Drug experience reports; advertisements and promotional labeling; distributor statements
Records and Reports Concerning Experience with Approved New Animal Drugs
0284 Screenshots
Drug experience reports; advertisements and promotional labeling; distributor statements
OMB: 0910-0284
Records and Reports Concerning Experience with Approved New Animal Drugs - 0910-0284
Screenshots Showing Sample of Proposed Electronic Submission System for Form FDA 2301
Table of Contents
1.0 DER Submission – CORE Template 2
1.1 Node 1.0 Type of submission 2
1.2 Node 2.0 Firm Information 3
1.3 Node 3.0 Responsible Official Information 5
1.4 Node 4.0 U.S Agent Information 7
1.5 Node 5.0 Submitter Information 9
1.6 Node 6.0 Submission Type 10
1.7 Node 7.0 Annual Submission Type 11
1.7.1 Node 7.1 Annual Complete Submission 12
1.7.2 Node 7.2 Annual Incomplete Submission 13
2.0 DER Submission – Semi-Annual Template 13
2.1 Node 1.0 Submission Selection – Semi-Annual (6 months) DER 13
2.2 Node 2.0 Adverse Drug Experiences (ADEs) 14
Node 3.0 Clinical Data (Animal Experience) 17
2.3 Node 4.0 Quantity Marketed 18
2.4 Node 5.0 Current Package Labeling 19
2.4.1 Node 5.1 Summary of Changes and Current Package Labeling 20
2.4.2 Node 5.2 Summary pf Labeling Changes 21
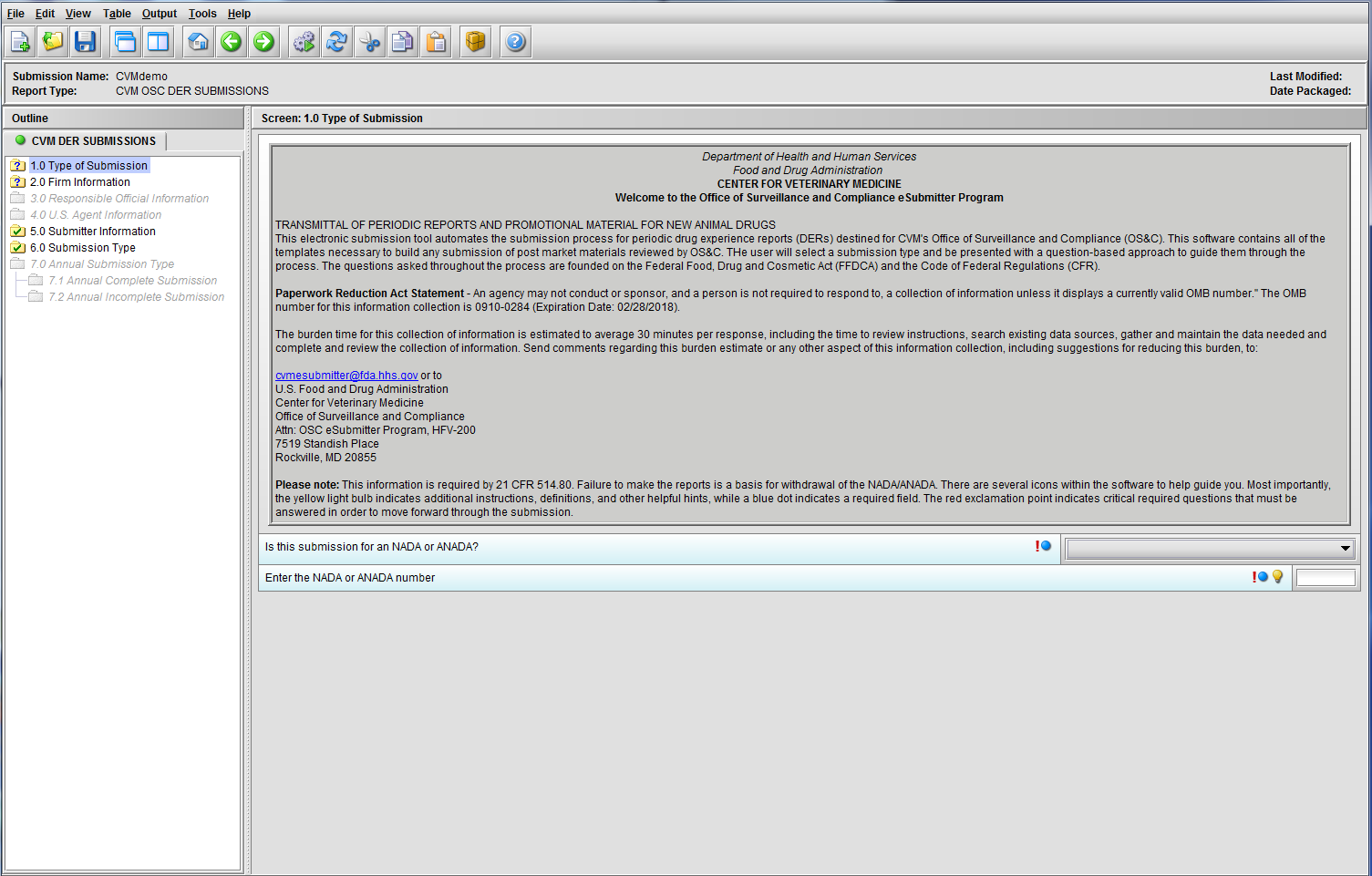
Figure 1.1(a): Initial screen for new submission
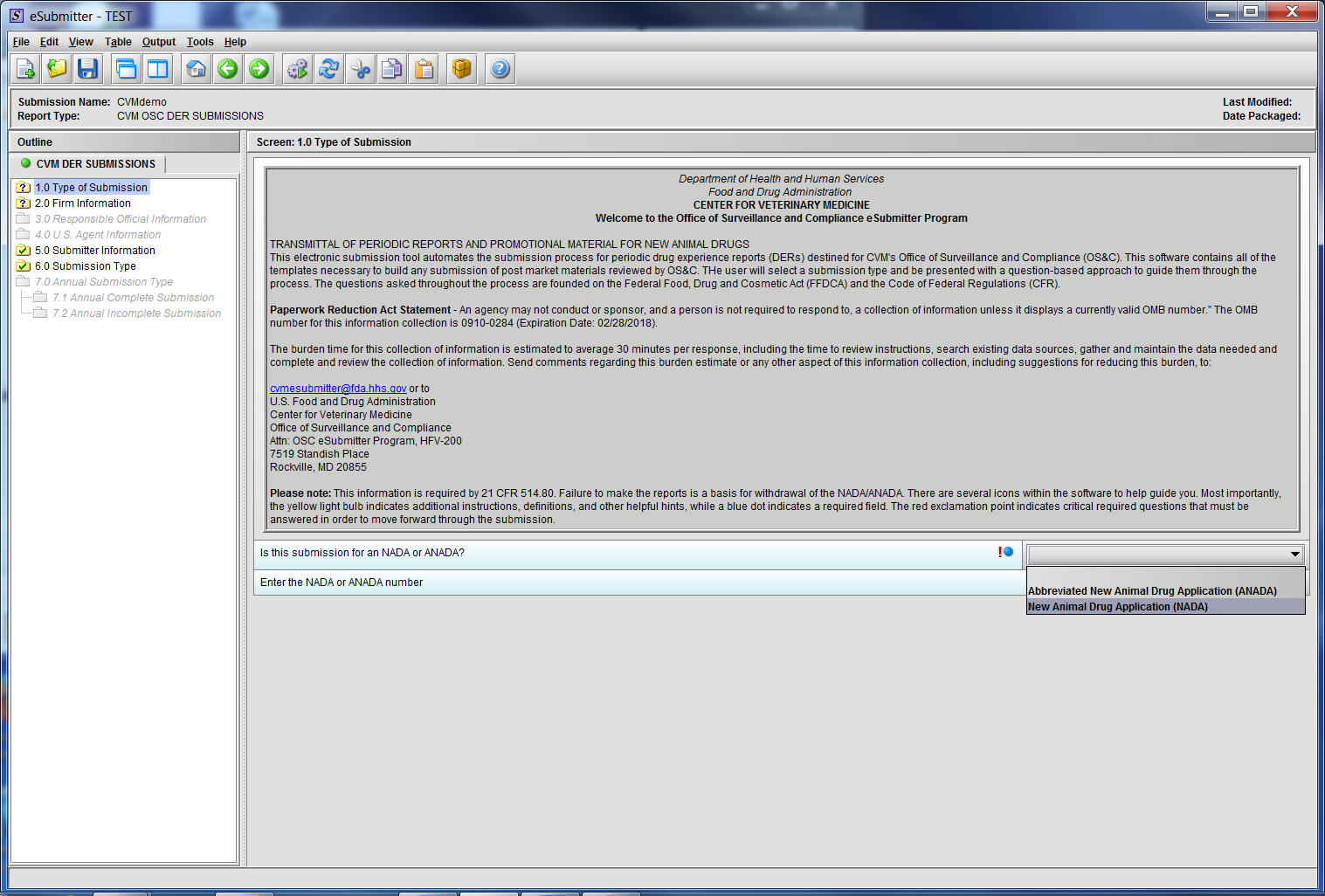
Figure 1.1(b): Drop Down selection for NADA or ANADA
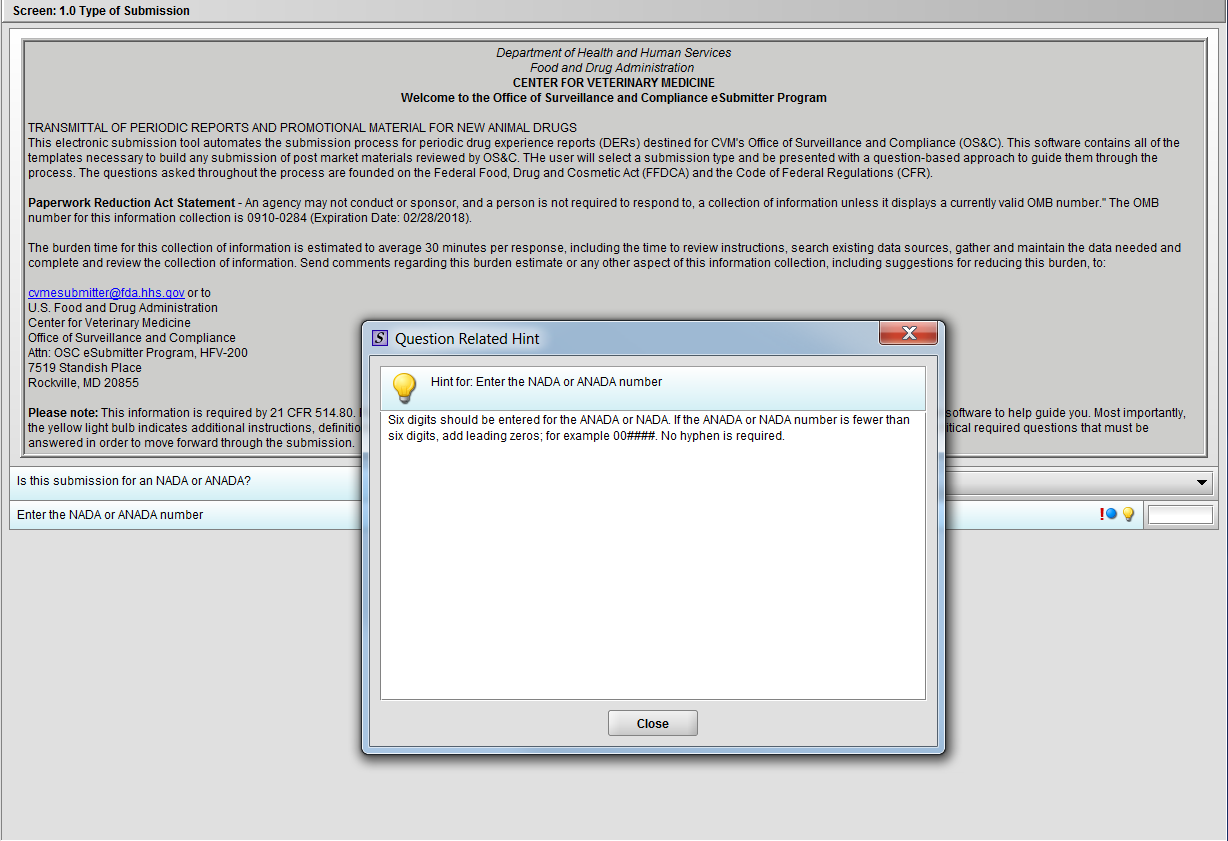
Figure 1.1(c): Hint associated with question ‘Enter the NADA or ANADA number’
