0841 SSA rev 040820_v2
0841 SSA rev 040820_v2.docx
Management Information System for Comprehensive Cancer Control Programs
OMB: 0920-0841
Reinstatement with Change
Management Information System for
Comprehensive Cancer Control Programs
OMB # 0920-0841
Supporting Statement: Part A
Contact:
Floyd ‘Trey’ Bonner, M.P.H.
Phone: (770) 488-2799
E-mail: [email protected]
Division of Cancer Prevention and Control
National Center for Chronic Disease Prevention and Health Promotion
4770 Buford Highway, NE, MS F76
Atlanta, GA 30341
April 8, 2020
Information Collection Request
TABLE OF CONTENTS
Justification
Circumstances Making the Collection of Information Necessary
Purposes and Use of Information Collection
Use of Improved Information Technology and Burden Reduction
Efforts to Identify Duplication and Use of Similar Information
Impact on Small Businesses or Other Small Entities
Consequences of Collecting the Information Less Frequently
Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
Explanation of Any Payment or Gift to Respondents
Assurance of Confidentiality Provided by Respondents
Justification for Sensitive Questions
Estimates of Annualized Burden Hours and Costs
Estimates of Other Total Annual Cost Burden to Respondents or Record Keepers
14. Annualized Cost to the Federal Government
Explanation for Program Changes or Adjustments
Plans for Tabulation and Publication and Project Time Schedule
17. Reason(s) Display of OMB Expiration Date is Inappropriate
18. Exceptions to Certification for Paperwork Reduction Act Submissions
List of Attachments
Authorizing Legislation for the National Comprehensive Cancer Control Program (NCCCP)
60-Day Federal Register Notice
3. List of NCCCP Awardees
4a. Current MIS Data Elements for NCCCP Awardees
4b. Summary of Changes to MIS Data Elements to Facilitate Reporting and Searching Standardized
Measures
4c. Data Entry Screenshot
4d. Login Page Screenshot
4e. Privacy Narrative
4f. Privacy Impact Assessment
4g. Non-Research Determination
4h. CDMIS User Guide: Action Plan tab
Goal: Collect, store, retrieve, share, and report accurate and timely information electronically from 66 cooperative agreement awardees that receive funding for participation in the National Comprehensive Cancer Control Program (NCCCP).
Intended Use: Monitor NCCCP awardee performance and provide timely and accurate responses to inquiries from Congress and other stakeholders.
Methods: Awardees will use the Management Information System (MIS) to monitor program outcomes and report progress to CDC yearly. CDC will retrieve information to respond to public and internal leadership inquiries.
Subpopulation: 66 NCCCP awardees
Data Analysis: Quantitative and qualitative analyses
A. JUSTIFICATION
1. Circumstances Making the Collection of Information Necessary
This statement supports the request for clearance of a revision to electronic collection of information by the National Comprehensive Cancer Control Program (NCCCP), funded by the Comprehensive Cancer Control Branch of the Centers for Disease Control and Prevention (CDC), Department of Health and Human Services (DHHS) (Management Information System for Comprehensive Cancer Control Programs, OMB No. 0920-0841, exp. 6/30/2019). OMB approval is requested for three years. This information collection is authorized by the Public Health Service Act, Section 301, 241(a) (see Attachment 1).
The Comprehensive Cancer Control Branch manages the NCCCP, which provides funding to 66 state, tribal, territorial, and U.S. Affiliated Pacific Island health departments to design, implement, and evaluate comprehensive cancer control plans to reduce the burden of cancer locally. Support for these programs is a cornerstone of CDC efforts to reduce the burden of cancer throughout the nation. Awards to individual applicants are made for a five-year budget period. Continuation awards for subsequent budget periods are made on the basis of satisfactory progress in achieving both national and program-specific goals and objectives, as well as availability of funds.
In 2017, 66 awardees were selected for funding for DP17-1701 (“Cancer Prevention and Control Programs for State, Territorial, and Tribal Organizations”) to implement cancer prevention and control programs to reduce morbidity, mortality, and related health disparities (see Attachment 3). Each awardee submits annual progress reports to CDC through the electronic Management Information System (MIS).
In this revision request, CDC seeks OMB approval to continue using the MIS to collect, store, retrieve, share, and report accurate and timely information to monitor awardee performance and resource use for three years. The request will cover the last three program years of DP17-1701.
Electronic reporting of core NCCCP data elements (see Attachment 4a) will continue for the new approval period. There are minor changes in content to increase standardization of reporting performance measures and to refine search capabilities (see Attachment 4b).
