CMS-10530 Data Collection insruments
Ambulatory Surgical Center Quality Reporting Program (CMS-10530)
Ambulatory Surgical Center Quality Reporting (ASCQR) Data Collection Ins...
Ambulatory Surgical Center Quality Reporting (ASCQR) Program
OMB: 0938-1270
Ambulatory Surgical Center Quality Reporting (ASCQR) Program Data Collection Instruments
The screen shots provided below are taken from the data collection instruments for the ASCQR Program on QualityNet.org. These program measures represent current requirements for data submitted via a web-based tool for facilities participating in the ASCQR Program.
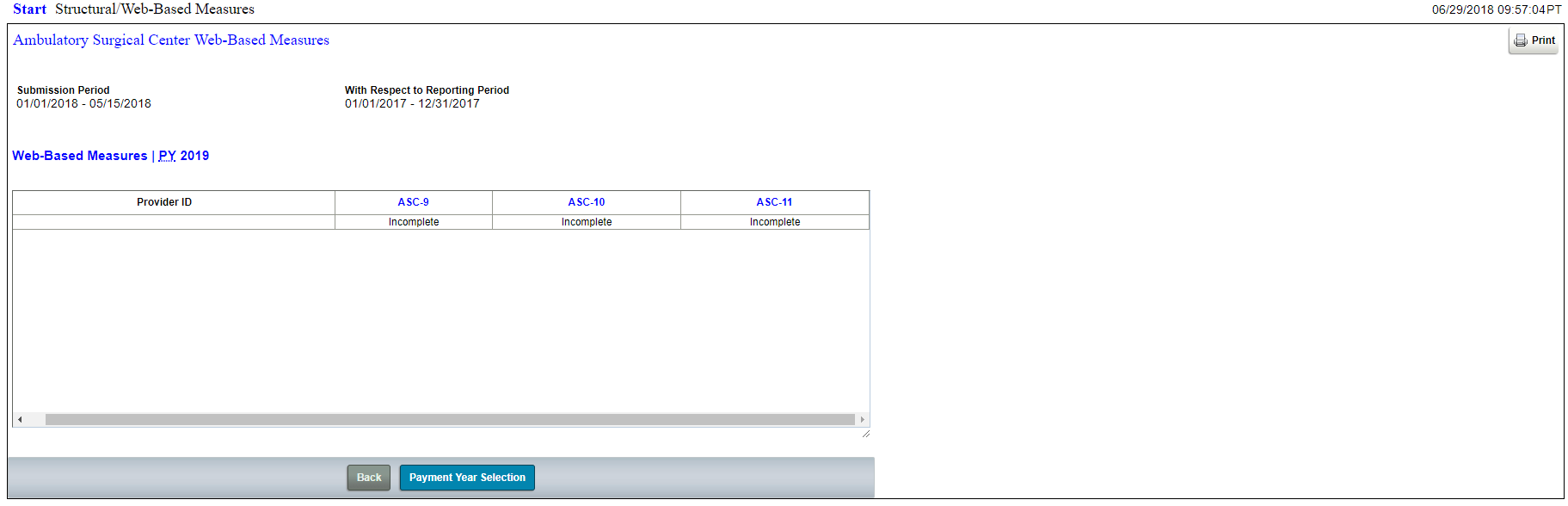