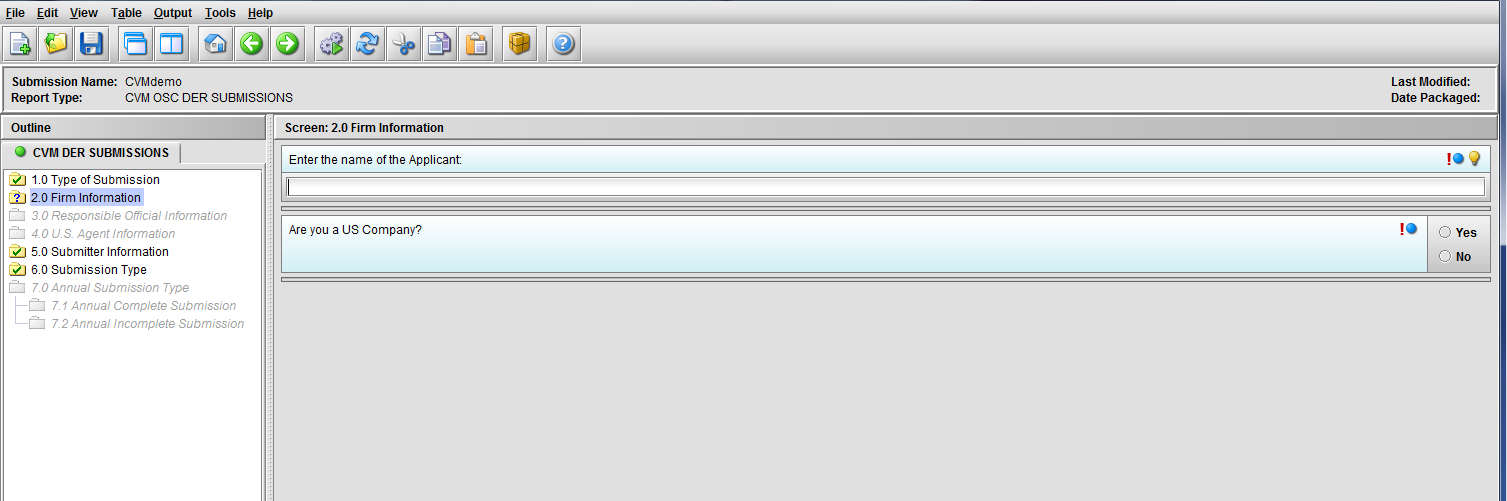
Figure 1.2(a): Firm information screen
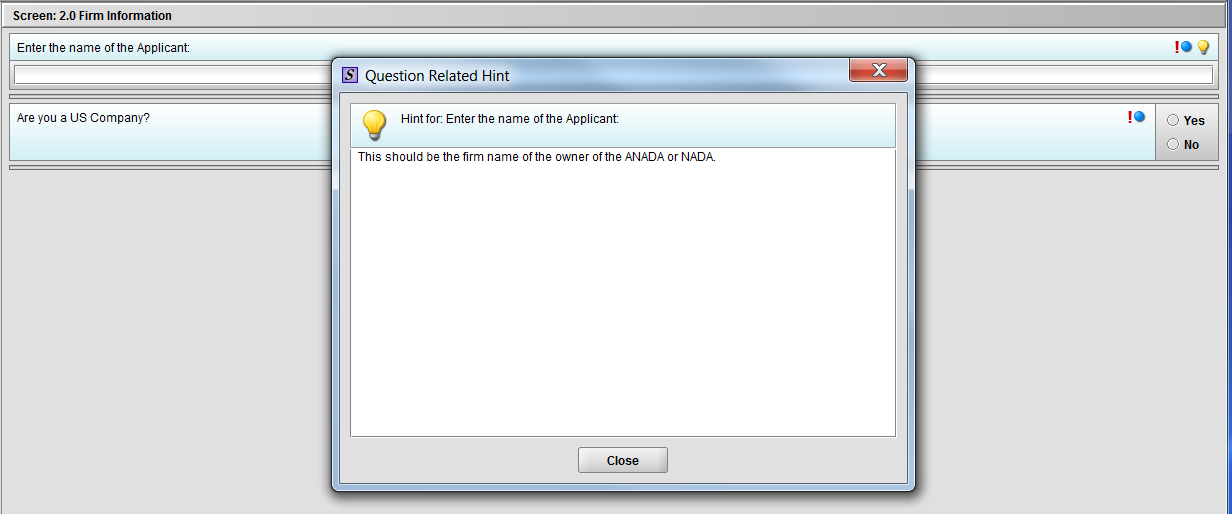
Figure 1.2(b): Hint related to question ‘Enter the name of the Applicant’
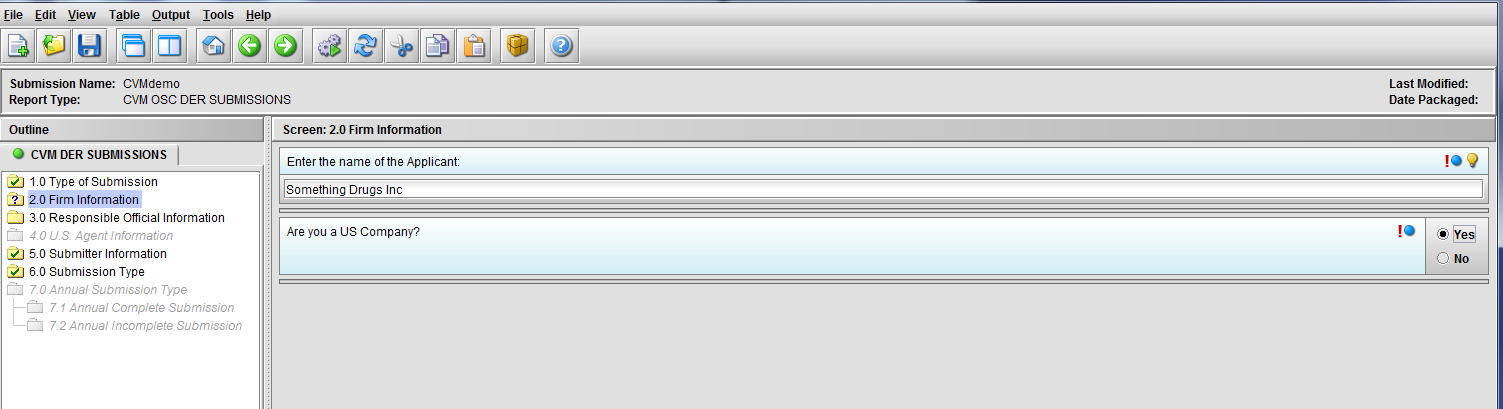
Figure 1.2(c): Selection of ‘Yes’ to question ‘Are you a US Company’ enables Node 3.0
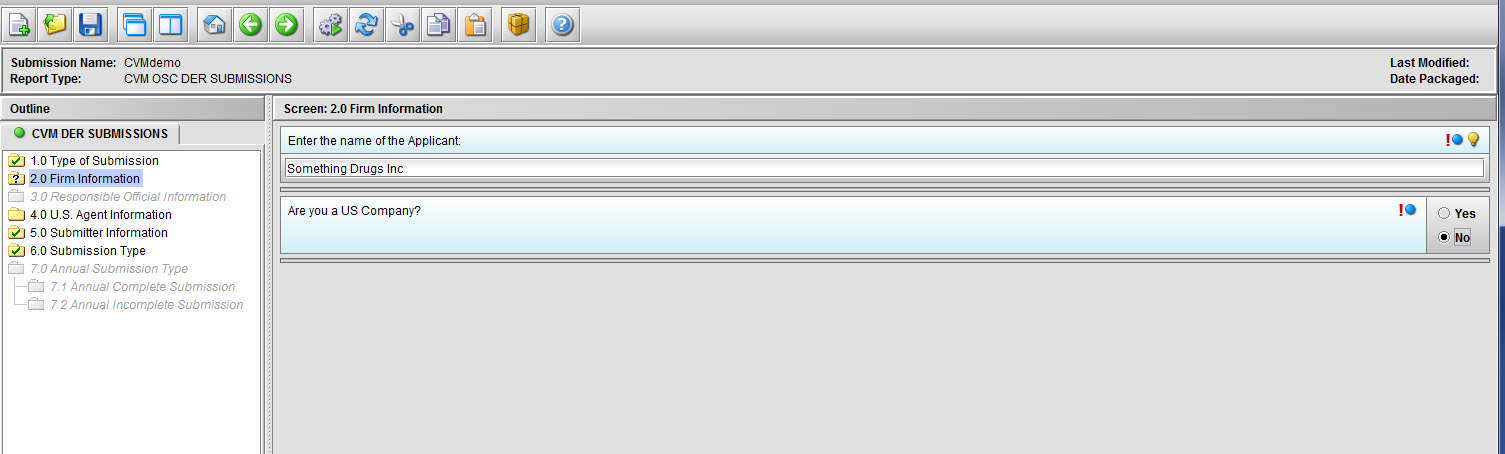
Figure 1.2(d): Selection of ‘No’ to question ‘Are you a US Company’ enables Node 4.0
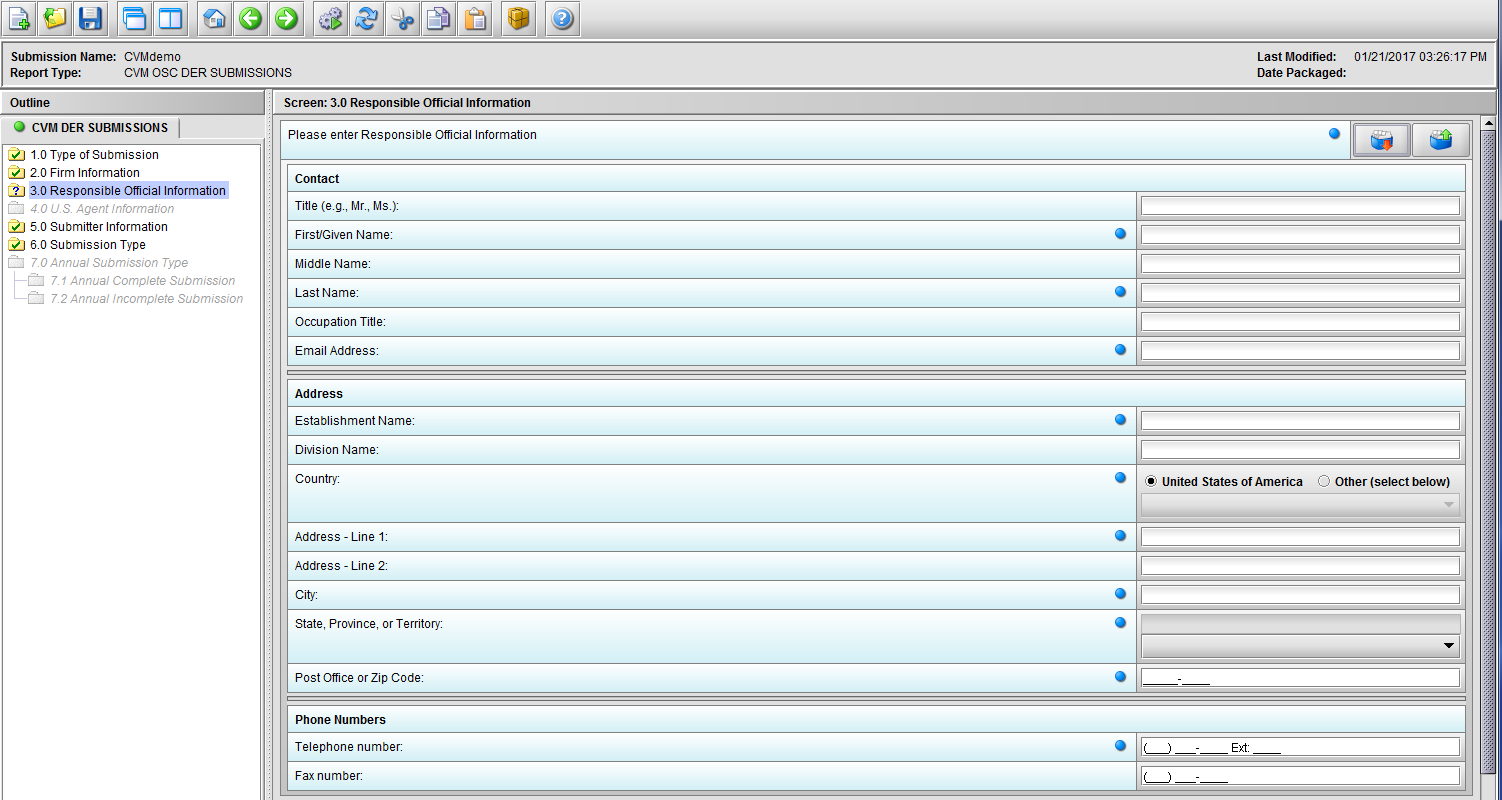
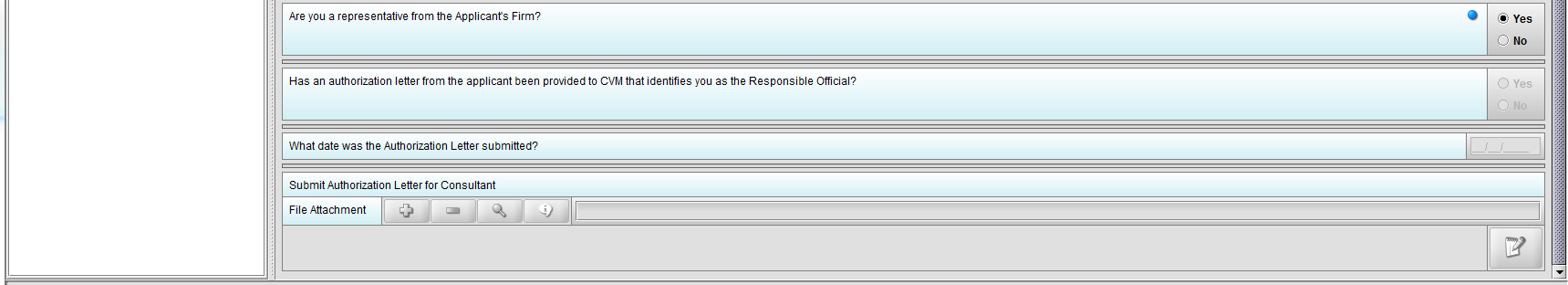
Figure 1.3(a): Responsible Official Information
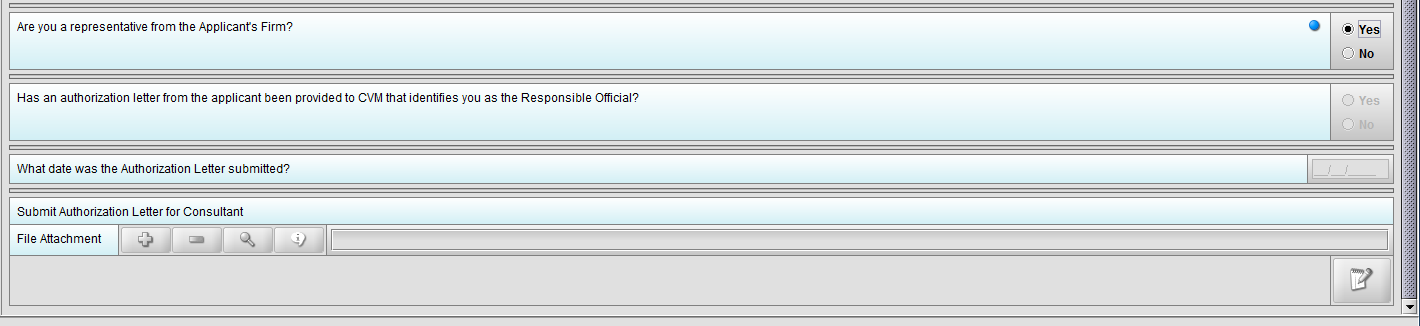
Figure 1.3(b): Selection of ‘Yes’ to question ‘Are you a representative from the Applicant’s Firm’ allow user to continue
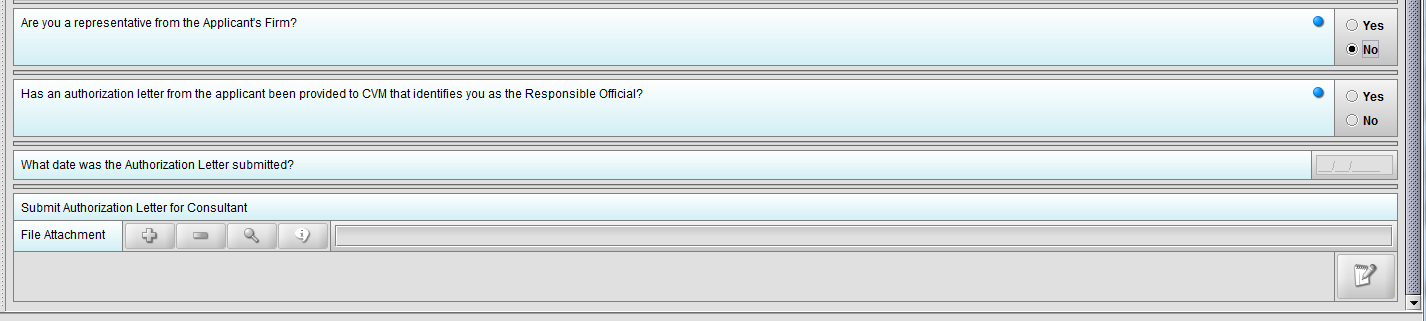
Figure 1.3(c): Selection of ‘No’ to question ‘Are you a representative from the Applicant’s Firm’ enables question ‘Has an authorization letter from the applicant been provided to CVM that identifies you as the Responsible Official?’
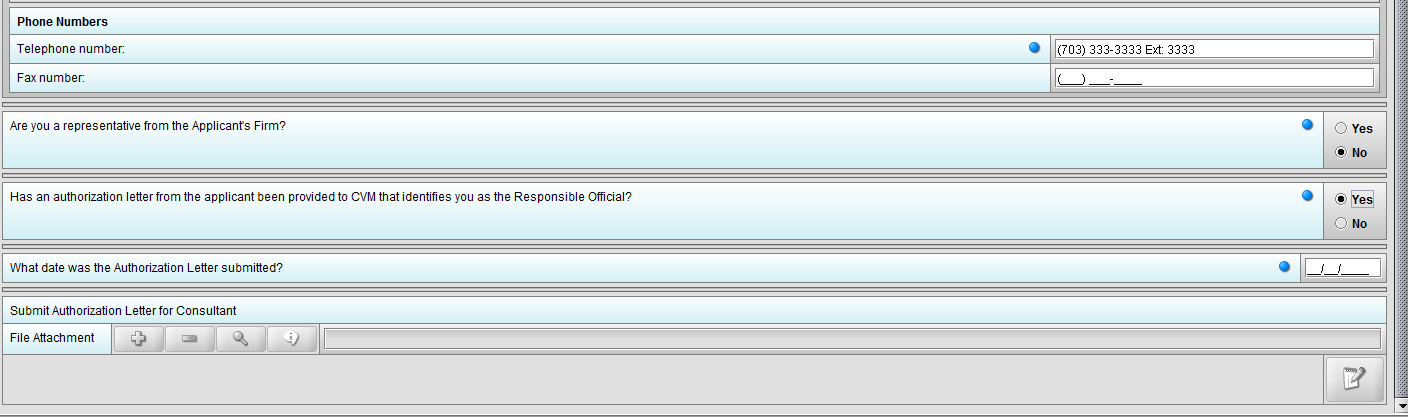
Figure 1.3(d): Selection of ‘Yes’ to question ‘Has an authorization letter from the applicant been provided to CVM that identifies you as the Responsible Official?’ enables question ‘What date was the Authorization Letter submitted?’
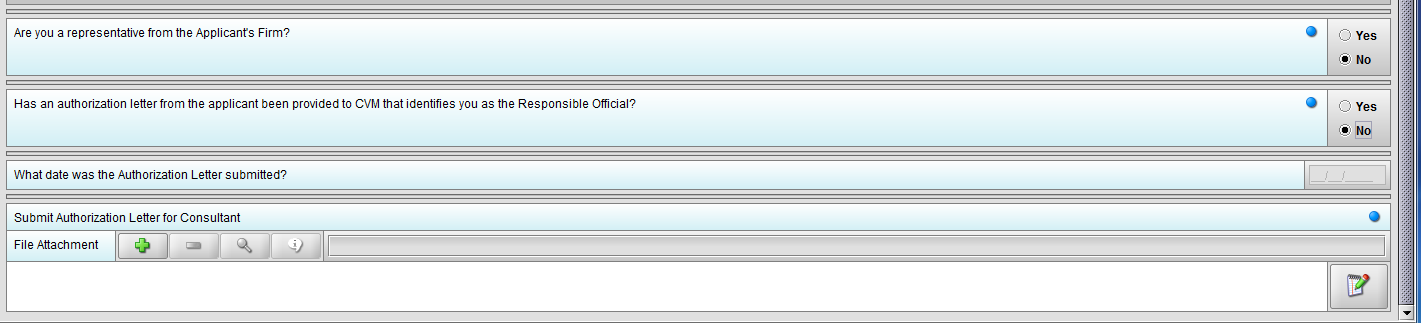
Figure 1.3(e): Selection of ‘No’ to question ‘Has an authorization letter from the applicant been provided to CVM that identifies you as the Responsible Official?’ enables question ‘Submit Authorization Letter submitted?’