2. Purpose and Use of the Information Collection
The MIS is a post award grants management web-based tool used to collect information about the financial and staffing resources dedicated to cancer control by each awardee; the types of cancer addressed by each awardee; their work plan objectives, activities, and partnerships; and their program evaluations, reports, and products. Awardees provide the information for resources and activities related to the cooperative agreement. Awardees provide this information for key program staff hired or retained to help implement the award (e.g. Program Coordinator). The contact person only provides information about the new program, not personal information.
The MIS is designed to improve the capacity of the CDC, as well as each NCCCP awardee, to efficiently report information needed to monitor program progress, report performance measures, track changes in work plans, and document and report information required as a condition of cooperative agreement funding. There are eight MIS tabs: (1) FOA & Recipients; (2) Program Information; (3) Resources; (4) Leadership Team; (5) Financial; (6) Planning; (7) Action Plan; and (8) Reports. The Program Information tab includes program contact information and a program summary. The Leadership Team tab includes a leadership team summary and the leadership team plan. Awardees action/work plans are housed in the Action Plan tab including work plan objectives and activities.
3. Use of Improved Information Technology and Burden Reduction
The MIS is based on well-defined information components and processes that foster consistency in data collection and reporting. The MIS takes advantage of technology to improve information quality by minimizing errors and redundancy. The MIS interface has been enhanced for usability, including increased use of drop-down menus and pre-formatted options. These modifications reduce the burden of entering data in open-ended format and facilitate the annual transfer of program and resource information that has not changed.
Special attention has been given to ensuring the system is easy to use and collects information that can later be queried and summarized through its reporting capabilities. The MIS is intended to accomplish the following functions:
Reduce both NCCCP awardee and CDC burden of program planning, reporting, and overall cooperative agreement administration.
Standardize the NCCCP awardee reporting process to facilitate development of evaluation methods.
Enable reporting information to be sorted and aggregated to assess the overall effectiveness of NCCCP and respond to stakeholder inquiries.
Support a common monitoring and evaluation framework for core cancer prevention and control program activities.
The MIS design also allows CDC and awardee staff to access the data entry pages for data entry, data review, and collaboration on technical assistance. Awardee staff have used MIS to review the completeness of data necessary to submit required reports, enter basic summary data for reports, and finalize and save required reports for upload into Grants Solutions.
4. Efforts to Identify Duplication and Use of Similar Information
The collection of an annual performance report is part of a federal reporting requirement for cooperative agreement awardees. The MIS consolidates information necessary for this report that also serves as a continuation application, so that information entered once can be used to generate a report that meets the federal reporting requirement without having to duplicate efforts. The MIS eliminates duplicative efforts under paper-based reporting systems. The information collected from NCCCP awardees is not available from other sources.
5. Impact on Small Businesses or Other Small Entities
No small businesses will participate in the MIS data collection.
6. Consequences of Collecting the Information Less Frequently
Annual performance reports are required for NCCCP awardees funded through the DP17-1701 cooperative agreement. The annual reporting schedule will be maintained during the first year of this three-year revision request.
7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
There are no special circumstances related to the continued use of the MIS, and the request fully complies with the regulation.
8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside Agency.
A. Federal Register Notice
A 60-day Notice was published in the Federal Register on November 4, 2019 (Volume 84, Number 213, pages 59378-59379) (see Attachment 2). No comments were received and no modifications were made to the information collection plan.
B. Other Consultations
The MIS was designed collaboratively by CDC staff and the data collection contractor. Consultation will continue throughout the system modification process.
9. Explanation of Any Payment or Gift to Respondents
Respondents do not receive payments or gifts for providing information.
10. Protection of the Privacy and Confidentiality of Information Provided by Respondent
NCCDPHP’s Information Systems Security Officer has reviewed this submission and has determined that the Privacy Act does not apply. Activities do not involve the collection of individually identifiable information. For additional details review the Privacy Impact Assessment attachment entitled CDMIS PIA . Respondents are state-, territorial/USAPIJ-, and tribal-based comprehensive cancer control programs. Although contact information is obtained for each program, the contact person provides information about the state, territorial, or tribal program, not personal information.
The MIS is a a post award grants management web-based application. There is no website content directed at children less than 13 years of age. Access is controlled by a password-protected login for authorized users.
Access levels vary from read-only to read-write, based on the user’s role and needs. Each NCCCP awardee has access to its own information and decides the level of access for each user. The extent to which local partners may access an NCCCP awardee’s information is decided by that awardee. Aggregated information is stored on an internal CDC SQL server subject to CDC’s information security guidelines. The MIS is hosted on NCCDPHP’s Intranet and Internet Application platforms, which undergo security certification and accreditation through CDC’s Office of the Chief Information Security Officer.
