SS A_clean 1.06.2020
SS A_clean 1.06.2020.docx
Comprehensive HIV Prevention and Care for Men Who Have Sex with Men of Color
OMB: 0920-1178
Comprehensive HIV Prevention and Care for Men Who Have Sex with Men of Color
OMB No. 0920-1178
Extension
SUPPORTING STATEMENT A
January 6, 2020
Project Officers
Ken Dominguez, MD, MPH
Phone: 404-639-6129
Fax: 404-639-6127
Email: [email protected]
Kashif Iqbal, MPH
Phone: 404-718-2038
Fax: 404-639-6127
Email: [email protected]
Mary Tanner, MD
Phone: 404-639-6376
Fax: 404-639-6127
Email: [email protected]
Centers of Disease Control and Prevention
National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention
Division
of HIV/ AIDS Prevention- Surveillance and Epidemiology
HIV
Epidemiology Branch
1600 Clifton Rd., MS E-45
Atlanta, GA 30333
TABLE OF CONTENTS
A. Justification
Circumstances Making the Collection of Information Necessary
Purpose and Use of the Information Collection
Use of Improved Information Technology and Burden Reduction
Efforts to Identify Duplication and Use of Similar Information
Impact on Small Businesses or Other Small Entities
Consequences of Collecting the Information Less frequently
Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
Explanation of Any Payment or Gift to Respondents
Protection of the Privacy and Confidentiality of Information Provided by Respondents
Institutional Review Board (IRB) and Justification for Sensitive Questions
Estimates of Annualized Burden Hours and Costs
Estimates of Other Total Annual Cost Burden to Respondents and Record Keepers
Annualized Cost to the Government
Explanation for Program Changes or Adjustments
Plans for Tabulation and Publication and Project Time Schedule
Reason(s) Display of OMB Expiration Date is Inappropriate
Exceptions to Certification for Paperwork Reduction Act Submissions
Exhibits
Exhibit 12.A Estimated Annualized Burden Hours
Exhibit 12.B Estimated Annualized Burden Costs
Exhibit 14.A Estimated Cost to the Government
LIST OF ATTACHMENTS
Attachment 1 Authorizing Legislation
Attachment 2 60-Day Federal Register Notice
Attachment 3 List of THRIVE Awardees
Attachment 4 Semi-annual Reporting of Monitoring and Evaluation (M&E) Variables – File Specifications
Attachment 5 Annual Collaborative Process and Outcome Evaluation – File Specifications
Attachment 6 Annual Collaborative Assessment Tool (CAT) - File Specifications
Attachment 7 Annual Funding Allocation Report – File Specifications
Attachment 8 Data Security Guidelines
Attachment 9 Project Determination
Attachment 10 THRIVE data collection summary from April 2017 to April 2019
Attachment 11 Screenshots
Attachment 12 Privacy Impact Assessment (PIA) Form

Abstract
• Goal of the study: To support state and local health departments to develop and implement demonstration projects for provision of comprehensive human immunodeficiency virus (HIV) prevention and care services for men who have sex with men (MSM) of color by creating a collaborative with community based organizations (CBOs), clinics and other health care providers, and behavioral health and social services providers in their jurisdiction. Navigators will be hired to assist clients reach next steps in the HIV prevention and care continuums of care and link to behavioral health and social services.
• Intended use of the resulting data: To assist health departments and their collaborative in monitoring and evaluating their activities to help them develop, deliver, and refine successful HIV prevention and care interventions for MSM of color. These data are also used to report key program performance indicators from the collaborative to the health department and then to CDC to show whether the funded programs are efficient and effective in achieving their stated goals.
• Methods: CDC has established guidelines for monitoring and evaluation content, but is permitting a flexible approach to reporting, recognizing that capacity building and technical assistance are activities under the cooperative agreement. The funded health departments and their collaborative will determine how data are to be collected. Many grantees will use their own data system. A qualitative approach through interviews and surveys will be used to evaluate community collaborative and funding allocation to meet programmatic goals.
• Subpopulation: The population targeted for this project are MSM of color at risk for and living with HIV infection in the funded health departments and their collaborative.
• How data will be analyzed: Descriptive analyses will be conducted using appropriate statistical software (i.e. SAS) on client-level data variables related to HIV prevention and care services, the collaborative assessment (qualitative and quantitative), and funding allocation in attachments 4-7. Monitoring and Evaluation variables will be reported to CDC twice a year by all THRIVE grantees, while qualitative assessments related to the community collaborative and funding allocation will be reported annually.
Circumstances Making the Collection of Information Necessary
CDC requests a 3-year OMB approval to extend the information collection entitled, “Comprehensive HIV Prevention and Care for Men Who Have Sex with Men of Color” (0920-1178, expiration April 30, 2020). CDC is authorized to conduct the information collection under Section 306 of the public Health Services Act [42 U.S.C.A.2. (Attachment 1).”
THRIVE has successfully collected information for monitoring and assessing the demonstration programs since the OMB approval was granted in April 2017. However, the project will be entering a no-cost extension year from September 30, 2019 to September 29, 2020 and will need to collect information from the demonstration programs after the current approval expires on April 30, 2020 (see attachment 10 for an update of the data collections from April 2017 to April 2019).