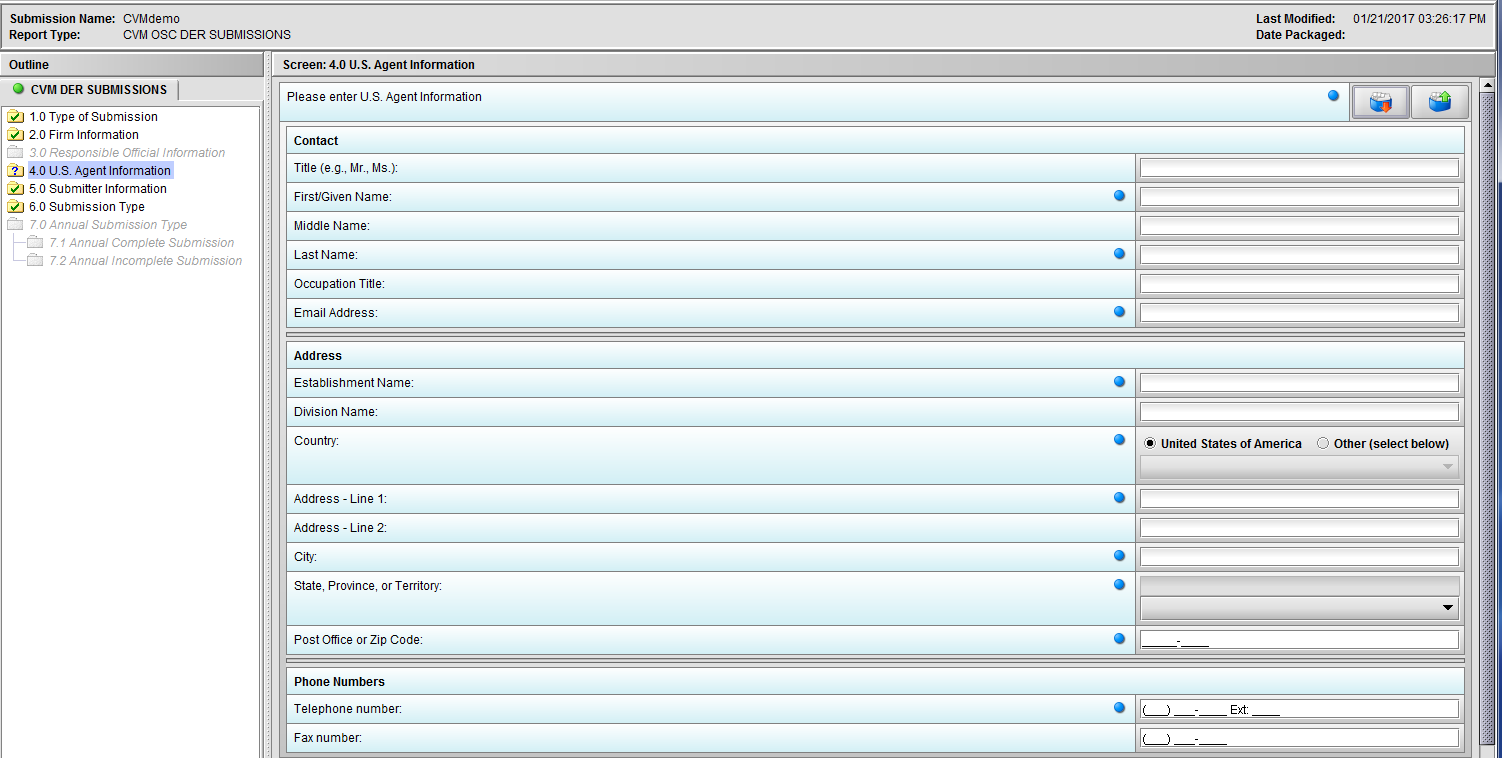
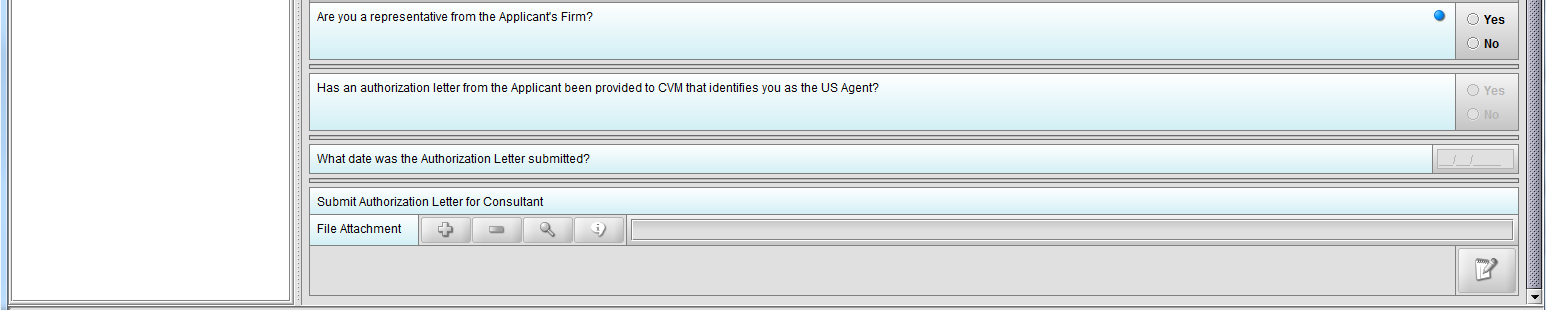
Figure 1.4(a): U.S. Agent Information
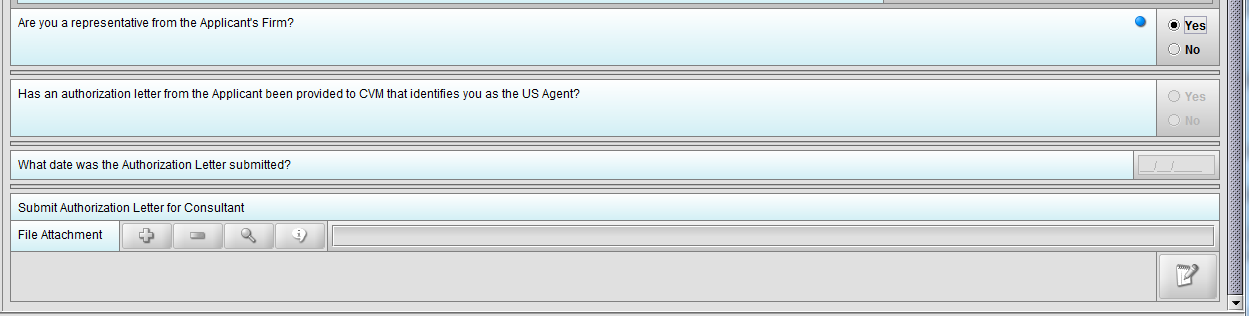
Figure 1.4(b): Selection of ‘Yes’ to question ‘Are you a representative from the Applicant’s Firm’ allow user to continue
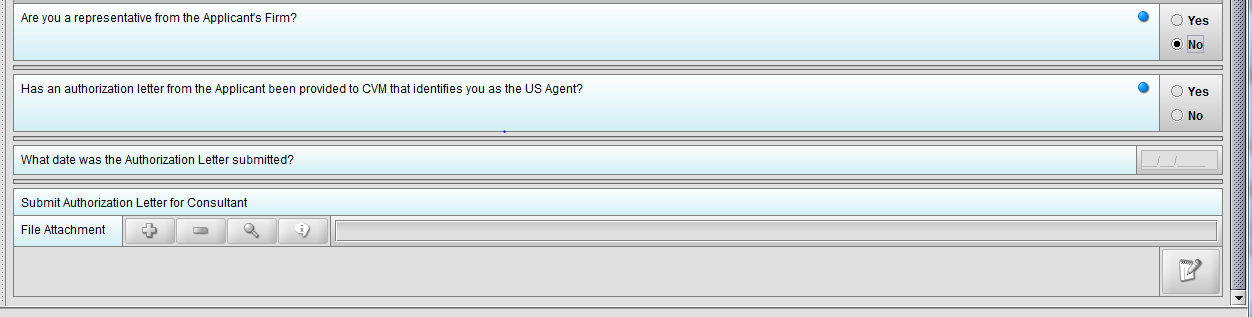
Figure 1.4(c): Selection of ‘No’ to question ‘Are you a representative from the Applicant’s Firm’ enables question ‘Has an authorization letter from the applicant been provided to CVM that identifies you as the US Agent?’
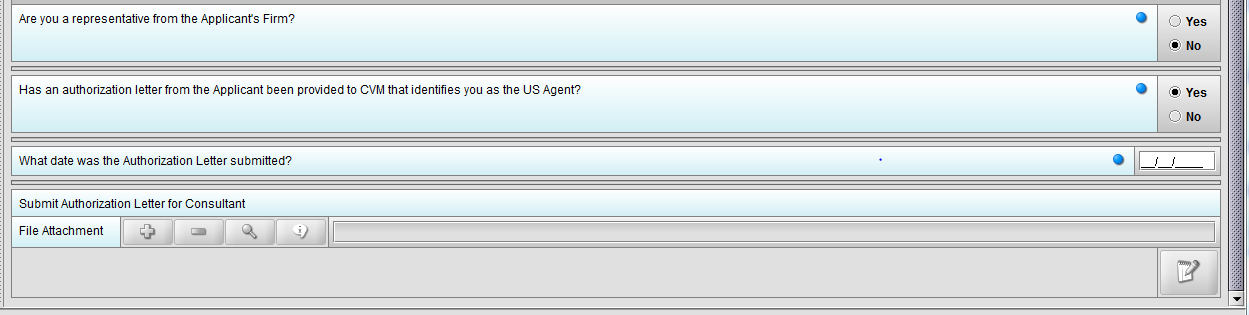
Figure 1.4(d): Selection of ‘Yes’ to question ‘Has an authorization letter from the applicant been provided to CVM that identifies you as the US Agent?’ enables question ‘What date was the Authorization Letter submitted?’
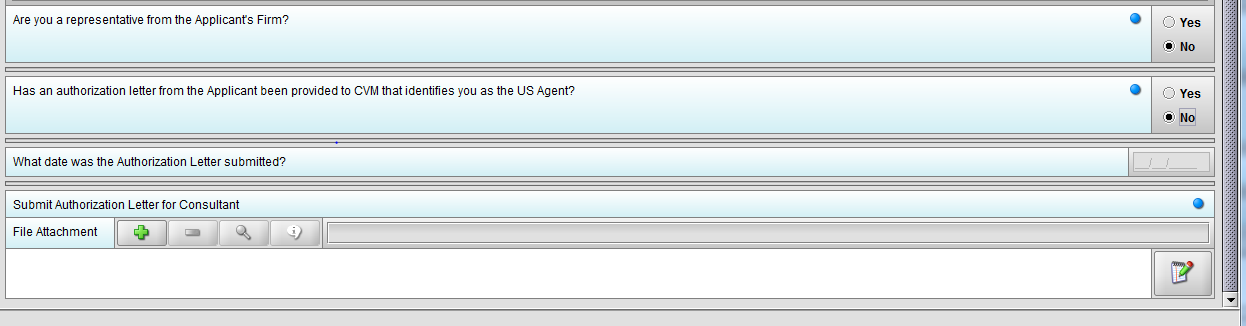
Figure 1.4(e): Selection of ‘No’ to question ‘Has an authorization letter from the applicant been provided to CVM that identifies you as the Responsible Official?’ enables question ‘Submit Authorization Letter submitted?’
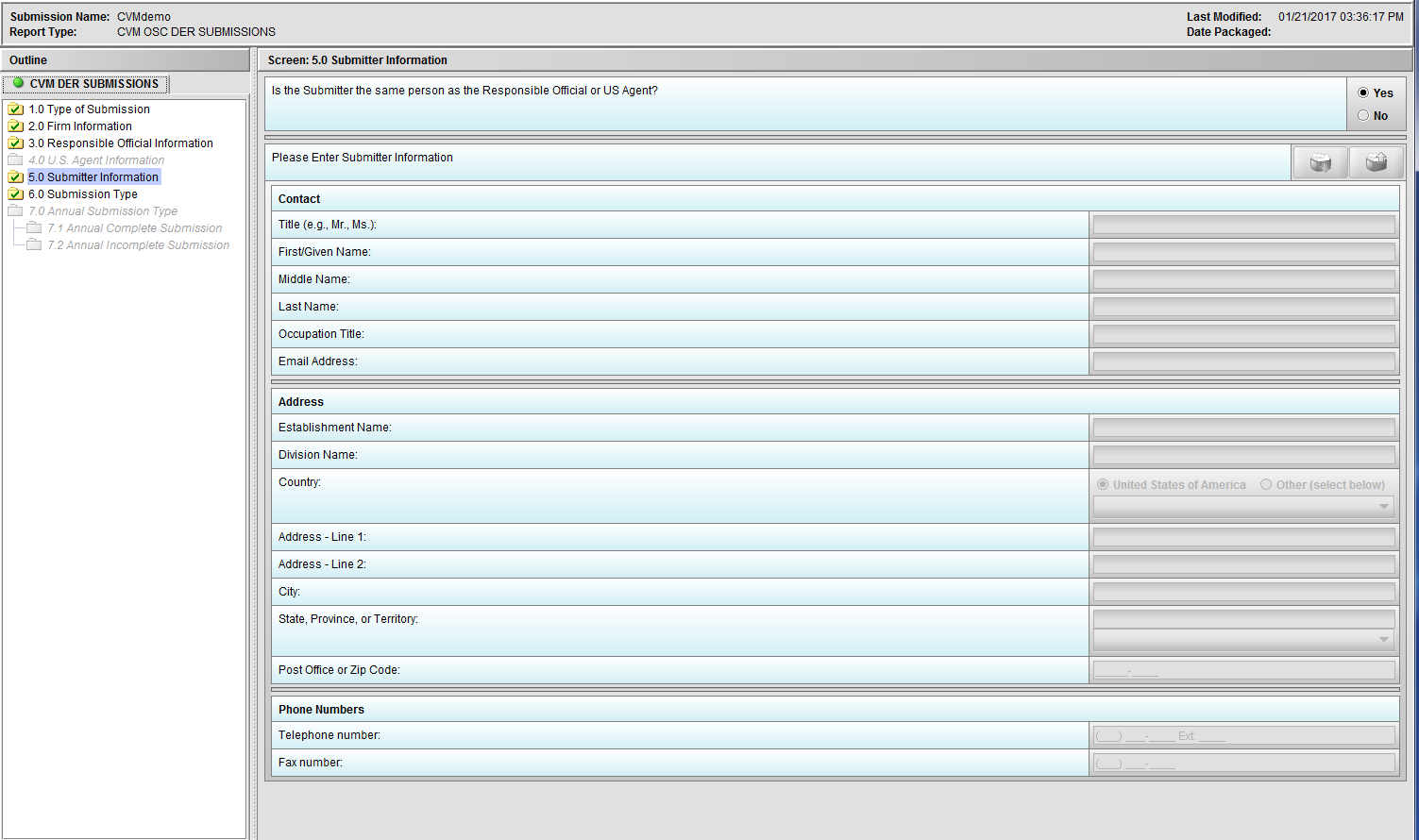
Figure 1.5(a): Submitter Information. Selection of ‘Yes’ to question ‘Is the Submitter the same person as the Responsible Official or US Agent?’ allow user to continue
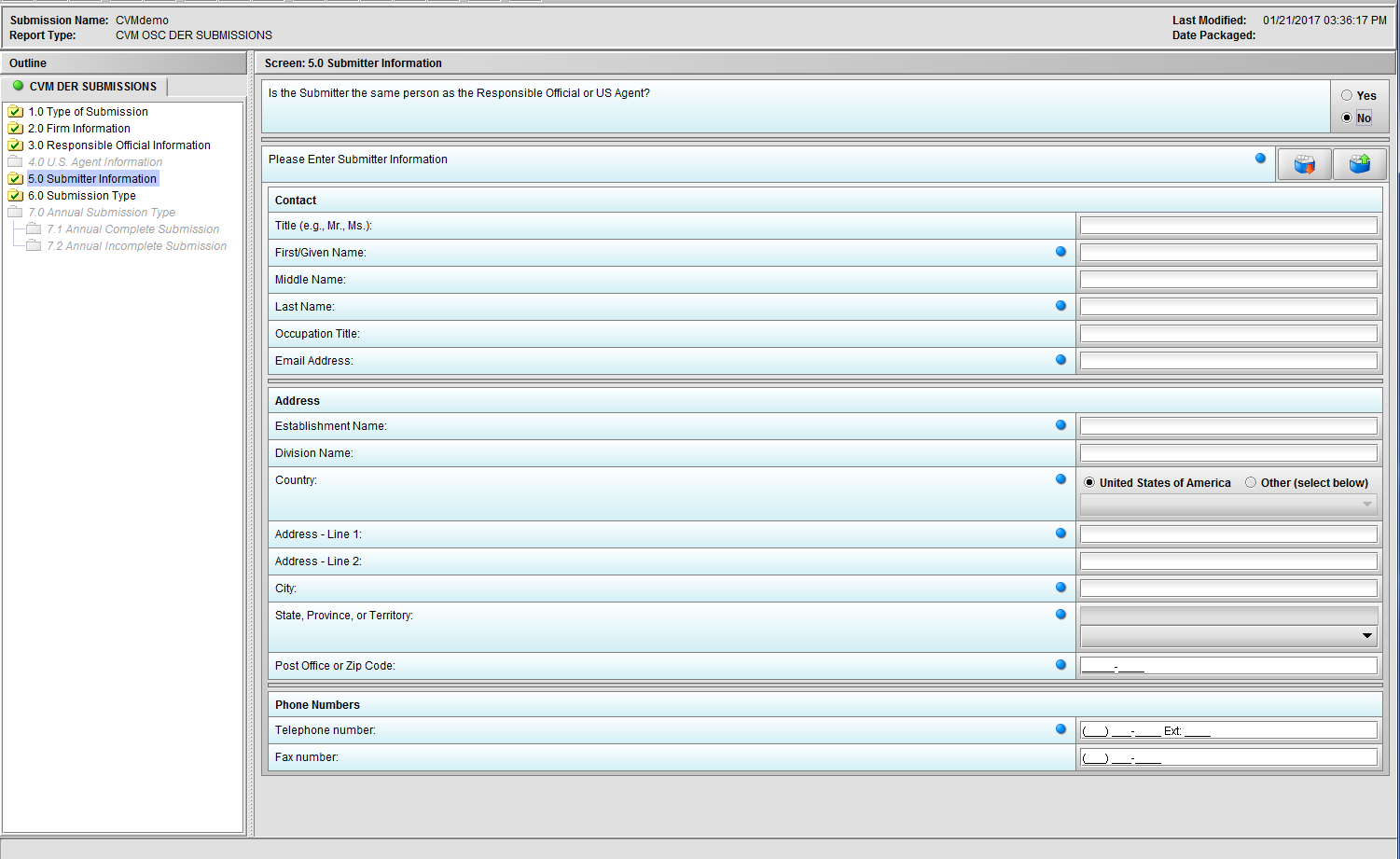
Figure 1.5(b): Selection of ‘No’ to question ‘Is the Submitter the same person as the Responsible Official or US Agent?’ enables the group of questions associated with question ‘Please Enter Submitter information.’