Structural Measures for the ASCQR Program
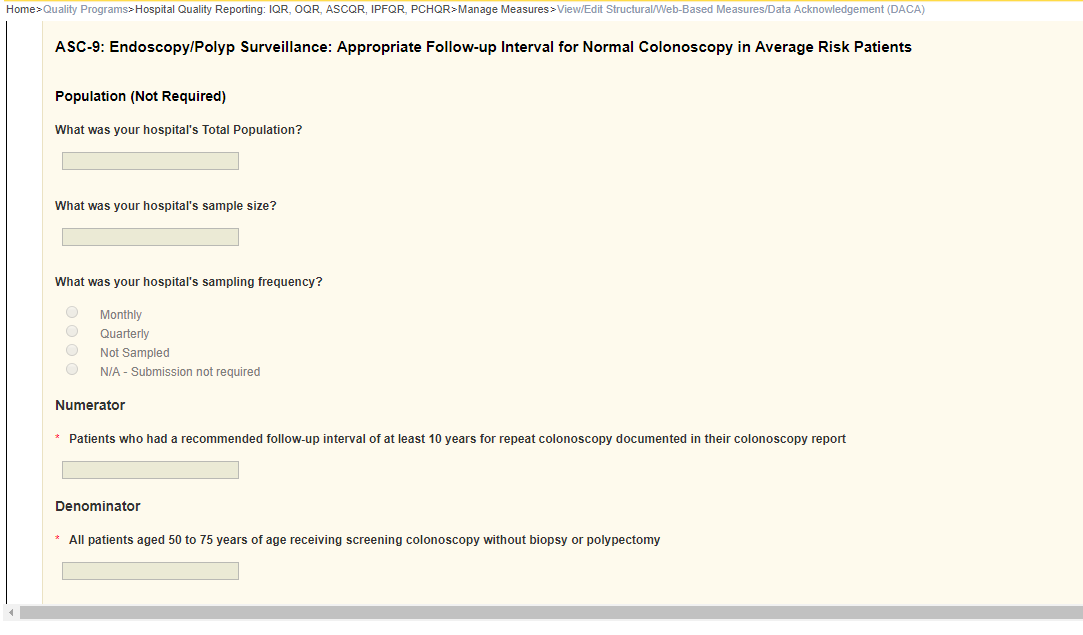
ASC-9: Endoscopy/Polyp Surveillance: Appropriate Follow-up Interval for Normal Colonoscopy in Average Risk Patients
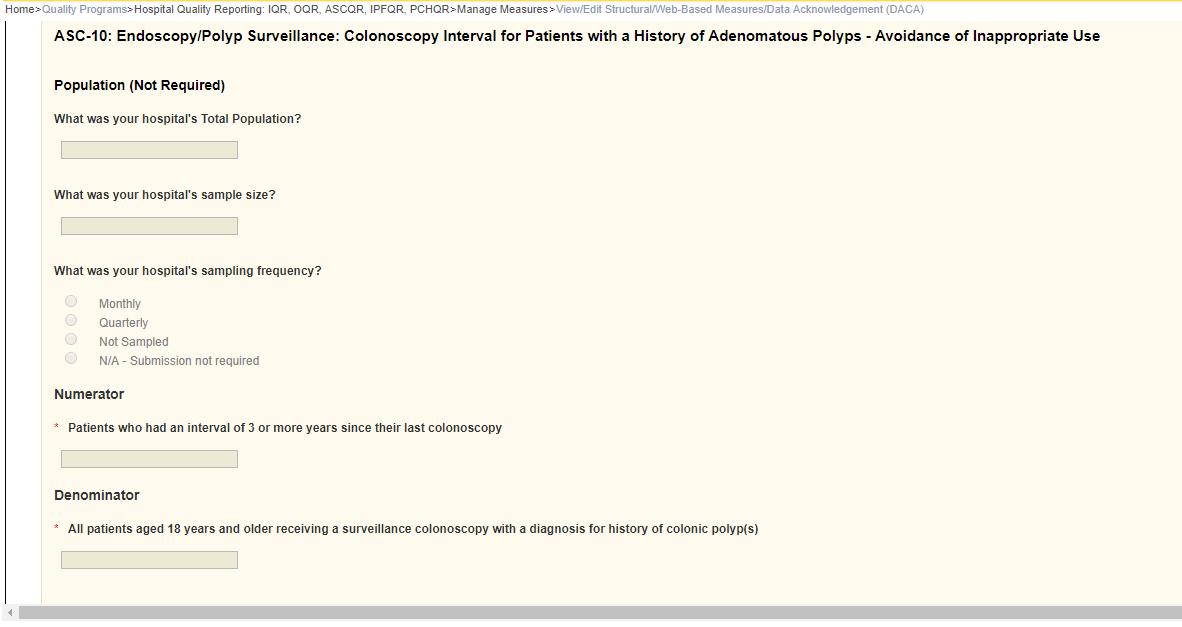
ASC-10: Endoscopy/Polyp Surveillance: Colonoscopy Interval for Patients with a History of Adenomatous Polyps – Avoidance of Inappropriate Use
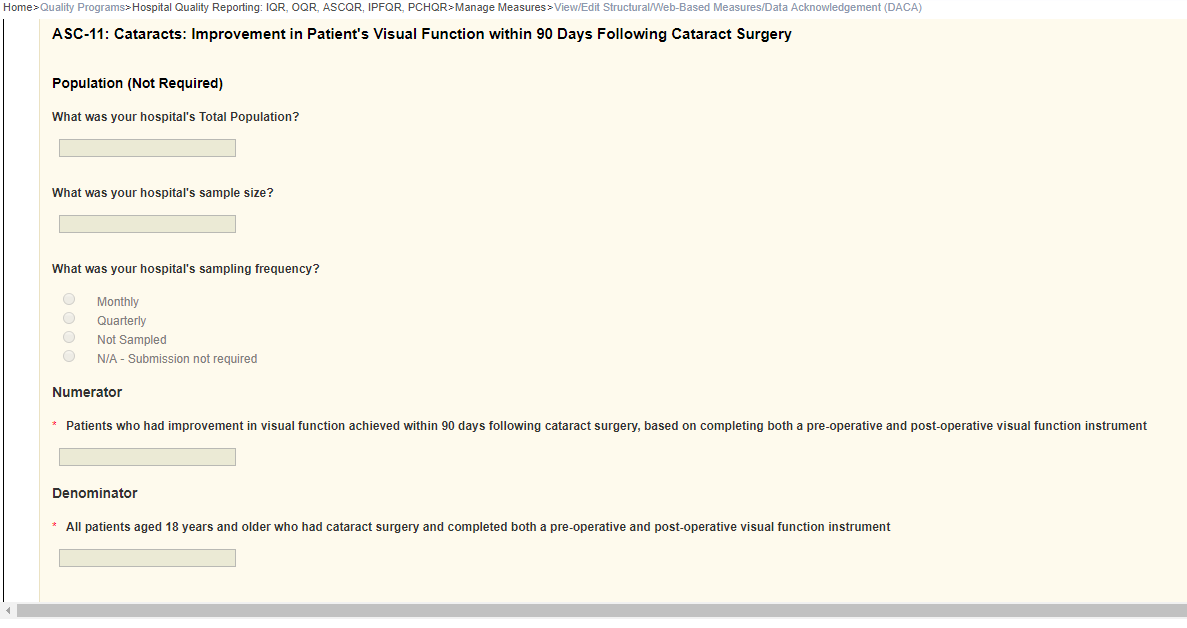
ASC-11: Cataracts: Improvement in Patient’s Visual Function within 90 Days Following Cataract Surgery
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Daniel Raj |
| File Modified | 0000-00-00 |
| File Created | 2021-01-15 |
© 2026 OMB.report | Privacy Policy