Background:
Approximately 40,000 people in the United States are newly infected with HIV each year. Gay, bisexual, and other men who have sex with men (MSM) remain the population most affected by HIV infection in the United States (US). Among MSM, those who are black and Hispanic comprise 64% of all new infections. Goals of the National HIV Prevention Strategy and the new initiative “Ending the HIV Epidemic: A Plan for America” include increasing the number of MSM of color living with HIV infection who achieve HIV viral suppression with antiretroviral treatment (ART), and decreasing the number of new HIV infections among MSM of color at risk of acquiring an HIV infection.
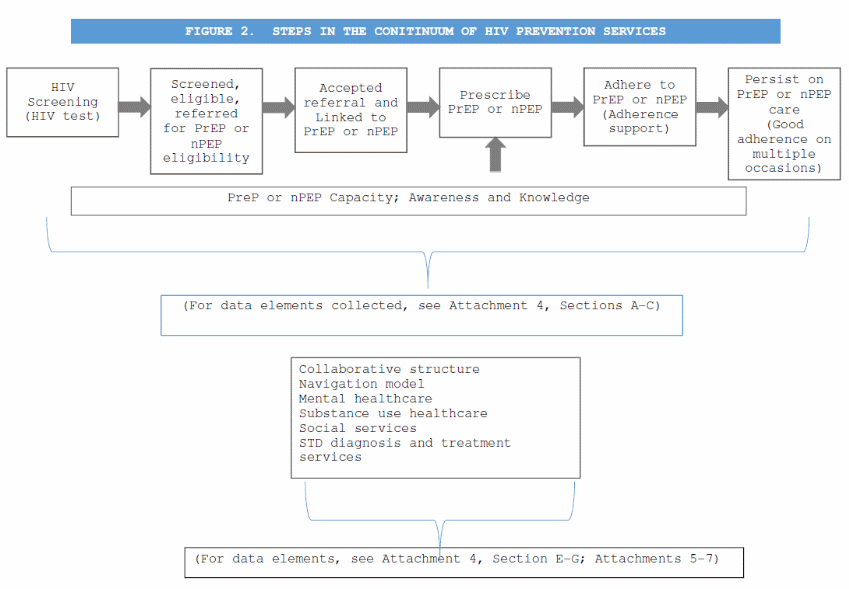
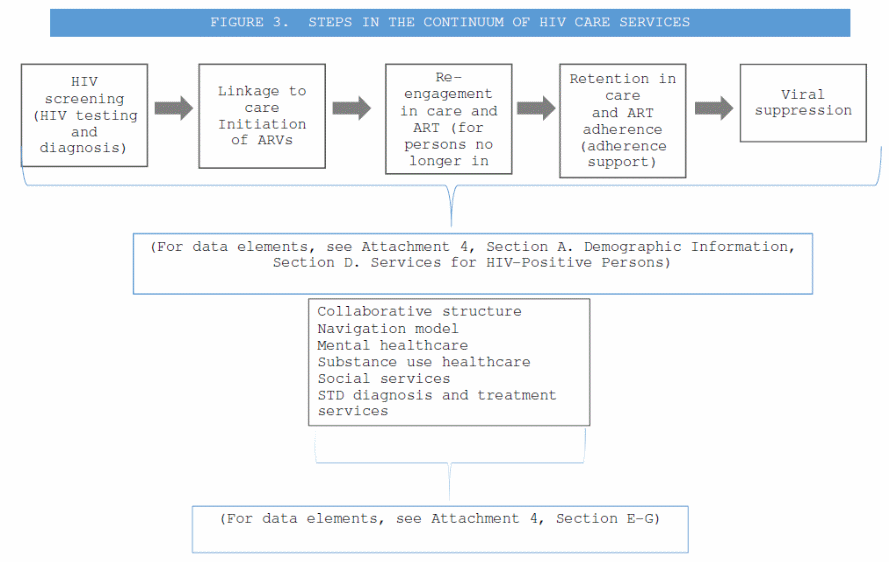
Achieving these outcomes requires that men utilize a broad variety of HIV prevention and care services. The continuum of HIV prevention services includes HIV testing,[1] offering HIV-negative men at risk for acquiring HIV infection biomedical interventions such as preexposure prophylaxis (PrEP)[2] or postexposure prophylaxis (PEP),[3] prescribing the medications needed for PrEP or PEP, adhering to these medications as prescribed, returning for follow-up visits to support adherence, and monitoring potential medication side effects. 4th generation HIV testing[4] is recommended because it is the most sensitive HIV diagnostic test, allowing acute HIV infection to be diagnosed, allowing health department to prioritize initiation of treatment early in the course of infection to improve long-term health outcomes and prevent secondary HIV transmission. State and local health departments are authorized to provide HIV testing, prevention, and care services, and so are essential partners in carrying out Thrive’s programmatic goals. The continuum of HIV care services[5] includes HIV testing and notifying HIV-positive men of their results, linking HIV-positive men to their initial HIV clinic visit, prescribing antiretroviral medications to treat HIV infection, adhering to antiretroviral medications, being retained[6] or re-engaged[7] in care, and achieving HIV viral suppression[8].