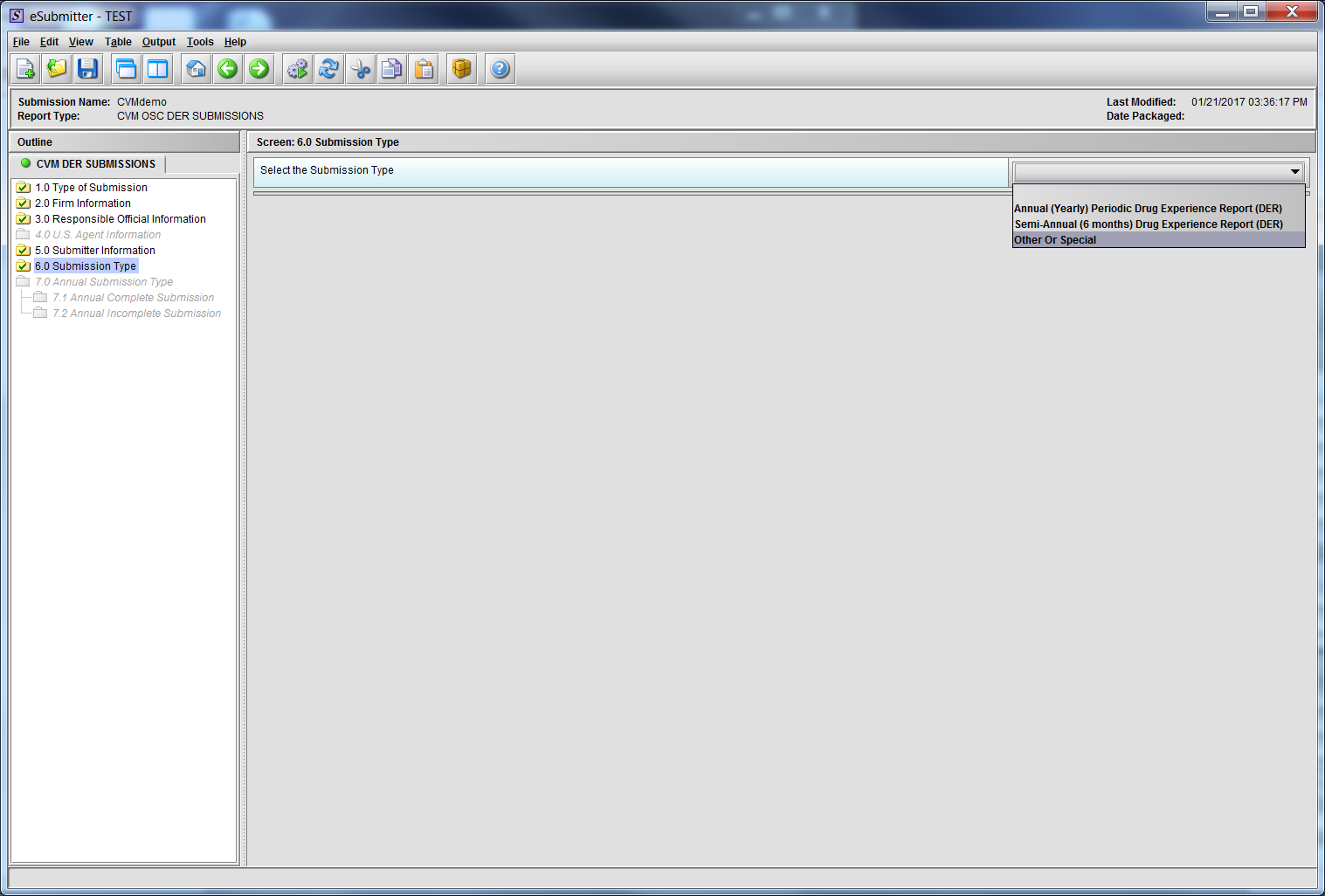
Figure 1.6(a): Submission Type. Drop Down selection for Annual, Semi-Annual or Other/Special Report
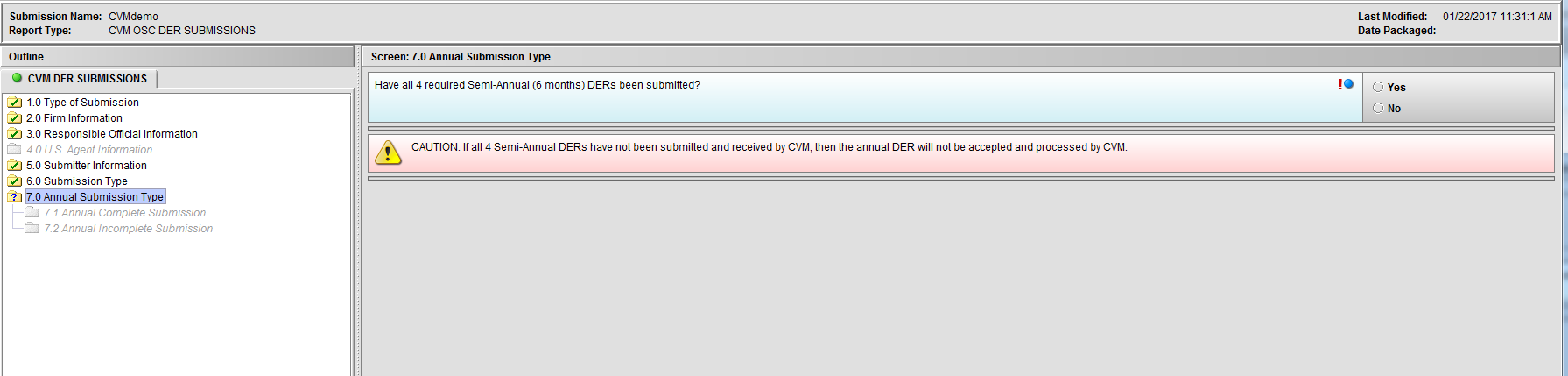
Figure 1.7(a): Annual Submission Type. Selection of ‘Annual (Yearly) Periodic Drug Experience Report (DER)’ from Node 6.0 enables Node 7.0
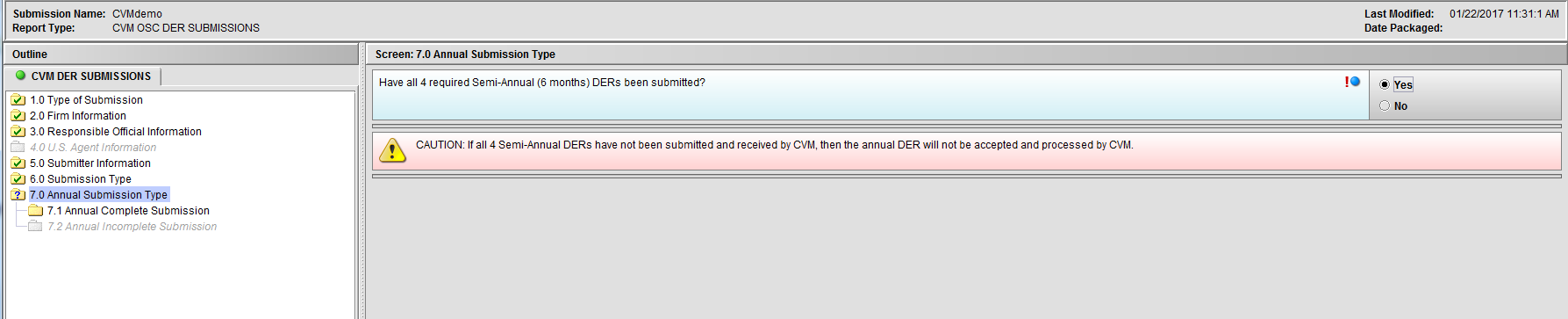
Figure 1.7(b): Selection of ‘Yes’ to question ‘Have all 4 required Semi-Annual (6 months) DERs been submitted?’ enables Node 7.1

Figure 1.7(c): Selection of ‘No’ to question ‘Have all 4 required Semi-Annual (6 months) DERs been submitted?’ enables Node 7.2
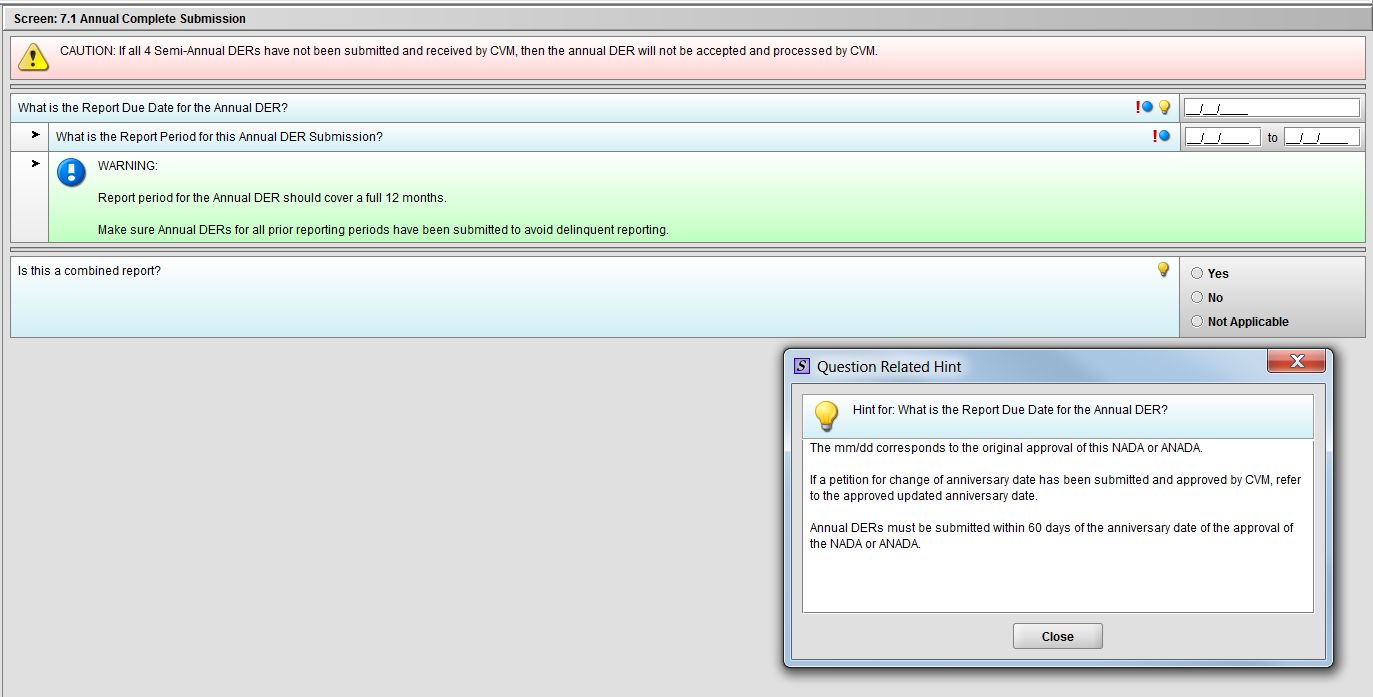
Figure 1.7.1(a): Hint for question ‘What is the Report Due Date for the Annual DER?’
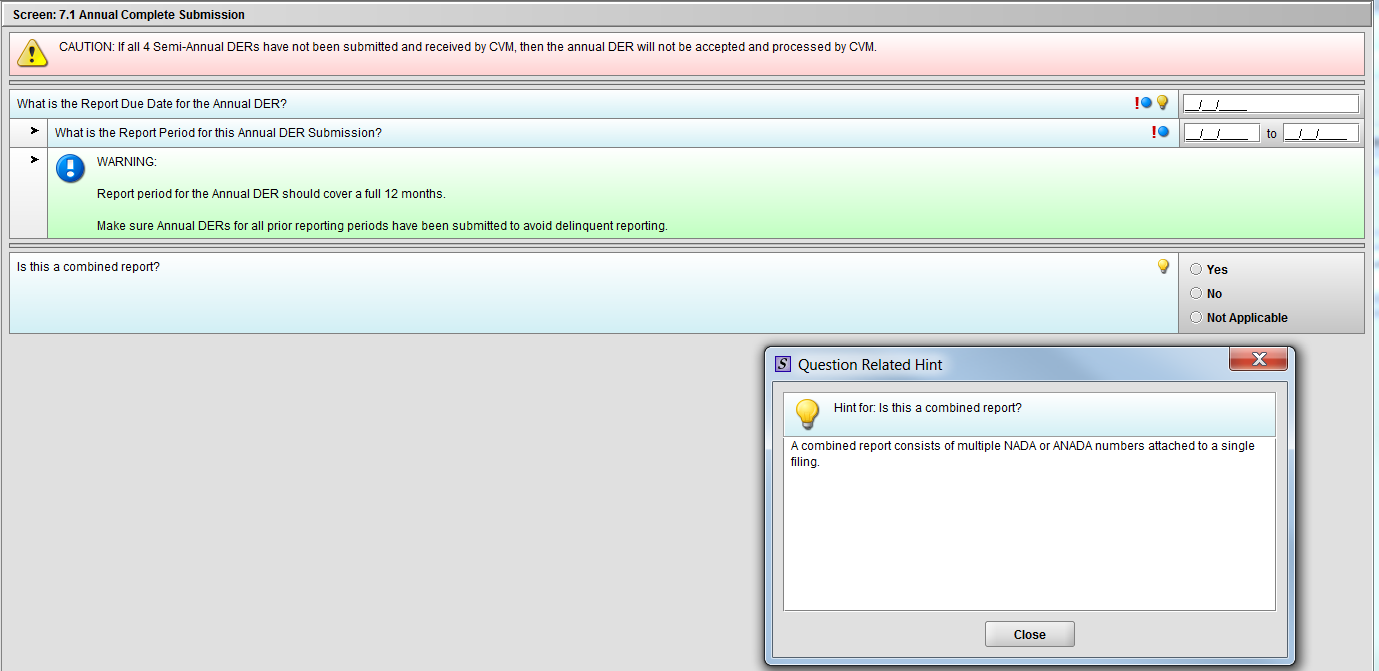
Figure 1.7.1(b): Hint for question ‘Is this a combined report?’
***NOTE: There are three (3) option in the figure above. The ‘Not Applicable’ option is only there for demonstrative purpose only. It will be removed after selection of Annual Template choice.
