA has identified possible farms in Salinas, California. FDA is deploying investigators to the farms in question to determine the source and extent of the contamination. More information will be forthcoming as the investigation proceeds.
Recommendations for Consumers
Consumers are advised not to eat Ready Pac Bistro® Chicken Caesar Salad, lot #255406963, UPC 0 77745 27249 8, “Best By” date Oct. 31, 2019, purchased from Sam’s Club stores in Maryland. State testing of unopened salad purchased by an ill person identified the presence of E. coli O157 in the romaine lettuce. It should be noted that the “Best By” date was almost 3 weeks ago, so this product is not likely in stores. Consumers should not eat this specific product, regardless of where it was purchased.
To report a complaint or adverse event (illness or serious allergic reaction), you can
Call an FDA Consumer Complaint Coordinator if you wish to speak directly to a person about your problem.
Complete an electronic Voluntary MedWatch form online.
Complete a paper Voluntary MedWatch form that can be mailed to FDA.
Visit www.fda.gov/fcic for additional consumer and industry assistance.

Outbreak Advisory for Romaine Lettuce
Template 3

Outbreak Investigation of lllnesses caused by E. coli O157:H7 (November 2019)
FDA working to determine the source of contamination found in Ready Pac Bistro Chicken Caesar Salad bought in Maryland
R ecommendations
as of November 20, 2019
ecommendations
as of November 20, 2019
FDA, CDC and state health authorities are investigating an outbreak of illnesses caused by E. coli O157:H7 in the U.S.
FDA, CDC and state health authorities are investigating an outbreak of illnesses caused by E. coli O157:H7 in the U.S.
Consumers are advised not to eat Ready Pac Bistro® Chicken Caesar Salad, lot #255406963, UPC 0 77745 27249 8, “Best By” date Oct. 31, 2019, purchased from Sam’s Club stores in Maryland. State testing of unopened salad purchased by an ill person identified the presence of E. coli O157 in the romaine lettuce. It should be noted that the “Best By” date was almost 3 weeks ago, so this product is not likely in stores. Consumers should not eat this specific product, regardless of where it was purchased.
Case Counts
Total Illnesses: 17
Hospitalizations: 7
Deaths: 0
Last illness onset: November 8, 2019
States with Cases: AZ (1), CA (2), CO (1), ID (3), MD (2), MT (1), WA (1), WI (6)
Background & Previous Updates
According to the CDC, as of November 18, 2019, 17 people infected with the outbreak strain of E. coli O157:H7 have been reported from eight states. The case patients report that illnesses started on dates ranging from September 24, 2019 to November 8, 2019.
Two cases reported from Maryland have been linked to this outbreak by Whole Genome Sequencing (WGS), through analysis of clinical samples taken from those patients. The Maryland Department of Health identified E. coli O157 in an unopened package of Ready Pac Bistro® Chicken Caesar Salad collected from an ill person’s home in Maryland which was purchased from a Sam’s Club in that state. Preliminary information indicates that romaine lettuce used in the product that tested positive was harvested in mid-October and is no longer within current expiration dates. To date, the food sample has not yet been definitively linked to the Maryland cases or other E. coli O157 illnesses in the multi-state outbreak. WGS analysis is currently underway for this sample to determine if it is closely related genetically to the E. coli found in people in this outbreak.
As analysis is underway, FDA is tracing back the supply of the romaine lettuce in the Caesar salad. FDA has identified possible farms in Salinas, California. FDA is deploying investigators to the farms in question to determine the source and extent of the contamination. More information will be forthcoming as the investigation proceeds.
Although the ill people interviewed in Maryland reported eating Ready Pac Bistro® Chicken Caesar Salad, at this time, ill people in other states have not reported eating this particular salad. Therefore, exposure to this product alone does not fully explain other cases in the outbreak.
State and local public health officials are interviewing ill people to determine what they ate and other exposures of interest in the week before their illness started.
What is E. coli?
E. coli are mostly harmless bacteria that live in the intestines of people and animals and contribute to intestinal health. However, eating or drinking food or water contaminated with certain types of E. coli can cause mild to severe gastrointestinal illness. Some types of pathogenic (illness-causing) E. coli, such as Shiga toxin-producing E. coli (STEC), can be life-threatening.
People infected with pathogenic E. coli can start to notice symptoms anywhere from a few days after consuming contaminated food or as much as nine days later. Generally, the symptoms include severe stomach cramps, diarrhea, fever, nausea, and/or vomiting.
The severity or presence of certain symptoms may depend on the type of pathogenic E. coli causing the infection. Some infections can cause severe bloody diarrhea and lead to life-threatening conditions, such as a type of kidney failure called hemolytic uremic syndrome (HUS), or the development of high blood pressure, chronic kidney disease, and neurologic problems. Other infections may have no symptoms or may resolve without medical treatment within five to seven days.
Due to the range in severity of illness, people should consult their health care provider if they suspect that they have developed symptoms that resemble an E. coli infection, including HUS, but even healthy older children and young adults can become seriously ill.
People of any age can become infected with pathogenic E. coli. Children under the age of 5 years, adults older than 65, and people with weakened immune systems are more likely to develop severe illness as a result of an E. coli infection. However, even healthy older children and young adults can become seriously ill.
General Food Safety Tips for Retailers
Restaurants and retailers should always practice safe food handling and preparation measures. It is recommended that employees wash hands, utensils, and surfaces with hot, soapy water before and after handling food.
Regular frequent cleaning and sanitizing of food contact surfaces and utensils used in food preparation may help to minimize the likelihood of cross-contamination.
Wash and sanitize display cases and refrigerators regularly.
Wash and sanitize cutting boards, surfaces, and utensils used to prepare, serve, or store food.
Wash hands with hot water and soap following the cleaning and sanitation process.
General Food Safety Tips for Consumers
People should consult their health care provider if they suspect that they have developed symptoms that resemble an E. coli infection.
Consumers should follow these steps for preventing foodborne illness:
Wash the inside walls and shelves of the refrigerator, cutting boards and countertops, and utensils that may have contacted contaminated foods; then sanitize them with a solution of one tablespoon of chlorine bleach to one gallon of hot water; dry with a clean cloth or paper towel that has not been previously used.
Wash and sanitize surfaces used to serve or store potentially contaminated products.
Wash hands with warm water and soap following the cleaning and sanitation process.
Consumers can also submit a voluntarily report, a complaint, or adverse event (illness or serious allergic reaction) related to a food product.
Who to Contact Consumers
who have symptoms should
contact their health care provider to report their symptoms and
receive care. To
report a complaint or adverse
event (illness
or serious allergic reaction), you can Call
an FDA Consumer
Complaint Coordinator if
you wish to speak directly to a person about your problem. Complete
an electronic
Voluntary MedWatch form online. Complete
a paper
Voluntary MedWatch form that
can be mailed to FDA. Visit www.fda.gov/fcic for
additional consumer and industry assistance.
Additional Information
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Wu, Fanfan |
| File Modified | 0000-00-00 |
| File Created | 2021-01-14 |
© 2026 OMB.report | Privacy Policy