In many cases, men do not progress through the continuum to achieve the desired outcome of preventing HIV infection or achieving viral load suppression. For example, in 2011, although 84% of the estimated 647,700 MSM were diagnosed with HIV, only 38% were later engaged[9] in HIV care. In addition STDs have been associated with increased acquisition and transmission of HIV, so STD services are an essential adjunct to HIV prevention and care.[10]
Utilization of HIV and STD testing services, and adherence to treatment regimens can be enhanced by targeted referrals to ancillary services including mental health services, substance use services, and other social services that promote access to housing, job counseling, and employment opportunities. However, many of the service providers are independently funded and managed, i.e., they are not part of the health department organizational structure or co-located within a health department facility or clinic where HIV testing and treatment services are delivered. The lack of a unified administrative framework for all services is a barrier to the delivery of coordinated, tailored, and maximally effective HIV prevention and treatment plans.
Billing Services and Capacity Building are two other important components of assuring access and proper delivery prevention and care services. Historically, CDC has supported HIV testing conducted by health departments and its partners. However, the US Preventive Services Task Force graded routine HIV testing as an “A”, which ensures that health insurance plans must provide this service without patient cost sharing. Similarly, PrEP, PEP and STD services can also be billed to health insurance. Health departments, CBOs, and other partners can bill for these services and should be supported to develop the infrastructure for this. Also sustainability of these services will be supported in this project by training of health department and collaborative workforce.
In 2015, The Division of HIV/AIDS Prevention (DHAP) of the National Center for HIV, Viral Hepatitis, STD, and TB Prevention (NCHHSTP) of the Centers for Disease Control and Prevention (CDC) developed a cooperative agreement program to address the perceived fragmentation of HIV prevention and care services for MSM of color (“Comprehensive HIV Prevention and Care for Men Who Have Sex with Men of Color,” also referred to as THRIVE – Targeted Highly-Effective Interventions to Reverse the HIV Epidemic). The program is funded by the CDC and the Department of Health and Human Services (DHHS) Secretary’s Minority AIDS Initiative Funds. Awardees are state and local health departments (Attachment 3).
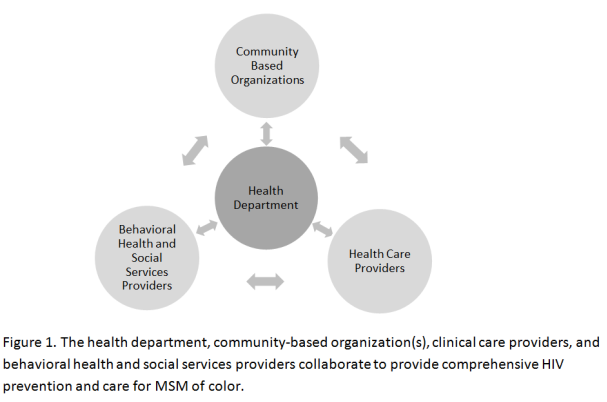
Each THRIVE awardee is working with partner organizations in its jurisdiction to provide enhanced coordination of comprehensive HIV prevention and care services for MSM of color. Each demonstration program is based on the concept of a health care collaborative involving the health department, community-based organizations (CBO), clinics and other health care providers, and behavioral health and social services providers (see Figure 1).
These services will be provided by a community collaborative[11] led by the health department, and that consists of several partners: community-based organizations (CBOs), healthcare providers, behavioral healthcare providers, and social service providers. But because of the fragmented nature of U.S. healthcare system, and the independent management and diverse funding streams and administration of each collaborative member, the health department will work cooperatively with CDC to form and sustain this collaborative and to provide technical assistance and support for them to deliver high quality HIV prevention and care services to men who require them. CDC needs to understand facilitators and barriers to developing and sustaining a community collaborative to guide the technical assistance it provides to the collaborative so it can optimally deliver all of the required HIV prevention and care services to all men who require them.
Because many of the service providers in the collaborative are not part of the health department organizational structure or co-located within a health department facility or clinic, the health departments will support navigators to help MSM of color to utilize all of the services. In order to provide technical assistance to ensure effective navigation models, CDC must understand the characteristics of the various models; how they integrate service utilization within the collaborative structure, and the number of men who they assist in utilizing services.
2. Purpose and Use of the Information Collection
The information collection plan includes a quantitative component that will allow CDC and THRIVE awardees to describe the number and types of services that health departments and collaborative members provide to clients, to identify opportunities for improving referrals, navigation, and coordination throughout the care continuum, and to account for costs of program implementation. The data will provide local program managers with information regarding intervention processes (e.g., who delivered what to whom, how many, where, and when) and client-level information (e.g., client demographics, utilization of services, and verified referrals to other services) for monitoring and enhancing local HIV prevention and care efforts. Other goals include maximizing sustainability of all services through ensuring billing and reimbursement for services and building capacity of local CBOs and other organizations to provide services. In addition, CDC will collect qualitative information from both THRIVE awardees and collaborative members to improve understanding of the processes used to establish the collaborative, and participants’ perceptions of those processes. The qualitative information collection will provide essential context for interpreting findings and developing recommendations for program improvement.
Semi-annual Services Report of Monitoring and Evaluation (M&E)Variables – File specifications (Attachment 4): Client level data will be transmitted electronically to CDC twice a year. Attachment 4 represents guidance for transmitting data. CDC has established guidelines for M&E content, but is permitting a flexible approach to reporting recognizing that capacity building and technical assistance are activities under the cooperative agreement. The data elements will include:
Population characteristics (Attachment 4, Section A. Demographic Information, data elements A1-A5). Semiannual services reports will be electronically transmitted by health departments for both persons at risk for acquiring HIV and HIV-positive persons when possible. The data sources for these data elements are from existing health department data collecting systems.