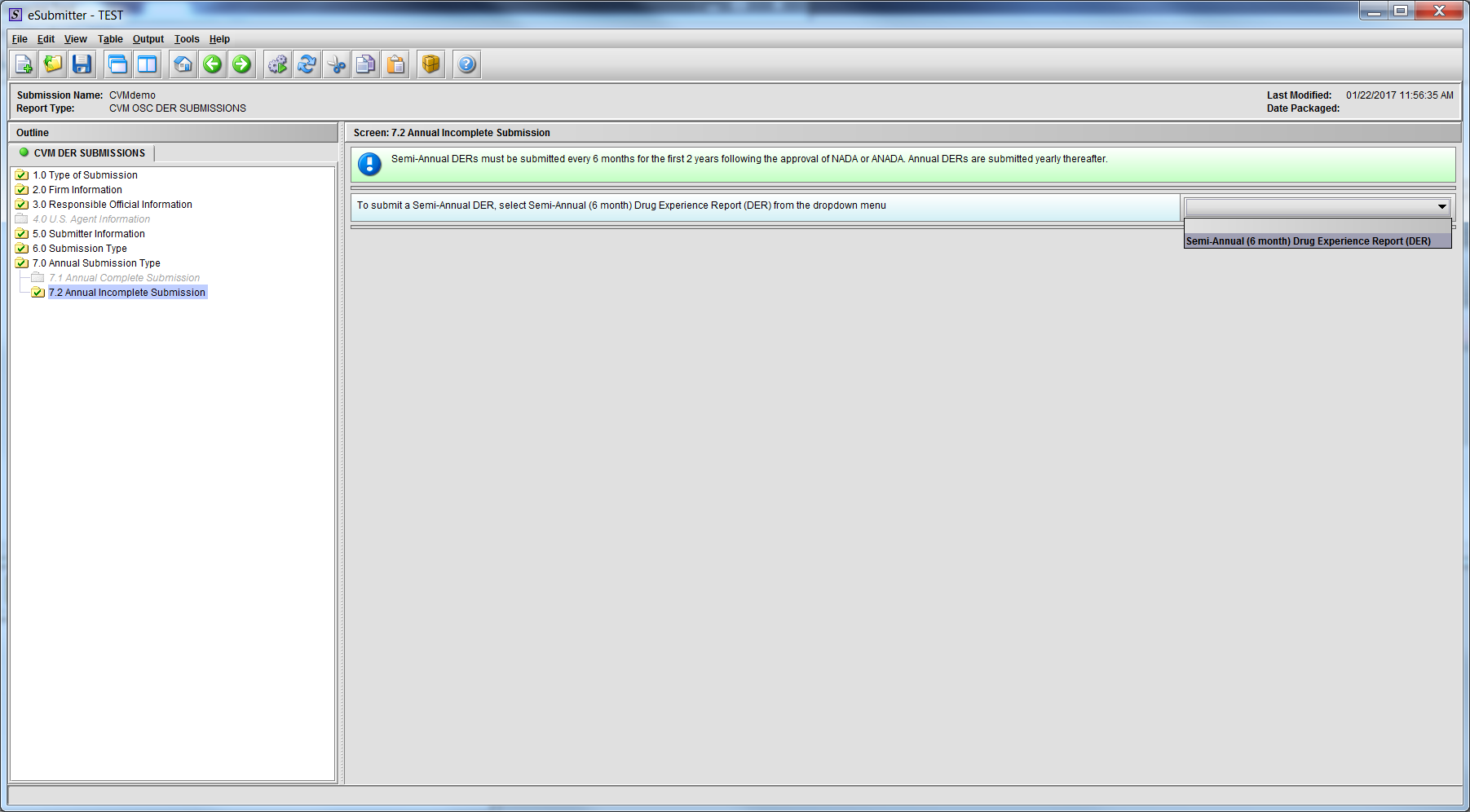
Figure 1.7.2(a): Drop Down navigates to Semi-Annual Template
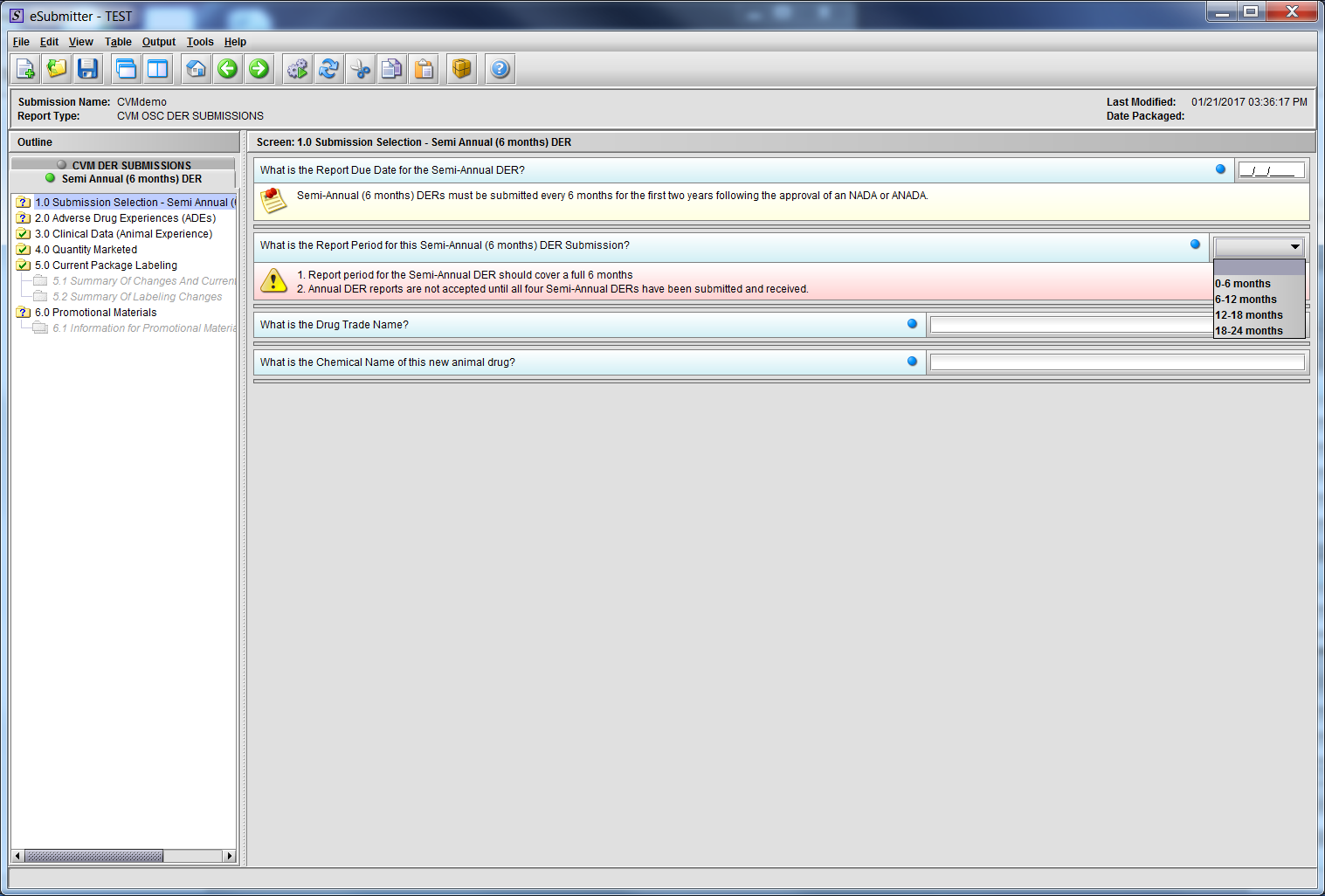
Figure 2.1(a): Submission Selection – Semi-Annual (6 months) DER. Drop Down selection for question ‘What is the Report Period for this Semi-Annual (6 months) DER Submission?’
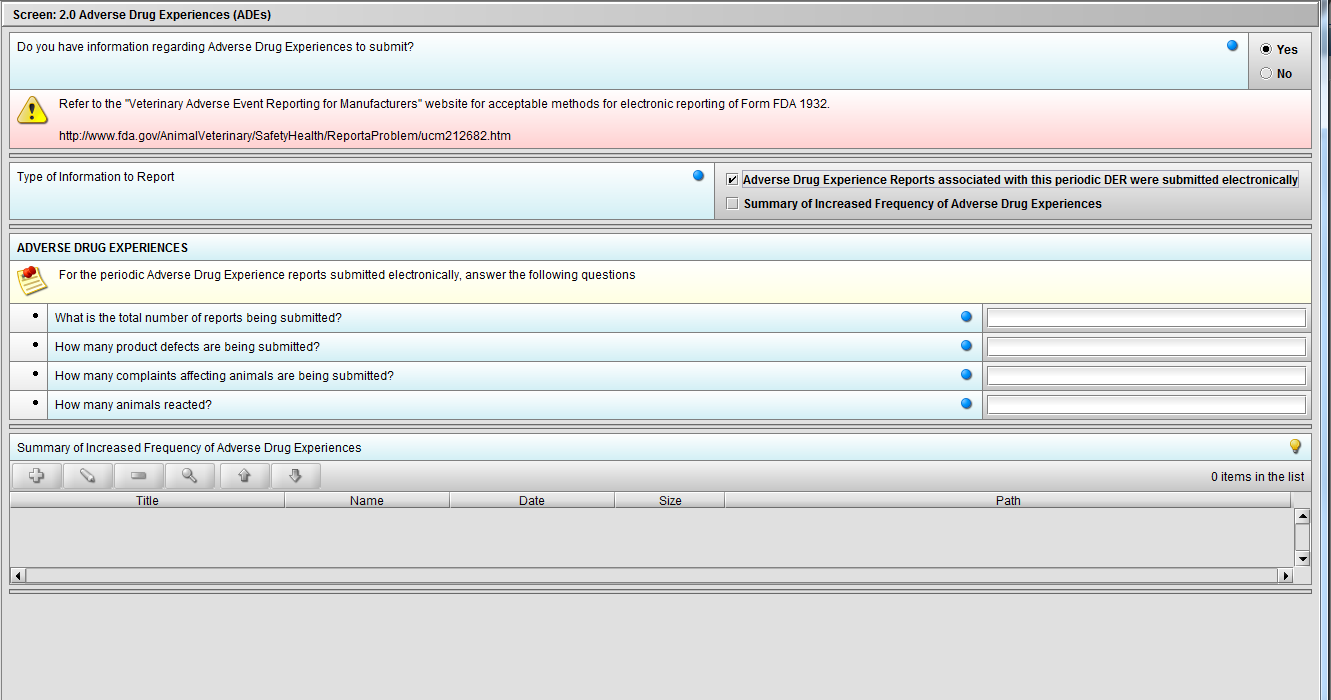
Figure 2.2(a): Adverse Drug Experiences (ADEs). Selection of ‘Yes’ to question ‘Do you have information regarding Adverse Drug Experiences to submit?’ enables question ‘Type of Information to Report’.
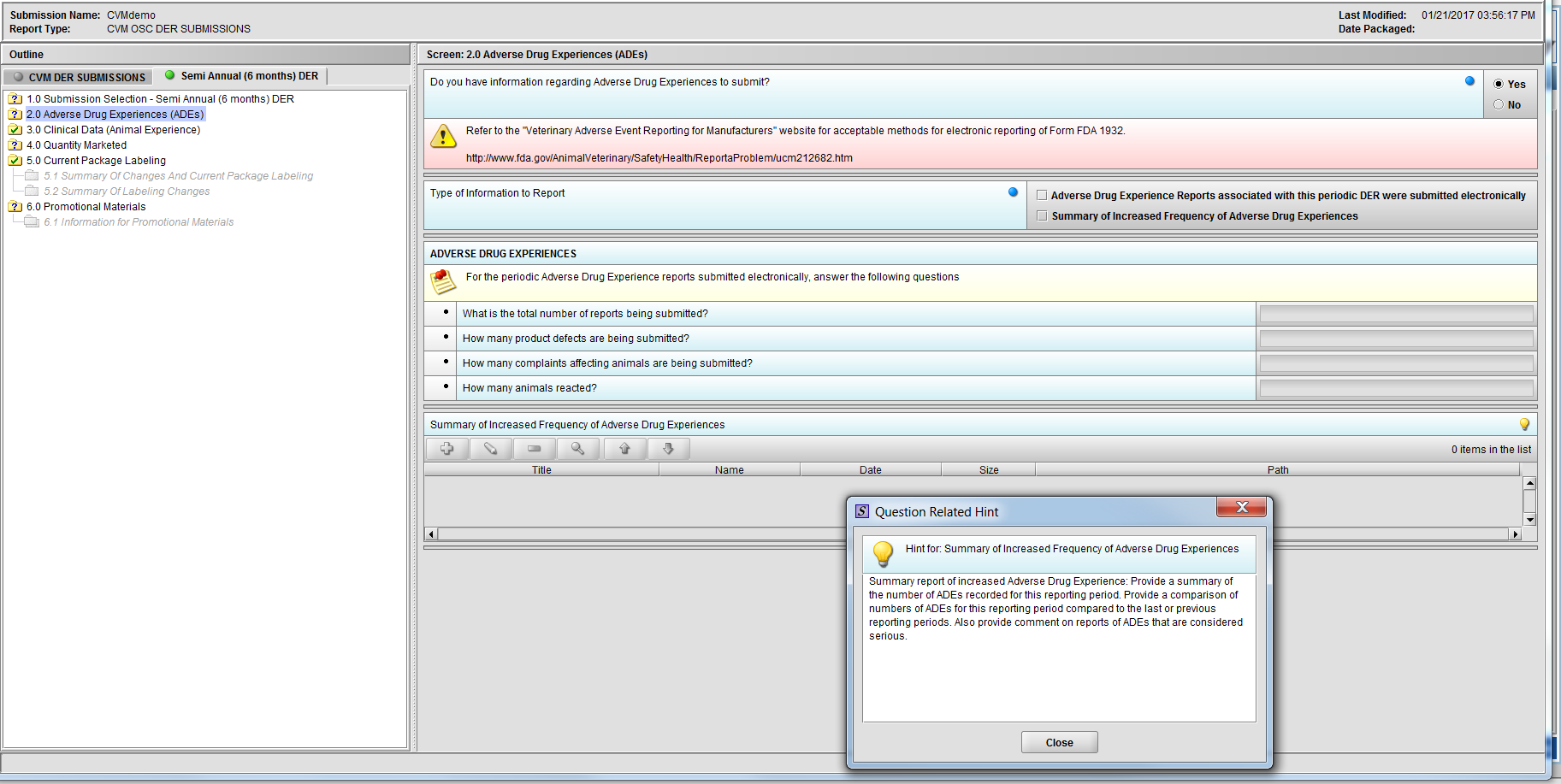
Figure 2.2(b): Hint for question ‘Summary of Increased Frequency of Adverse Drug Experiences’

Figure 2.2(c): Selection of ‘Adverse Drug Experience Reports……’ from question ‘Type of Information to Report’ enables the follwing four (4) questions: ‘What is the total number of reports being submitted?’, ‘How many product defects are being submitted?’, ‘How many complaints affecting animals are being submitted?’ and ‘How many animals reacted?’.