Continuum of HIV prevention services (Attachment 4, Section B. Services for Persons at Risk for HIV. Section C. Services for HIV-Negative Persons). Semiannual services reports will be submitted by health departments and those data will include data elements describing the steps in the continuum as seen in Figure 2. HIV Prevention Continuum.
Continuum of HIV care services (Attachment 4, Section B. Services for Persons at Risk for HIV, Section D. Services for HIV-Positive Persons). Semiannual services reports will be submitted by health departments and those data will include data elements describing the steps in the continuum as seen in Figure 3.
Other Integral Services for HIV-positive and HIV-negative persons (Attachment 4, Section E. Services for HIV-positive and HIV-negative persons). Semiannual services reports will be submitted by health departments and those data will include data elements describing other integral services for both HIV-positive and HIV-negative services (e.g. STD services, partner services, behavioral health screening and linkage, social service screening and linkage, etc.)
Navigation services (Attachment 4, Section F. Navigation Services). Semiannual services reports will be submitted by health departments and those data will include data elements describing navigation services for both HIV-positive and HIV-negative services (e.g. navigation for linkage of services [PrEP initiation and ongoing PrEP care, navigation to support retention of HIV-positive persons in HIV care] navigation for assessing health insurance needs and enrolling in a health insurance plan.
Billing/Re-imbursement, Capacity Building, and Collaborations (Attachment 4, Section G. Billing/Reimbursement, Capacity Building, and Collaborations). Semiannual services reports will be submitted by health departments and those data will include data elements describing contracts awarded to implement projects, and numbers of CBOs and other organizations. These reports will also include data elements describing number of HIV tests, STD tests, nPEP health care services, and PrEP health care services that were reimbursed by a third party payer; number of trainings, staff hired, and contracts and partnerships executed for each health department and its collaborative workforce to provide culturally competent services.
Annual Services Reports
Reports to assist in the evaluation of the collaborative process and funding allocations related to HIV prevention and care activities will be submitted annually.
Collaboration Assessment and Evaluation (Attachments 5 and 6): To assess how successful grantees have been in creating, engaging, and sustaining collaborative partnerships and to understand how these partnerships contributed to achieving the goals of the project, we will use open-ended questions (Annual Collaborative Process and Outcome Evaluation – Attachment 5), and a survey (Annual Collaboration Assessment Tool – Attachment 6)(CAT). The health departments will use the guidance related to specific data elements from attachments 5 and 6 to develop their own electronic versions (e.g. word document) of these instruments. Both of these instruments will be administered by the health department and/or collaborative staff (e.g., CBOs, healthcare providers, social service providers). The Annual Collaborative Process and Outcome Evaluation will be administered as a face-to-face or telephone interview, with responses being typed into the electronic version and submitted electronically by the health department to the CDC. Similarly, The Annual Collaboration Assessment Tool will be administered as a survey by the Health Department and/or collaborative staff, with responses being typed into an electronic version and submitted by the health department to the CDC. We are in the process of developing an online version of the Collaborative Assessment Tool. When development of the online survey is complete, we plan to request OMB approval through the Change Request mechanism.
Annual Collaborative Process and Outcome Evaluation (Attachment 5, Annual Collaborative Process and Outcome Evaluation – File specifications, Data Elements CQ01-CQ12). Process questions include queries about reasons for involvement; clarity of collaborative goals; leadership support; resource input; collaborative activities; and collaborative operational success. Outcome questions include queries about collaborative success in achieving goals; impact on the collaborative; positive, negative, or unintended consequences of the collaboration; benefits from participating in the collaborative barriers to successful collaboration; and lessons learned from participating in the collaborative.
Collaboration Assessment (Attachment 6, Annual Collaboration Assessment Tool [CAT]- File specifications, Data Elements A1-H8). To understand the development and function of the collaborative and how they are related to the needs of the collaborators in the communities in which they operate, health department and collaborative staff will be asked to complete a survey. In order to assess collaborative processes and outcomes, several questions will be included in the survey. These will focus on the cultural, political, and legislative context of the community in which the collaborative is located; collaborative organizational processes including communication, defining goals and objectives, available resources, leadership capacity; and perception of collaborative success.
Funding Allocation (Attachment 7, Annual Funding Allocation Report – file specifications, Data Elements FA01-FA18). Annual summary reports of funds allocated for HIV prevention services for MSM of color at substantial risk of acquiring HIV infection; HIV care services for MSM of color living with HIV infection; navigation and linkage services; HIV or STD partner services; behavioral risk reduction counseling or interventions; behavioral health services; social services; program planning; program monitoring and evaluation; capacity building; general operations and administration; CBO contracts; other collaborative activities; and activities to reach MSM of color will be electronically submitted by health departments to CDC. The health departments will use the guidance related to specific data elements from attachment 7 to develop their own electronic instrument (e.g. word document) to report this funding information. Once this information is collected on an electronic version of the instrument, the health department will forward a completed copy of this electronic instrument to CDC.
3. Use of Improved Information Technology and Burden Reduction
Each of the 7 funded Health Departments and their collaborative will determine how data are to be collected. CDC has established guidelines for M&E content, but is permitting a flexible approach to reporting, recognizing that capacity building and technical assistance are activities under the cooperative agreement. For some types of data, CDC provides optional, modifiable data collection templates that grantees can change and adapt for their own procedures and additional local data needs (Attachment 4). Many grantees use their own data system and extract data in specified formats for upload into standard data reporting systems.
All M&E data are to be transmitted to CDC electronically. While grantees may collect the data by whatever means they choose, data must be transmitted to CDC electronically using a Secure File Transfer Protocol (FTP). Grantees are given the option of using their own software system to collect M&E data. Grantees who use their own software must collect the standardized M&E data and then transmit data electronically to CDC. For qualitative interview or survey data related to the collaborative and funding allocation, grantees will be unable to use existing software to collect this data, however CDC will provide technical assistance to help grantees develop methods for electronic data collection and transmission to CDC that meet NCHHSTP data security guidelines (Attachment 8).
4. Efforts to Identify Duplication and Use of Similar Information
Due diligence was applied to identify duplication of M&E data include the assessment of existing or previously used HIV prevention data collection systems used by CDC, other federal agencies, as well as health department jurisdictions and community-based organizations. It should be noted that because the M&E data reporting requirements are specific to CDC-funded HIV prevention activities, the only possible duplication is if other federal or state organizations or entities are also funding the same HIV prevention activities to be performed by the same grantees.
In addition to systems at CDC, other federal systems were reviewed. Specifically, consultations were held with the Health Resources and Services Administration (HRSA) and the Substance Abuse and Mental Health Services Administration (SAMHSA) to identify and match similar data elements to avoid duplication. Given that HRSA and SAMHSA do not collect detailed HIV prevention program data, very few similarities were identified. The only overlap detected was in the collection of HIV testing data, and SAMHSA determined that they would use the M&E HIV testing data variables to collect data from their grantees. SAMHSA submitted a separate ICR for this data collection. The burden for the SAMHSA data collection is not included in the burden calculations for this ICR.
If the number of new HIV infections is to be reduced, the quality of HIV prevention programs must be improved. The M&E data significantly advance the monitoring and evaluation of HIV prevention programs. On the local level, use of the standardized M&E variables will enhance the capacity of HIV prevention programs to thoroughly assess and refine their HIV prevention interventions and to identify unmet needs and redundancies while providing accountability to their stakeholders.
Impact on Small Business or Other Small Entities
No small businesses will be involved in this study.
Consequences of Collecting the Information Less Frequently
Respondents are asked to submit data to the CDC on a semiannual basis. Less frequent data submission would result in a lag time between the occurrence of program problems and their identification. This lag time could result in costly program inefficiencies, defects, and failures to continue or worsen without a timely opportunity for CDC to provide valuable assistance and corrective measures to agencies funded to prevent the spread of HIV. There are no legal obstacles to reducing the burden.
Special Circumstances relating to the Guidelines of 5 CFR 1320.5
This request fully complies with the guidelines of 5 CFR 1320.5.
Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
A 60-day federal register notice to solicit public comments was published in the Federal Register on 08/13/2018, Volume 84, Number 156, Pages 40059-40060 (attachment 2). CDC received no comments.
CDC consulted with Dr. Ron Valdiserri ([email protected]) Deputy Assistant Secretary for Health, Infectious Diseases, and Director of the Office of HIV/AIDS and Infectious Disease Policy, U.S. Department of Health and Human Services in the development of project.
Explanation of Any Payment or Gift to Respondents
No payments or gifts will be provided to respondents.
Protection of the Privacy and Confidentiality of Information Provided by Respondents
The CDC Privacy Office has determined that the information collected under this request will include personally identifiable information (PII) covered by the Privacy Act such as the name and address information, etc., which health jurisdictions may collect identifiers (name, address, etc.) on clients who receive HIV prevention services, including HIV testing as part of clinical care and follow up (attachment 12). This activity is covered under the Privacy Act System Notice 09-20-0136, “Epidemiologic Studies and Surveillance of Disease Problems. HHS/CDC”, which enables the Centers for Disease Control and Prevention (CDC) officials to collect information to better understand disease patterns in the United States, develop programs for prevention and control of health problems, and communicate new knowledge to the health community.
The M&E data is collected by health departments and partners as identifiable information for service delivery; but the data received by CDC is de-identified and coded. The information collected through the CAT process might be identifiable but to the extent that it’s about organizations, and not the individual respondents, the Privacy Act doesn’t apply. Similarly, the Privacy Act does not apply to the information collected through the annual funding allocation report as it does not include information about individual respondents.
Data collection and management will be conducted consistent with Attachment 8, which is the “Data Security and Confidentiality Guidelines” plan that has been reviewed by CDC’s Office of the Chief Information Security Officer (OCISO).
CDC will receive only secure de-identified M&E data from health jurisdictions from health jurisdictions for evaluation purposes. No patient identifiers will be transmitted to CDC. Attachment 8 includes a copy CDC’s National Center for HIV, Viral Hepatitis, STD, and TB Prevention (NCHHSTP) Data Security and Confidentiality Guidelines for its programs to “facilitate the secure collection, storage, and use of data while maintaining confidentiality.”
Institutional Review Board (IRB) and Justification for Sensitive Questions
IRB Approval
The Centers for Disease Control and Prevention, Division of STD, determined this collection does not involve human subjects research and therefore, IRB review and approval is not required(Attachment 9).