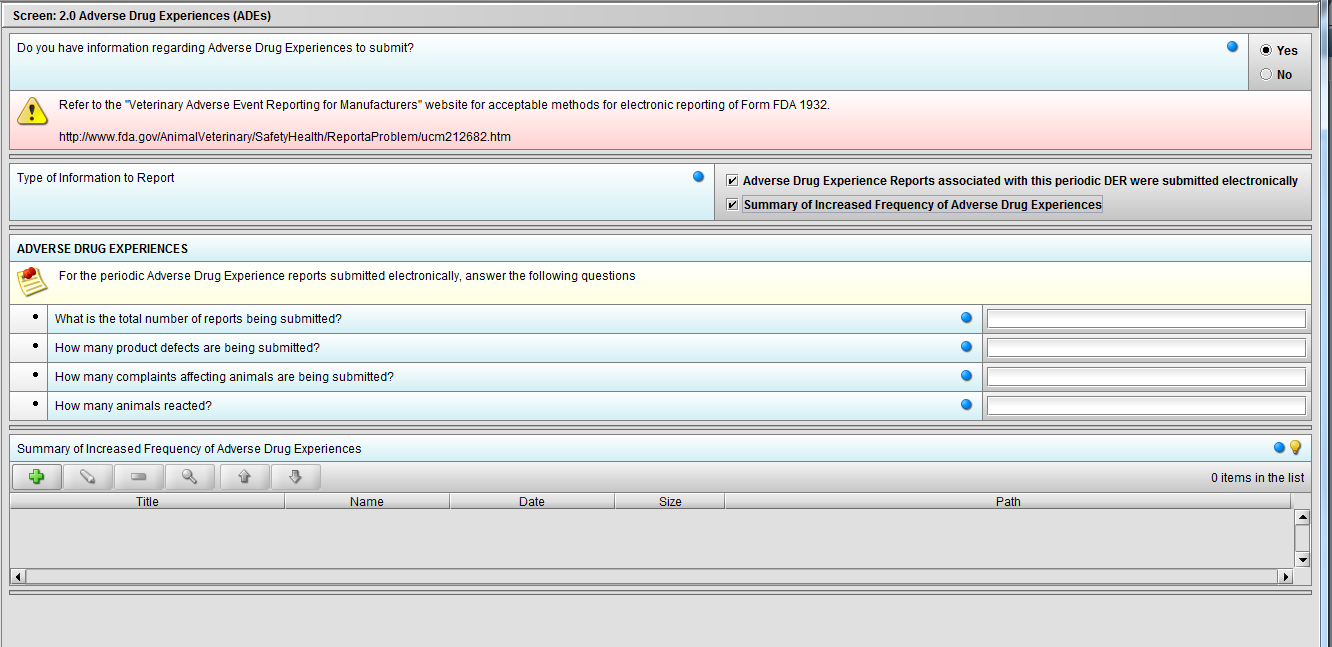
Figure 2.2(d): Selection of ‘Summary of Increased Frequency……’ from question ‘Type of Information to Report’ enables the question ‘Summary of Increased Frequency of Adverse Drug Experiences’
***Note: Selection to question ‘Type of Information to Report’ can be either or both.
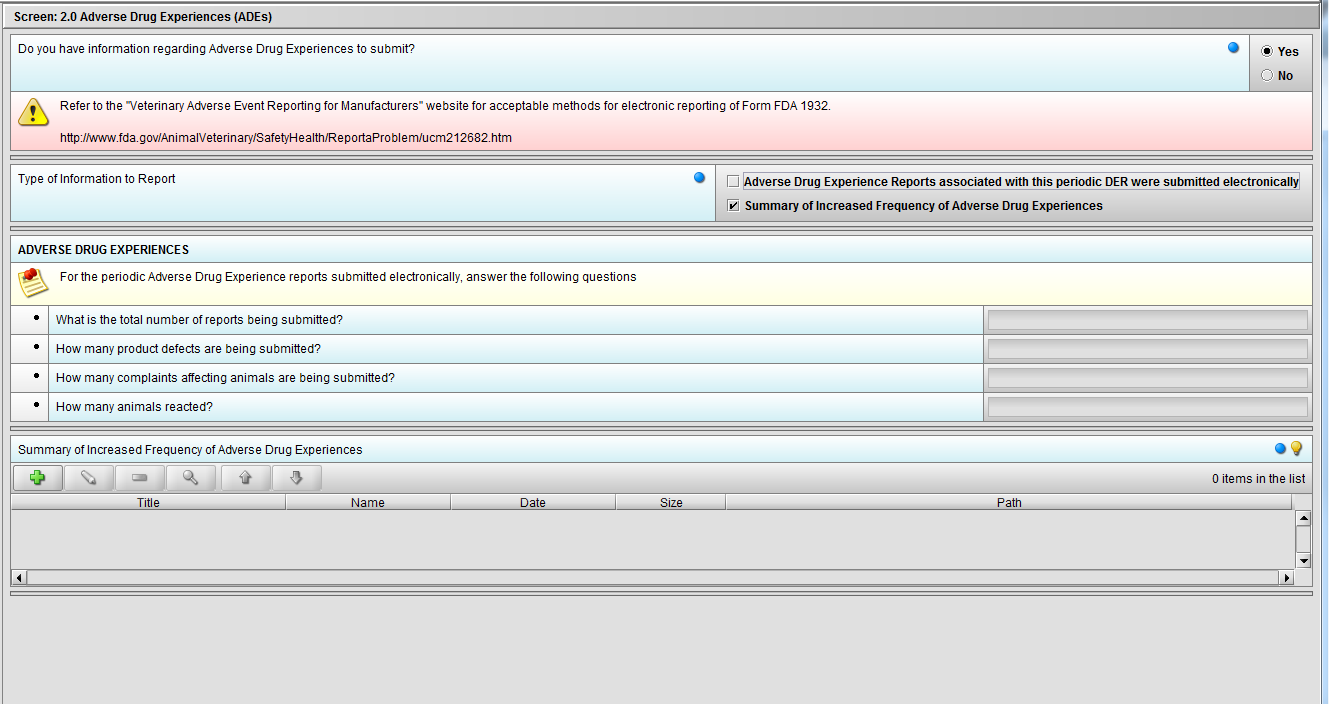
Figure 2.2(e): Selection of ‘Summary of Increased Frequency……’ from question ‘Type of Information to Report’ enables the question ‘Summary of Increased Frequency of Adverse Drug Experiences’
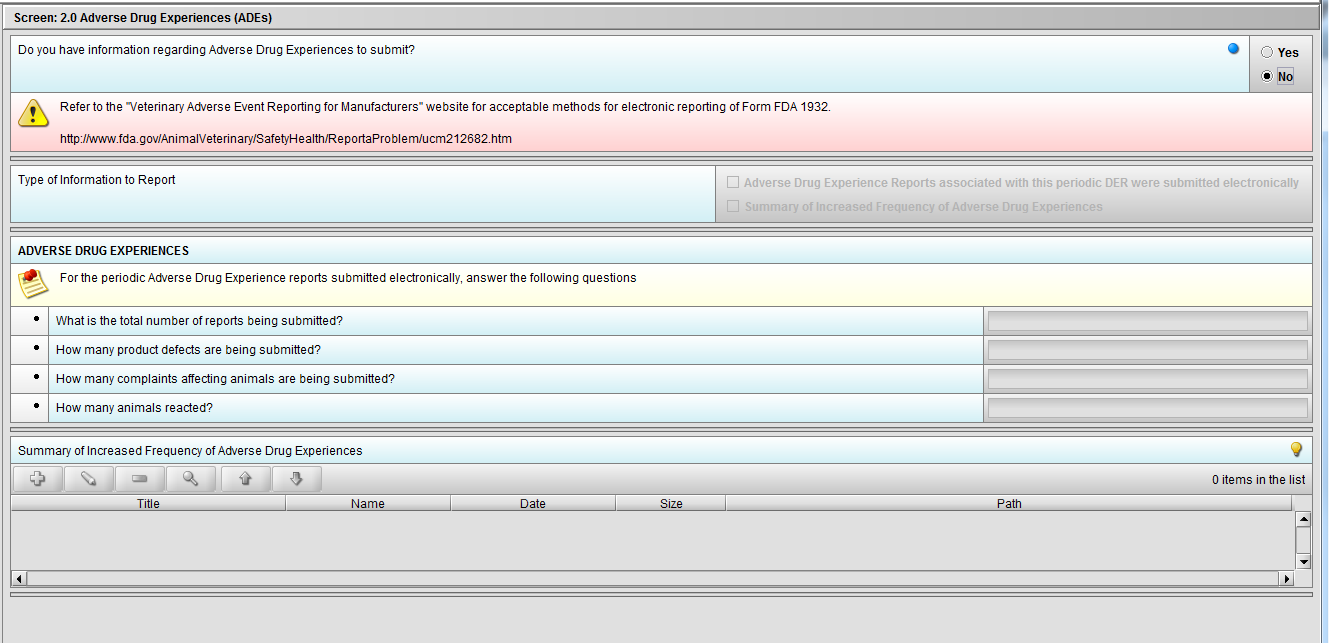
Figure 2.2(f): Selection of ‘No’ to question ‘Do you have information regarding Adverse Drug Experiences to submit?’ navigates to next Node.
Node 3.0 Clinical Data (Animal Experience)
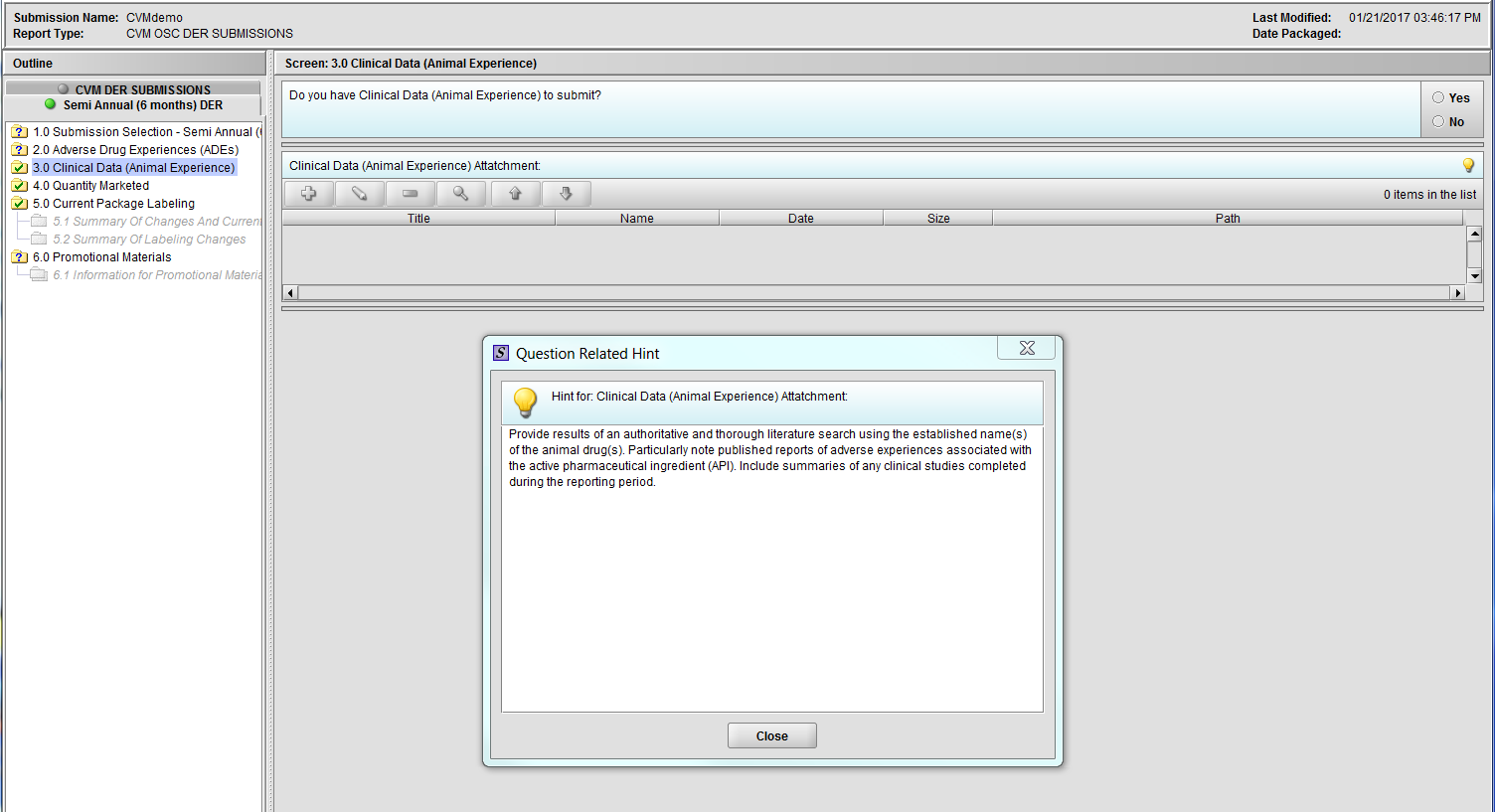
Figure 2.3(a): Clinical Data (Animal Experience). Hint for question ‘Clinical Data (Animal Experience) Attachment’.
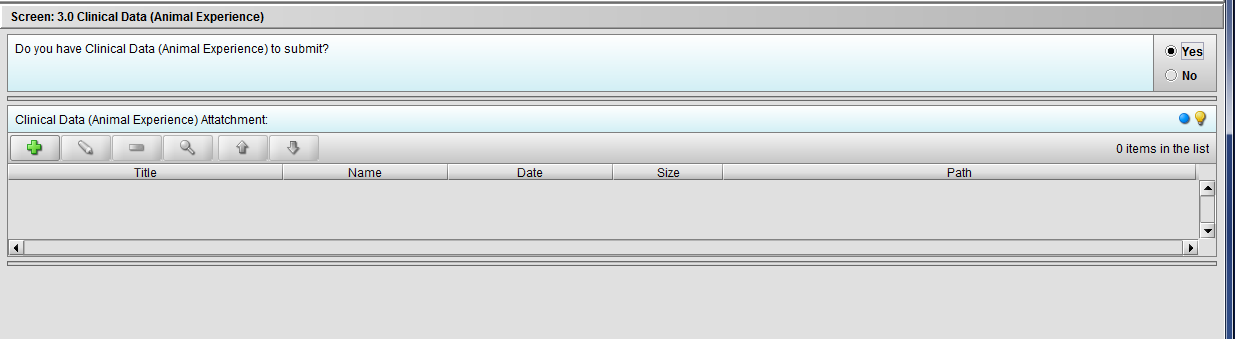
Figure 2.3(b): Selection of ‘Yes’ to question ‘Do you have Clinical Data (Animal Experience) to submit?’ enables question ‘Clinical Data (Animal Experience) Attachment’.
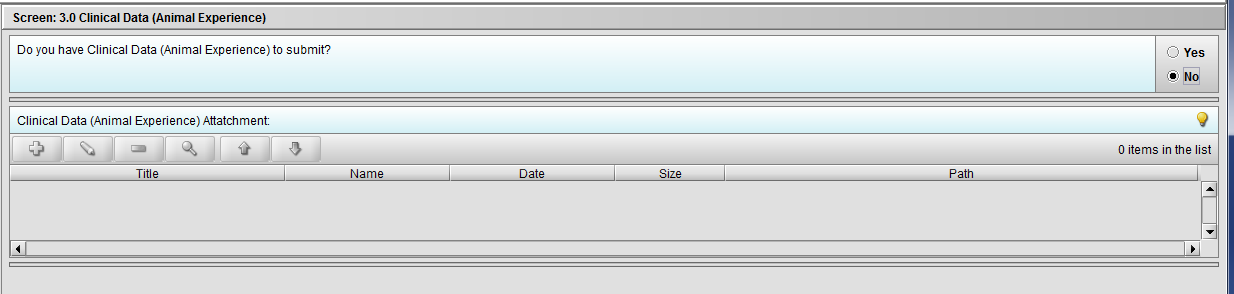
Figure 2.3(c): Selection of ‘No’ to question ‘Do you have Clinical Data (Animal Experience) to submit?’ navigates to next Node.
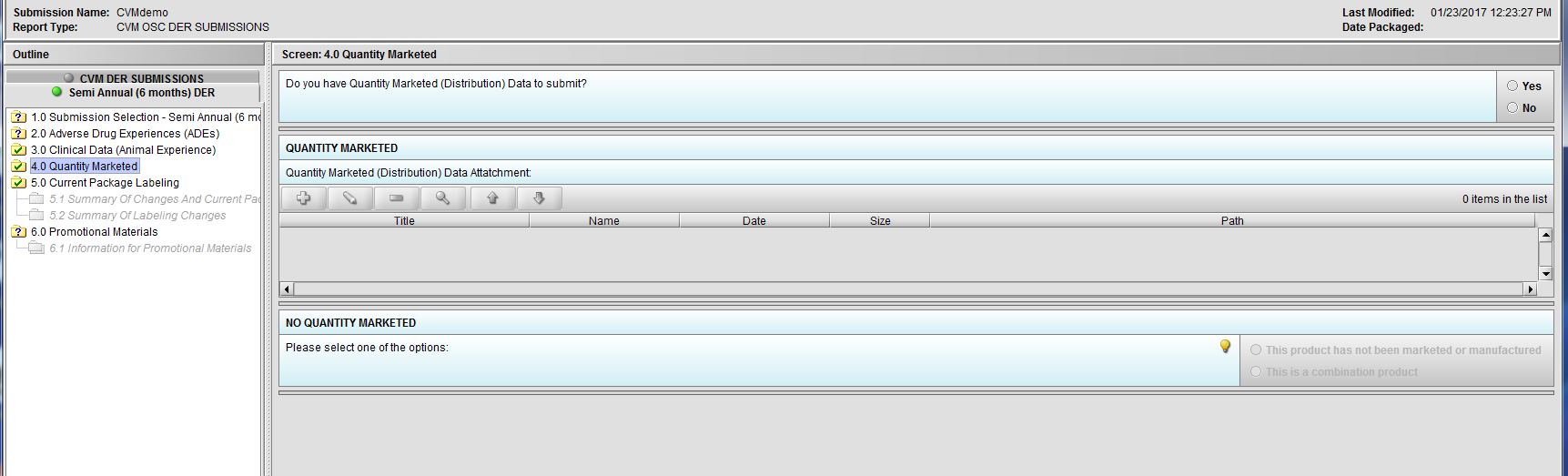
Figure 2.4(a): Quantity Marketed
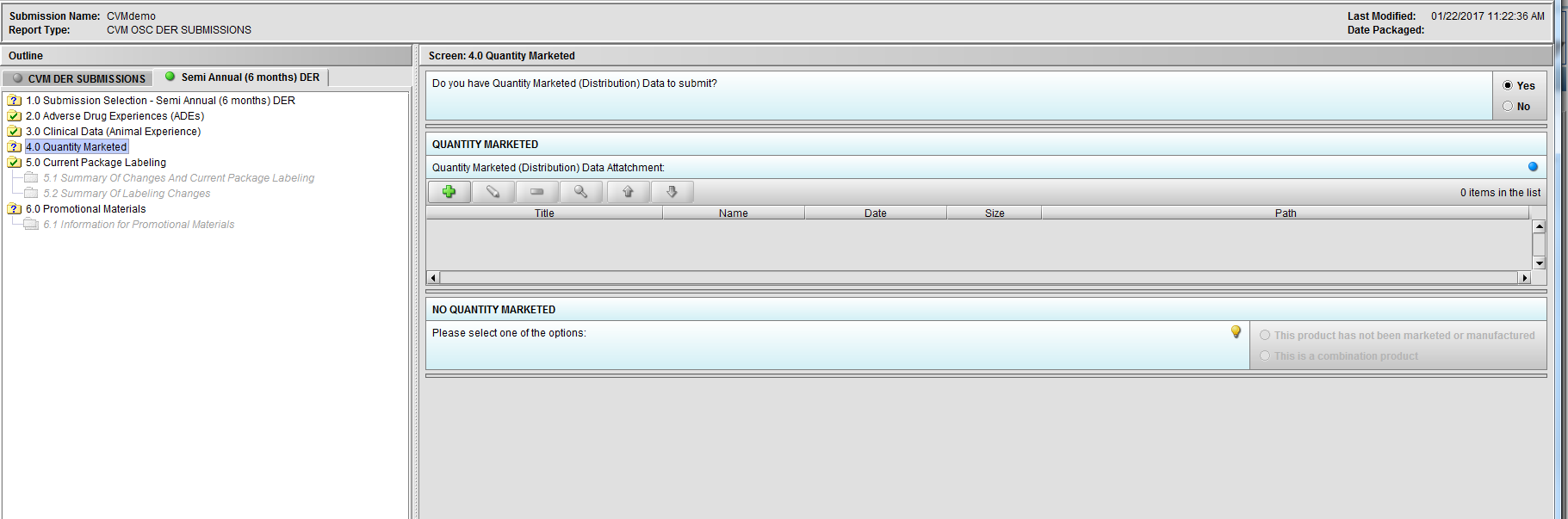
Figure 2.4(b): Selection of ‘Yes’ to question ‘Do you have Quantity Marketed (Distribution) Data to submit?’ enables question ‘Quantity Marketed (Distribution) Data Attatchment:’.