Sensitive Questions
Some of the client-level data to be collected are highly sensitive. HIV can be transmitted through sexual contact and the sharing of HIV contaminated needles and syringes. These modes of transmission necessitate the collection of sensitive data regarding sexual behaviors as well as alcohol and drug use. Because collection of these data will be used to provide improved HIV prevention services to high-risk populations, to enhance HIV prevention programs at the local level, and to reduce high-risk behaviors in persons most likely to acquire or transmit HIV, specific information about client demographics and client risk profiles is essential to designing appropriate interventions and programs and to monitoring and evaluating these programs.
This data collection also includes race and ethnicity questions, which may also be viewed as sensitive by some respondents, for use in data analysis (e.g., designing and evaluating programs, as discussed above).
12. Estimates of Annualized Burden Hours and Costs
The burden estimate is based on the participation of 7 THRIVE Awardees and 80 THRIVE Collaborative Partner organizations providing services to approximately 1,400 clients. To construct the burden table, we have allocated and rounded as follows:
Each Awardee is facilitating coordination, communication, and client-level reporting for an average of 11 Collaborative Partner organizations.
[(7 Awardees)*(11 Collaborative Partners per Awardee)] ~ 80 Collaborative Partners
Each Awardee and its Collaborative of 11 Partners will provide enhanced coordination of services to ~ 200 clients.
[(1,400 total clients) / (7 Awardee-based coalitions)] = 200 clients per Awardee-based coalition
The average number of clients per Collaborative Partner is 18.
[(18 clients per Collaborative Partner)*(11 Collaborative Partners per coalition)] = 198 total clients per coalition.
The majority of information collection will be conducted in 2 steps, where the first step involves each THRIVE Collaborative Partner reporting to its respective Awardee, and the second step involves the Awardee preparing and submitting client-level data to CDC. The exception is the Funding Allocation Report, which is only required for THRIVE Awardees.
Burden is assessed for each information collection step. To minimize burden, CDC has established the specifications for information collection content, but in many cases will allow Collaborative Partners and Awardees to select the reporting method best suited to each organization’s needs.
Each THRIVE Collaborative Partner will prepare a Semi-Annual Report of Monitoring and Evaluation (M&E) Variables (Attachment 4). This report provides client-level data on services by category (the number of services provided for persons at risk for HIV, the number of services for HIV-negative persons, etc.) and aggregate program-level data on capacity building. Each THRIVE Partner’s M&E Report may be submitted to the Awardee electronically in the format that is appropriate. The estimated burden for each Partner’s M&E report is 9 hours. We estimate 30 minutes per client to calculate the time needed to produce the Semi-Annual Report of Monitoring and Evaluation (M&E) Variables (Attachment 4)[(30 minutes per client)*(18 clients) = 540 minutes or 9 hours per M&E report].
Each Awardee will receive its Collaborative Partners’ M&E reports and then prepare client-level M&E data for submission to CDC. Awardees are expected to electronically transmit files that conform to NCHHSTP Data security confidentiality guidelines (Attachment 8). The estimated burden per response is 1 hour. For Collaborative Partners, the burden per response is based on the estimated time to extract information about clients from the system they normally use to support service delivery. For Awardees, the burden per response reflects the time needed to compile client-level data and to electronically transmit it to CDC according to NCHHSTP Data Security and Confidentiality Guidelines (Attachment 8).
Each THRIVE Collaborative Partner and Awardee will be asked to participate in an annual Qualitative Interview for Collaborative Process Evaluation. The same interview guide will be used for all interviews. The estimated burden per response is 40 minutes (Attachment 5).On an annual basis each THRIVE Partner will be asked to complete the Collaborative Assessment Tool (Attachment 6). The tool consists of a scoring rubric that complements the topics discussed in the qualitative interview. The estimated burden per response is 20 minutes. Awardees are expected to electronically transmit data to CDC according to NCHHSTP Data Security and Confidentiality Guidelines (Attachment 8).
Each THRIVE Awardee will submit an Annual Funding Allocation Report(FAR)(Attachment 7). The estimated burden per response is 20 minutes. All FAR information is submitted to CDC electronically.
Exhibit A.12-A. Estimated Annualized Burden Hours
Type of Respondent
Form Name
Number of Respondents
Number of Responses per Respondent
Average Burden per Response (in Hours)
Total Burden (in Hours)
THRIVE Partners
Monitoring and Evaluation Data Elements on HIV Prevention and Care Services
(att 4)
80
2
9
1,440
Qualitative Interview: Collaborative Process Evaluation
(att 5)
80
1
40/60
53
Collaborative Assessment Tool (att 6)
80
1
20/60
27
THRIVE Awardees
Monitoring and Evaluation Data Elements on HIV Prevention and Care Services
(att 4)
7
2
1
14
Qualitative Interview: Collaborative Process Evaluation (att 5)
7
1
40/60
5
Collaborative Assessment Tool (att 6)
7
1
20/60
2
Funding Allocation Report (att 7)
7
1
20/60
2
Total
1,543
B. Annualized Cost to Respondent
The collection and reporting of M&E data are part of the activities specified in the HIV prevention program announcements as part of the funded activities. Any expense incurred collecting and submitting the M&E data, above the routine collection of data required to conduct business, is supported by CDC funding through this project. There is no additional cost to the respondent.