Figure 2.4(c): Selection of ‘No’ to question ‘Do you have Quantity Marketed (Distribution) Data to submit?’ navigates to next Node. Hint to question ‘Quantity Marketed (Distribution) Data Attatchment:’
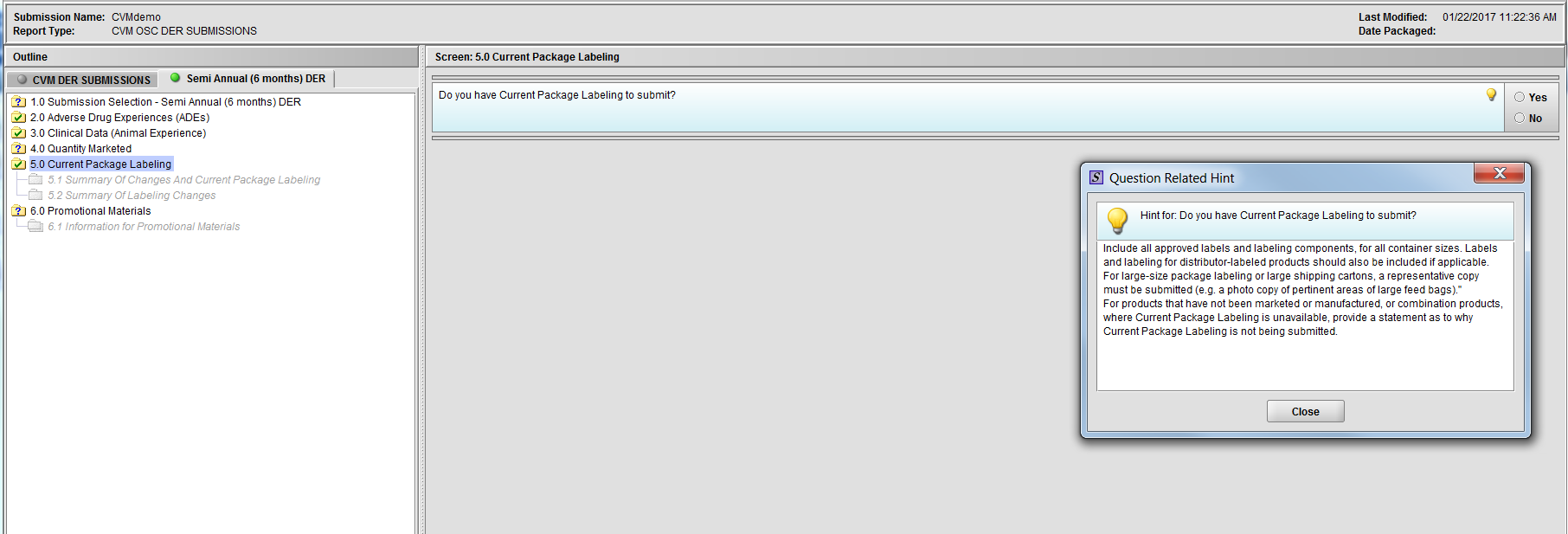
Figure 2.5(a): Current Package Labeling. Hint for question ‘Do you have Current Package Labeling to submit?’
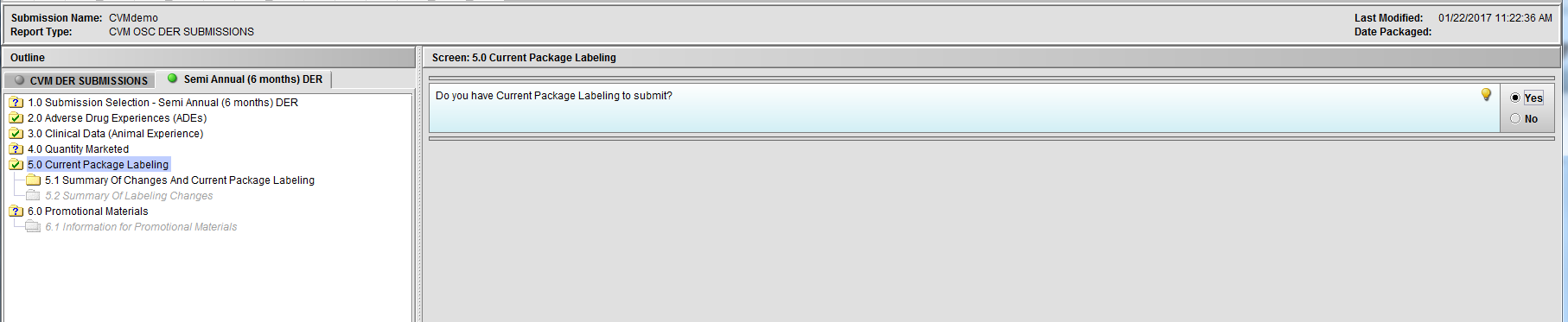
Figure 2.5(b): Selection of ‘Yes’ to question ‘Do you have Current Package Labeling to submit?’ enables Node 5.1
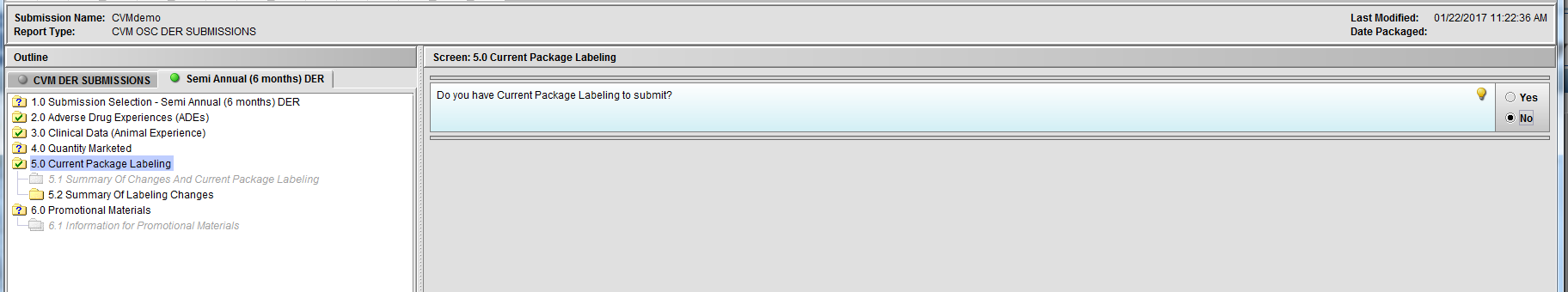
Figure 2.5(c): Selection of ‘No’ to question ‘Do you have Current Package Labeling to submit?’ enables Node 5.2
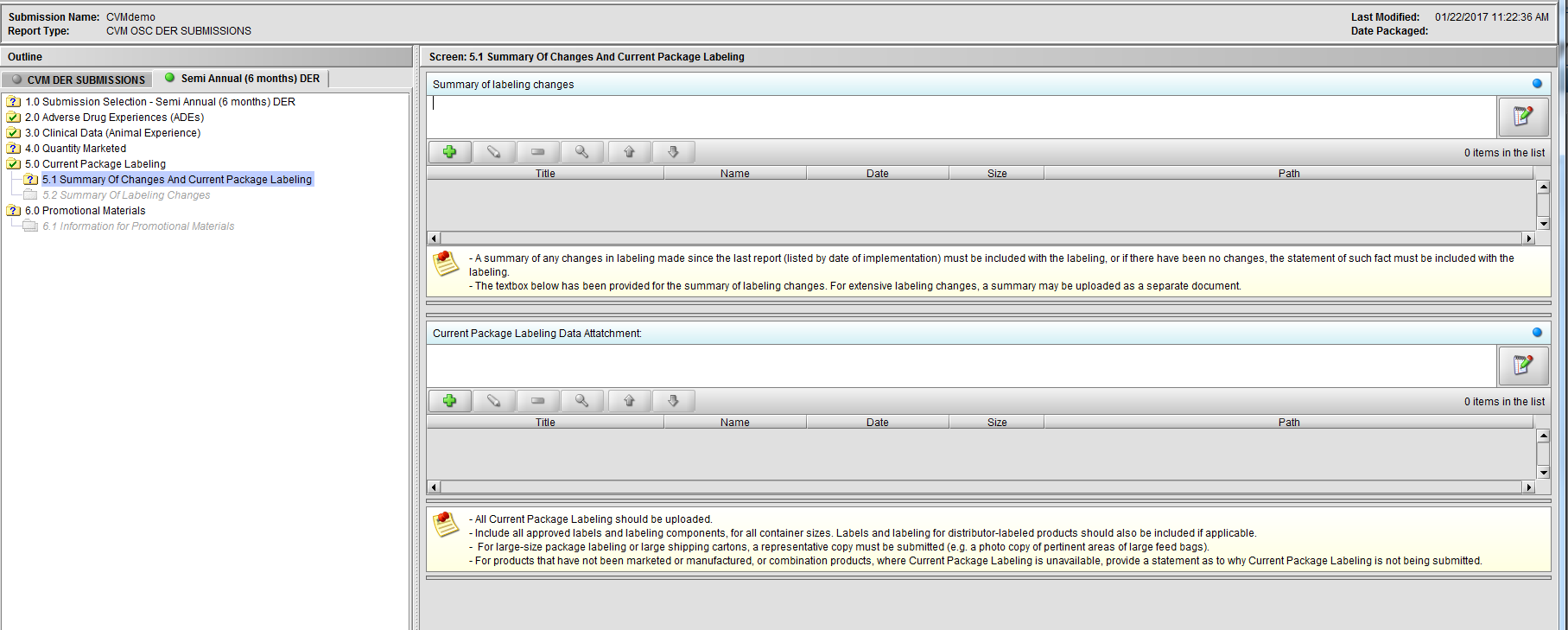
Figure 2.5.1(a): Summary Of Changes And Current Package Labeling
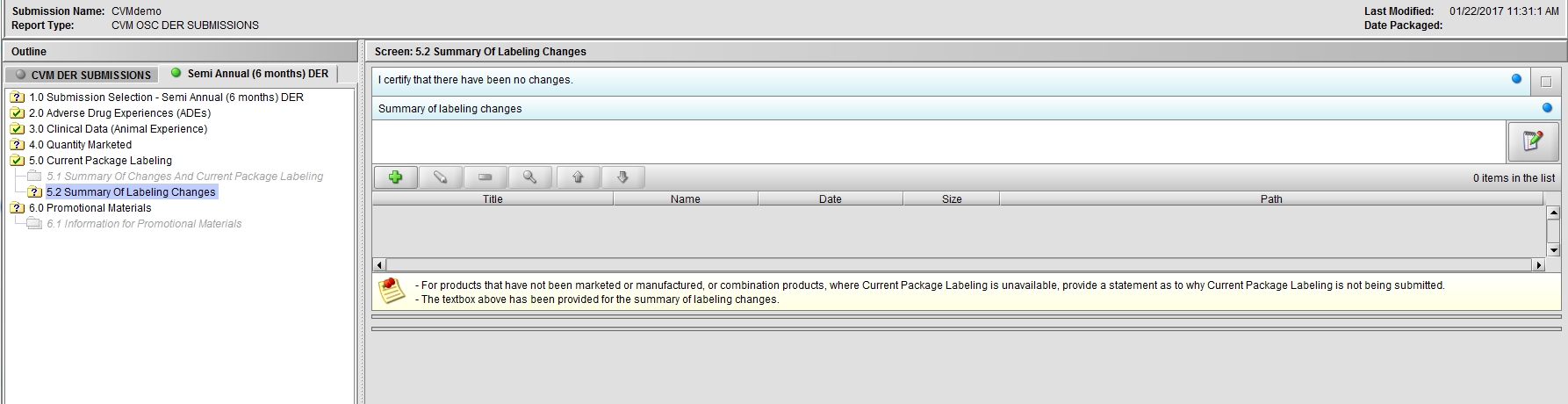
Figure 2.5.2(a): Summary Of Changes And Current Package Labeling
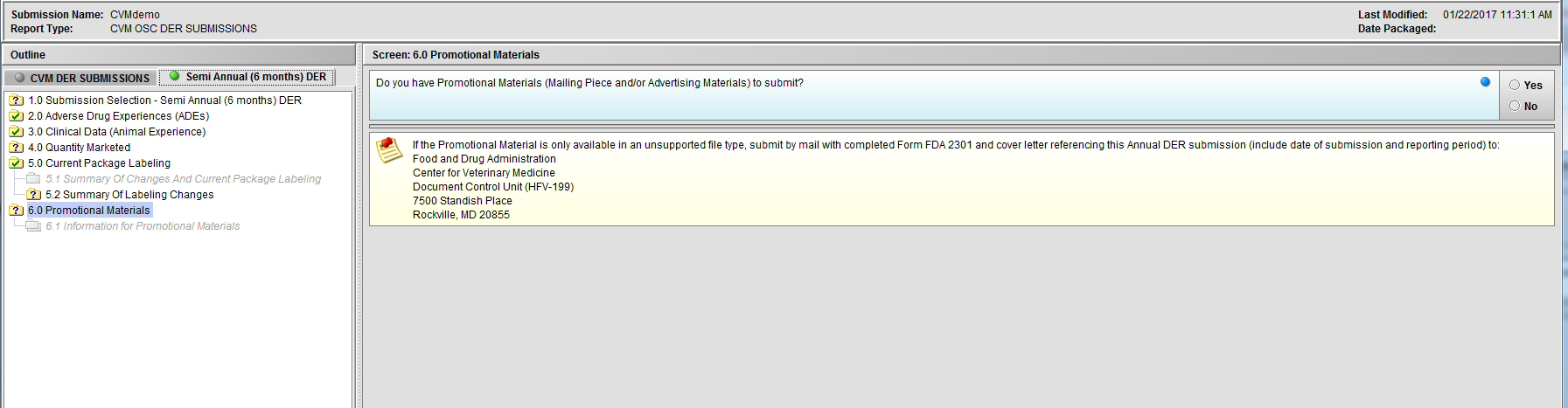
Figure 2.6(a): Promotional Materials
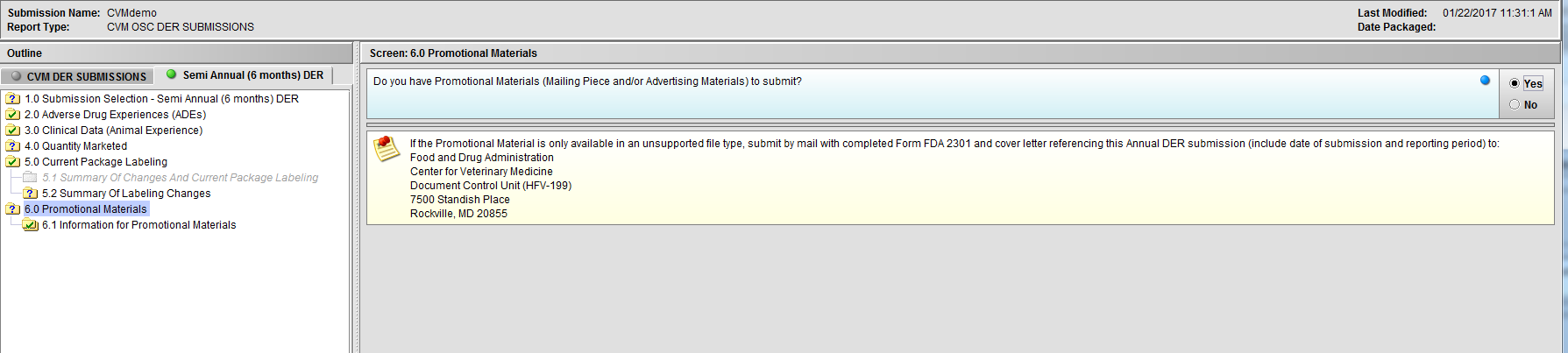
Figure 2.6(b): Selection of ‘Yes’ to question ‘Do you have Promotional Materials (Mailing Piece and/or Advertising Materials) to submit?’ enables Node 6.1
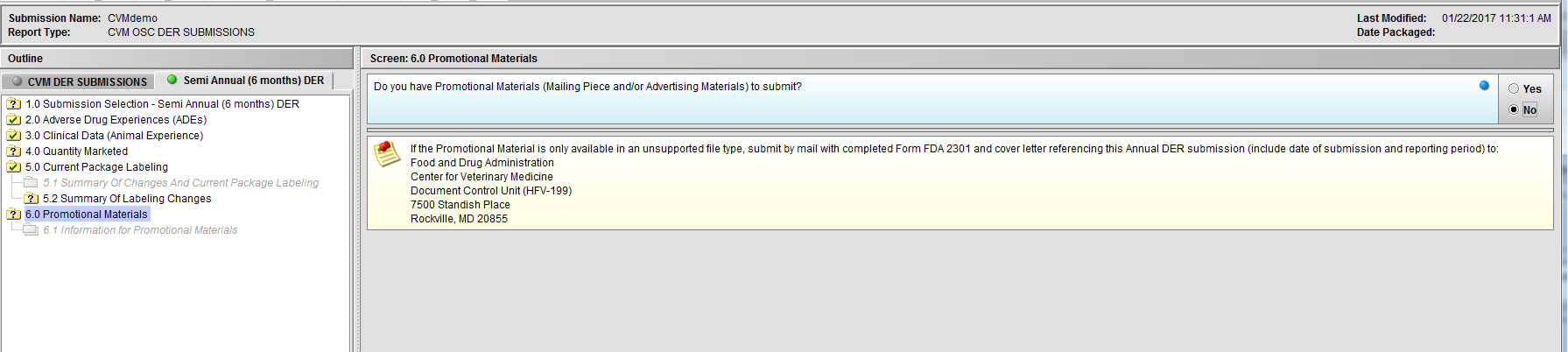