The estimated cost to be supported by CDC funding is as follows. Based on a review of salaries reported by seven health department jurisdictions and their eighty collaborative members representing a range of sizes and HIV prevalence in their funding applications, it is estimated that health jurisdiction staff and their collaborative staff who collect M&E information will be paid about $54,000 annually. Based on the OMB Pay Tables for the Atlanta area, comparable annual salary for Federal General Schedule (GS) employees is that of a GS-9 step 3 ($53,949 annually or $25.85/hour).
Exhibit A.12-B. Annualized Cost to Respondents
Type of Respondent
Form Name
Total Burden (in Hours)
Hourly Wage Rate
Total Respondent Cost
THRIVE Partners
Monitoring and Evaluation Data Elements on HIV Prevention and Care Services (att 4)
1,440
$25.85
$37,224
Qualitative Interview: Collaborative Process Evaluation (att 5)
53
$25.85
$1,370.05
Collaborative Assessment Tool (att 6)
27
$25.85
$697.95
THRIVE Awardees
Monitoring and Evaluation Data Elements on HIV Prevention and Care Services (att 4)
14
$25.85
$361.90
Qualitative Interview: Collaborative Process Evaluation (att 5)
5
$25.85
$129.25
Collaborative Assessment Tool (att 6)
2
$25.85
$51.70
Funding Allocation Report (att 7)
2
$25.85
$51.70
Total
$39,886.55
Source: http://www.opm.gov/policy-data-oversight/pay-leave/salaries-wages/salary-tables/pdf/2015/ATL.pdf
13. Estimates of Other Total Annual Cost Burden to Respondents and Record Keepers
There are no other costs to respondents or record keepers associated with this study.
14. Annualized Cost to the Federal Government
The total annualized cost to the government is $472,825.60. Training for grantees is currently available online. Instruction will include topics such as confidentiality and computer security, evaluation principles, and use of data for program improvement. The base Federal General Schedule (GS) salary for full-time employees (FTEs) with experience in these areas is estimated to be a GS-13 step 9. It is expected that the equivalent of two FTEs paid $52.94/hour will each expend approximately twenty-five percent (25%) of their time or 1040 hours/FTE annually to oversee these trainings.
Technical assistance will be provided through an e-mail and telephone service center overseen by a CDC FTE. It is expected that the equivalent of two GS-13 step 9 ($52.94/hour) FTEs will expend approximately twenty-five percent (25%) of working hours (1040 hours) to oversee this service center.
Monitoring, analyzing, and reporting the M&E data are projected to require the expertise of the equivalent of two data managers and two data analysts. The data managers would be at the pay scale of GS-13 step 5 ($47.36/hour) and the data analysts would be at the pay scale of GS-12 step 5 ($39.83/hour).
Exhibit 14.A Annualized Cost to the Government
-
Employee Function
Annual Burden
(in hours)
Hourly Wage Rate
Annual Cost
Training
1,040
$52.94
$ 55,057.60
Technical Assistance
1,040
$52.94
$ 55,057.60
Monitoring, Analyzing and Reporting
4,160 (Data Managers)
$47.36
$197,017.60
4,160 (Data Analysts)
$39.83
$165,692.80
TOTAL ANNUAL FEDERAL GOVERNMENT COSTS: $472,825.60
Source: http://www.opm.gov/policy-data-oversight/pay-leave/salaries-wages/salary-tables/pdf/2015/ATL.pdf
15. Explanation for Program Changes or Adjustments
The burden has not changed from the current approval.
16. Plans for Tabulation and Publication and Project Time Schedule
Data collection will be conducted during the 3-year period after OMB approval. M&E data will be submitted to CDC on a semiannual basis (once as an M&E report alone and once as part of the larger annual progress report). Data analysis will occur within 12 months of final data collection. The following is a brief overview of the M&E process:
Activity
Time Schedule
Status
1st Semi-annual Reporting of Monitoring and Evaluation (M&E) Variables – File Specifications
.5 years after OMB approval
Completed
December 2017
1st Annual Progress report (2nd Semi-annual Reporting of Monitoring and Evaluation (M&E) Variables – File Specifications)
1 years after OMB approval
Completed
June 2018
1st Annual Collaborative Process and Outcome Evaluation – File Specifications
1 year after OMB approval
Completed
June 2018
1st Annual Collaborative Assessment Tool (CAT)
1 year after OMB approval
Started August 2018 completed
January 2019
1st Annual Funding Allocation Report – File Specifications
1 year after OMB approval
Completed
June 2018
3rd Semi-annual Reporting of Monitoring and Evaluation (M&E) Variables – File Specifications
1.5 years after OMB approval
Completed
December 2018
2nd Annual Progress report (4th Semi-annual Reporting of Monitoring and Evaluation (M&E) Variables – File Specifications
2 years after OMB approval
Completed
June 2018
2nd Annual Collaborative Process and Outcome Evaluation – File Specifications
2 years after OMB approval
Completed
June 2018
2nd Annual Collaborative Assessment Tool (CAT)
2 years after OMB approval
Modified-only one CAT survey has been conducted
2nd Annual Funding Allocation Report – File Specifications
2 years after OMB approval
Completed
June 2018
5th Semi-annual Reporting of Monitoring and Evaluation (M&E) Variables – File Specifications
2.5 years after OMB approval
Completed
December 2018
3rd Annual Progress report (6th Semi-annual Reporting of Monitoring and Evaluation (M&E) Variables – File Specifications)
3 years after OMB approval
Completed
June 2019
3rd Annual Collaborative Process and Outcome Evaluation – File Specifications
3 years after OMB approval
Completed
June 2019
3rd Annual Collaborative Assessment Tool (CAT)
3 years after OMB approval
Modified-only one CAT survey has been conducted
3rd Annual Funding Allocation Report – File Specifications
3 years after OMB approval
Completed
June 2019
6th Semi-annual Reporting of Monitoring and Evaluation (M&E) Variables – File Specifications
3.5 years after OMB approval
Completed
December 2019
4th Annual Progress report (6th Semi-annual Reporting of Monitoring and Evaluation (M&E) Variables – File Specifications)
4 years after OMB approval
Outstanding per OMB approval for extension
4th Annual Collaborative Process and Outcome Evaluation – File Specifications
4 years after OMB approval
Outstanding per OMB approval for extension
4th Annual Collaborative Assessment Tool (CAT)
4 years after OMB approval
Modified-only one CAT survey has been conducted
3rd Annual Funding Allocation Report – File Specifications
4 years after OMB approval
Outstanding per OMB approval for extension
7th Semi-annual Reporting of Monitoring and Evaluation (M&E) Variables – File Specifications
4.5 years after OMB approval
Outstanding per OMB approval for extension
5th Annual Progress report (6th Semi-annual Reporting of Monitoring and Evaluation (M&E) Variables – File Specifications)
5 years after OMB approval
Outstanding per OMB approval for extension
5th Annual Collaborative Assessment Tool (CAT)
5 years after OMB approval
Modified-only one CAT survey has been conducted with possibility of one additional survey after project ends
4th Annual Funding Allocation Report – File Specifications
5 years after OMB approval
Outstanding per OMB approval for extension
17. Reason(s) Display of OMB Expiration Date is Inappropriate
CDC is not seeking approval to not display the expiration date.
18. Exceptions to Certification for Paperwork Reduction Act (PRA) Submissions 5CFR 1320.3(h)(1)-(10)
There are no exceptions to the certification.
[1][1] Moyer VA. Screening for HIV: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2013; 159:51–60. http://annals.org/aim/article/1700660/screening-hiv-u-s-preventive-services-task-force-recommendation-statement
[2][2] PrEP – Use of daily oral antiretroviral preexposure prophylaxis to reduce the risk of acquiring HIV infection in adults. http://www.cdc.gov/hiv/pdf/guidelines/PrEPguidelines2014.pdf
[3][3] PEP – This project focuses on the use nonoccupational postexposure prophylaxis (nPEP) for purposes of reducing the risk of acquiring HIV infection in persons with isolated exposure outside of healthcare settings to blood, genital secretions, or other body fluids potentially infected with human immunodeficiency virus. http://www.cdc.gov/hiv/pdf/programresources/cdc-hiv-npep-guidelines.pdf
[4][4] Centers for Disease Control and Prevention and Association of Public Health Laboratories. Laboratory Testing for the Diagnosis of HIV Infection: Updated Recommendations. Available at http://stacks.cdc.gov/view/cdc/23447. Published June 27, 2014.http://www.cdc.gov/hiv/pdf/HIVtestingAlgorithmRecommendation-Final.pdf
[5[5] Bradley H, Hall HI, Wolitski RJ, et al. Vital signs: HIV diagnosis, care, and treatment among persons living with HIV: United States, 2011. MMWR Morb Mortal Wkly Rep. 2014;63(47):1113-1117.
[6][6] Rentention in care has been previously defined by CDC in one study as “ having two or more CD4+ or viral load tests ≥3 months apart during a given calendar year” Dasgupta S, Oster AM, Li J, Hall HI. Disparities in Consistent Retention in HIV Care — 11 States and the District of Columbia, 2011–2013. MMWR Morb Mortal Wkly Rep 2016;65:77–82. DOI: http://dx.doi.org/10.15585/mmwr.mm6504a2
[7][7] The definition of reengagement in care will differ by study and was previously defined in one CDC study as “patients who had not been seen in the 12-month period immediately preceding the year in which their records were included in the analysis but who had been seen in clinic at least once (after a specified date) Gardner L, Marks G, Craw J, et al. A low-effort, clinic-wide intervention improves attendance for HIV primary care. Clin Infect Dis. 2012;55:1124–1134. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3657526/
[8][8] The definition of undetectable viral load varies by study and sensitivity of viral load tests used. Undetectable viral was defined in one national CDC study as “documentation in the medical record of viral load <200 copies/mL at last viral load test in the 12 months preceding interview.” Bradley et al. MMWR Morb Mortal Wkly Rep. 2014;63(47):1113-1117.
[9][9] Engaged in care - having had an HIV medical care visit during the survey's sampling period of January–April 2011. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6347a5.htm
[11][11] Kevin J. Robinson MSW MHA DrPH (2009) Community Collaborative Partnerships: The Foundation for HIV Prevention Research Efforts, by Mary M. McKay and Roberta L. Paikoff (Eds.), Social Work in Health Care, 48:5, 551-554, http://dx.doi.org/10.1080/00981380802580729
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Iqbal, Kashif (CDC/OID/NCHHSTP) |
| File Modified | 0000-00-00 |
| File Created | 2021-01-15 |
© 2026 OMB.report | Privacy Policy