Figure 2.6(c): Selection of ‘No’ to question ‘Do you have Promotional Materials (Mailing Piece and/or Advertising Materials) to submit?’ completes the submission questions
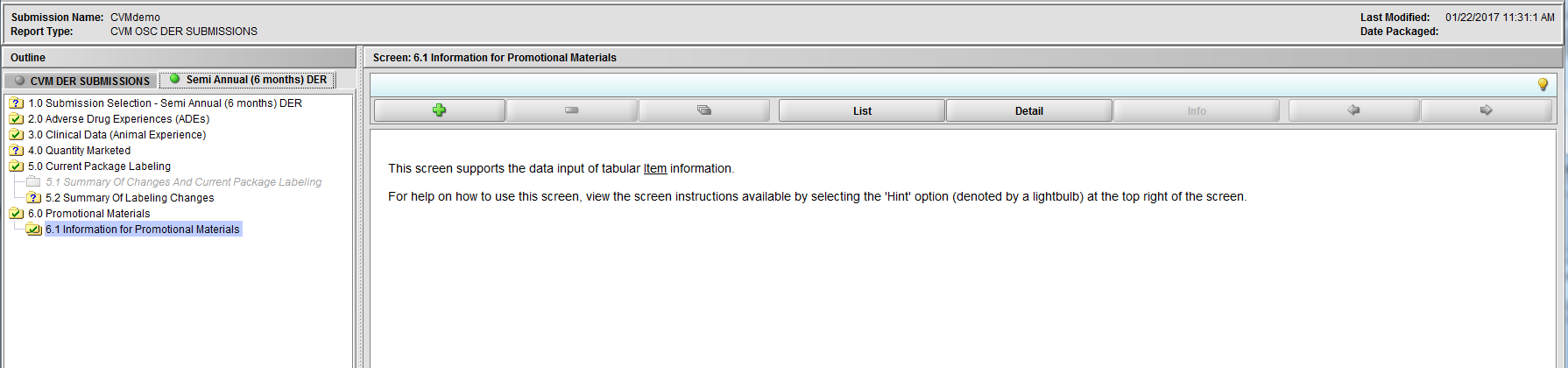
Figure 2.6.1(a): Information for Promotional Materials
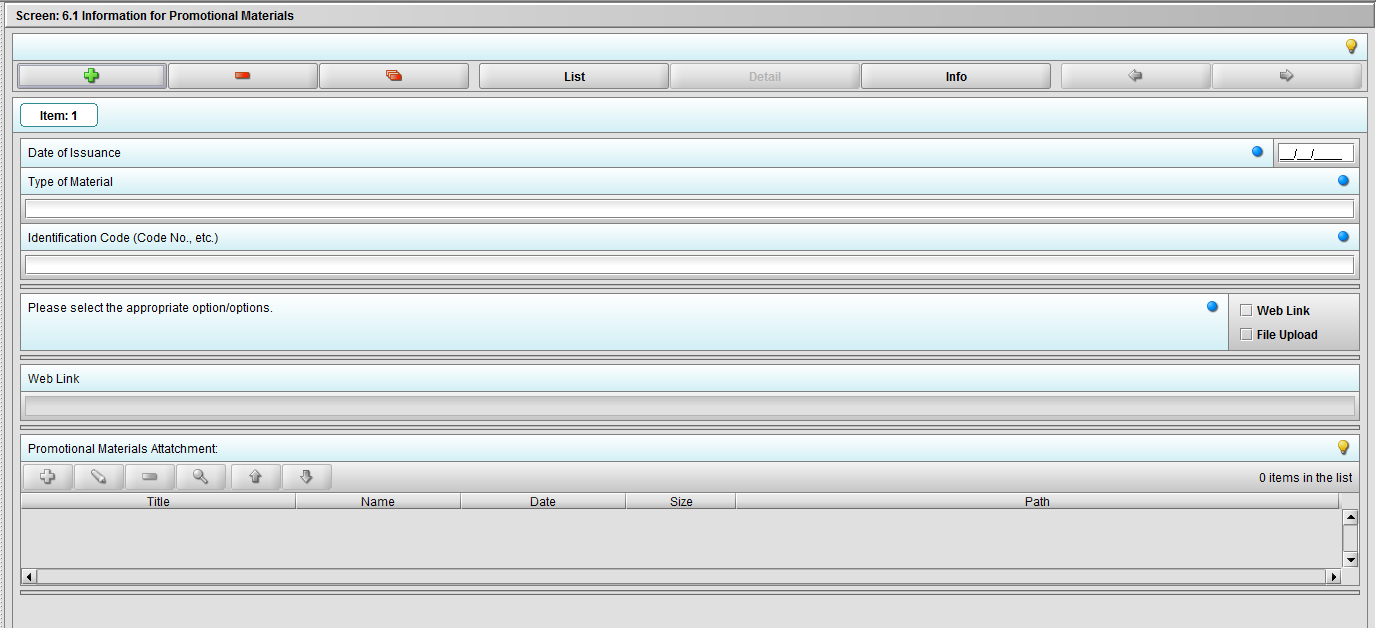
Figure 2.6.1(b): Clicking the ‘+’ navigates to the questions associated with Promotional Materials.
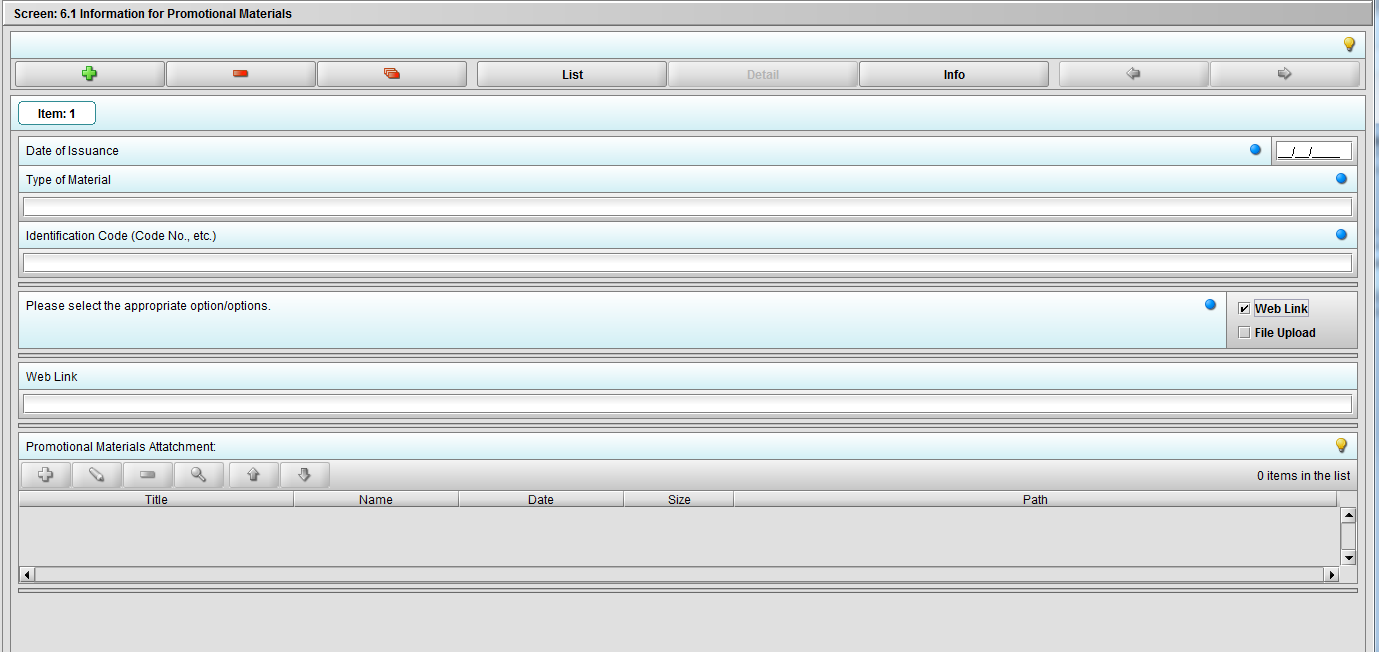
Figure 2.6.1(c): Selecting ‘Web link’ on question ‘Please select the appropriate option/options.’ enables question ‘Web link’.
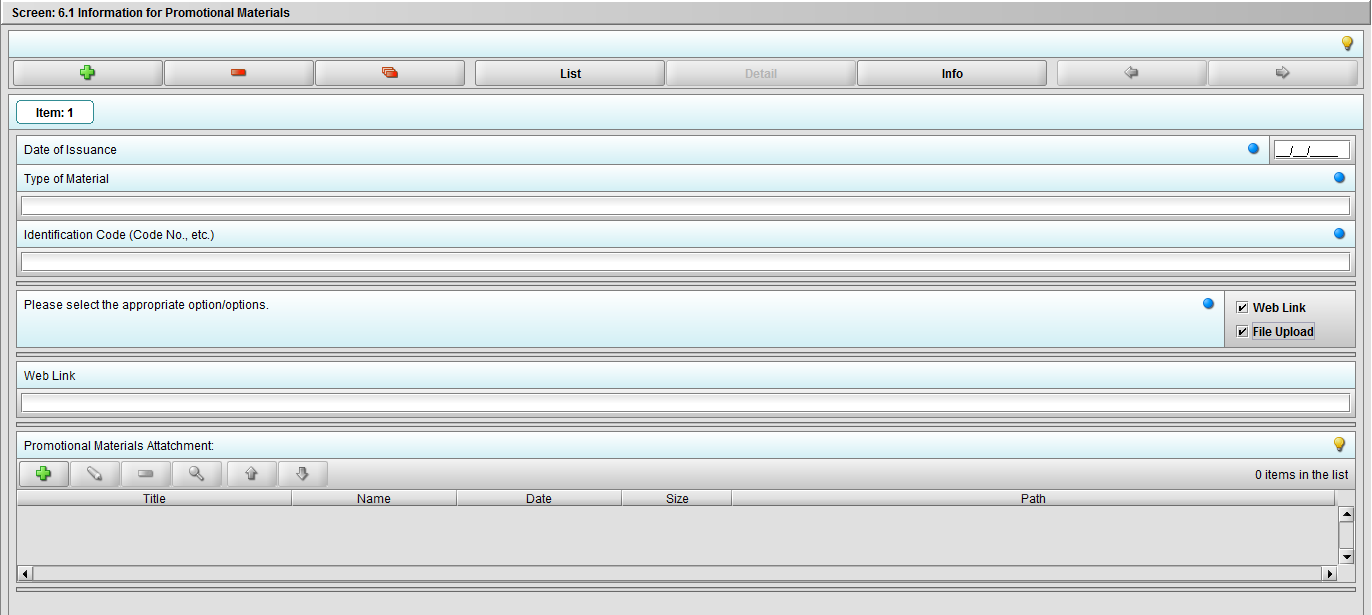
Figure 2.6.1(c): Selecting ‘File Upload’ on question ‘Please select the appropriate option/options.’ enables question ‘Promotional materials Attachment’.
***Note: Selection to question ‘Please select the appropriate option/options.’ can be either or both.
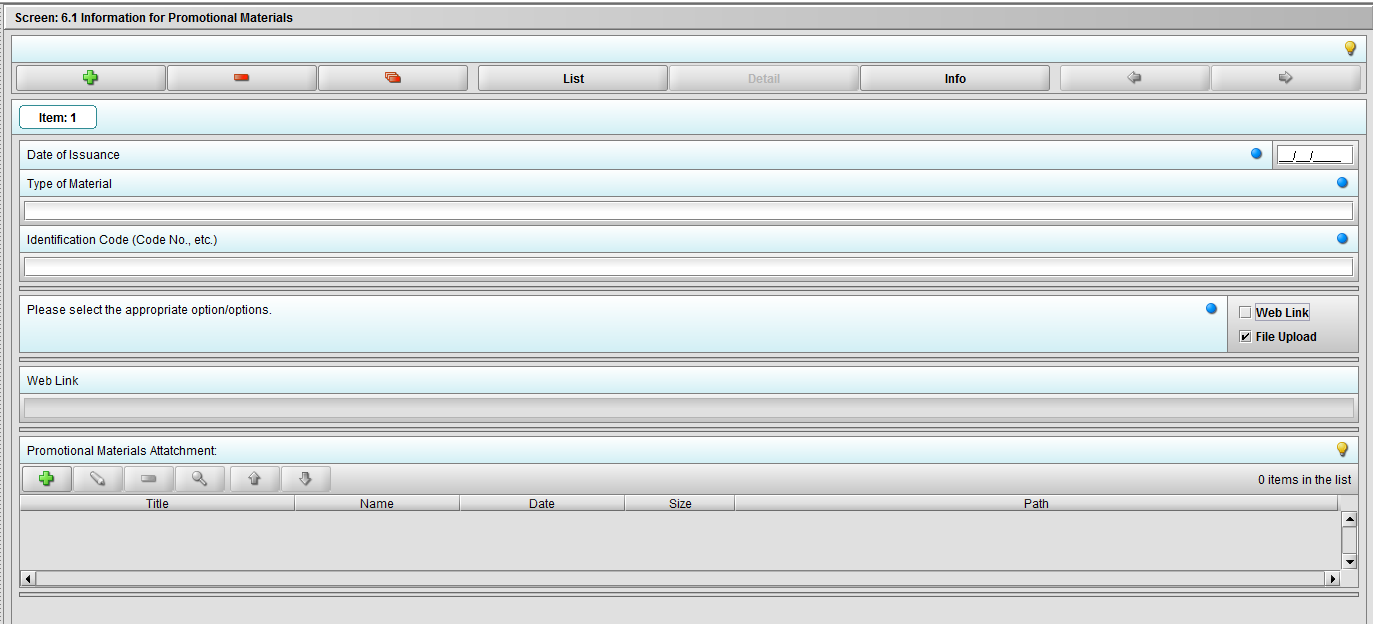
Figure 2.6.1(c): Selecting ‘File Upload’ on question ‘Please select the appropriate option/options.’ enables question ‘Promotional materials Attachment’.
Page
| File Type | application/msword |
| Author | Cheng, Chung * |
| Last Modified By | Mizrachi, Ila |
| File Modified | 2017-03-10 |
| File Created | 2017-03-10 |
© 2026 OMB.report | Privacy Policy