11. Institutional Review Board (IRB) and Justification for Sensitive Questions
IRB Approval
The MIS information collection has been determined to be public health practice and not research involving human subjects; therefore, neither IRB approval nor consent from individuals are required. However, awardees are required to respond as a condition of cooperative agreement funding.
Sensitive Questions
The MIS instrument does not collect sensitive information. No personal information is requested. The MIS collects a limited amount of information in identifiable form (IIF) for key program staff (e.g., Program Director, Coalition Chairperson). Awardees provide the names of these individuals as well as their professional contact information. The contact person only provides information about the NCCCP program, not personal information. For additional information refer to the non-research determination attachment (Attachment 4g).
12. Estimates of Annualized Burden Hours and Costs
A. Estimated Annualized Burden Hours
All 66 NCCCP awardees will submit Data Elements for All NCCCP Programs through a web-based Management Information System (MIS). Screenshots are provided in Attachment 4a.
Current requested changes are summarized in Attachment 4b. Respondents will report outcomes annually during this clearance period. For routine reporting, the estimated burden per response is 1 hour.
For all data collection and reporting for cancer prevention and control programs, the total estimated annualized burden to respondents is 66 hours, as summarized in Table A.12-1.
OMB approval is requested for 3 years. To avoid under-estimation of respondent burden, the burden table accounts for annual reporting throughout the 3-year clearance period.
Table A.12-1. Estimated Annualized Burden to Respondents
Type of Respondents |
Form Name |
No. of Respondents |
No. of Responses per Respondent |
Avg. Burden per Response (in hrs.) |
Total Burden (in hrs.) |
Program Director for State-, Tribal-, or Territorial-based Cancer Prevention and Control Program |
Data Elements for All CPC Programs: Annual Reporting |
66 |
1 |
1 |
66 |
Total |
66 |
||||
B. Estimated Annualized Burden Costs to Respondents
Table B.12-1 displays the estimated annualized cost to respondents for reporting program progress information. The hourly wage rates for program managers are based on wages for similar mid-to-high level positions in the public sector. The total estimated annualized cost to respondents is $2,581.26 [(66 x 1) + (1 x $39.11)].
Table B.12-1. Estimated Annualized Cost to Respondents
Type of Respondents |
Form Name |
No. of Respondents |
No. of Responses per Respondent |
Average Burden per Response (in hrs.) |
Average Hourly Wage |
Total Cost |
|
Program Director for State-, Tribal-, or Territorial-based Cancer Prevention and Control Program |
Data Elements for All CPC Programs: Semi-annual Reporting |
66 |
1 |
1 |
$39.11 |
$2,581.26 |
|
|
Total |
$2,581.26 |
|||||
*Hourly wage information is from the U.S. Department of Labor, Bureau of Labor Statistics website (www.data.bls.gov/cgi-bin/print.pl/oes/current/oes1191999.htm).
13. Estimates of Other Total Annual Cost Burden to Respondents and Record Keepers
The MIS is designed to use existing hardware within funded sites, and all respondents currently have access to the Internet to use the information system. No capital or maintenance costs have been required. Additionally, there have been no start-up, hardware or software costs.
14. Annualized Cost to the Federal Government
A. Development, Implementation, and Maintenance
The MIS developer and data collection contractor is Northrup-Grumman. Major cost factors related to deploying the MIS include development and testing costs, system maintenance costs, and the cost of oversight by CDC GS-14 program staff, requiring 5% level of effort equating to $6,814.75. The total estimated annualized cost of the MIS is $248,917.
Tables A.14.1 and A.14.2 provide detailed breakdowns of the estimated annualized cost for each program component.
Table A.14.1 Estimated Annualized Cost for Collection of MIS-based NCCCP (DP17-1701) Data
-
Annualized cost of system development and implementation*
$208,917
Annual system maintenance contract
$40,000
Total annualized cost to the government
$248,917
* The annualized cost of system development and implementation is based on costs for NCCCP for the period July 1, 2016 to February 2019.
Explanation for Program Changes or Adjustments
In the 2016 previous OMB approval period, the total estimated annualized burden is 304 hours. The existing burden hours for this request is 66 hours, which is a decrease of 238 hours. Since the DP17-1701 is in a current funding cycle, awardees will not be populating MIS with initial data, which results in a decrease of 44 hours. Also, the reporting frequency has changed from biannual to annual, the response time has decreased from 2 hours to 1 hour, and the number of respondents has increased from 65 to 66, which results in a decrease of 194 hours. Annual reporting includes data entry related to the action plan and leadership team tab. User acceptability testing was conducted related to the addition of the leadership team tab data which allowed for accurate estimates of burden per response.
Table A.15-1. Changes to Estimated Annualized Burden to Respondents
Form Name |
Previous Approval |
Current Revision Request |
Change |
||||||
Number of respondents |
Frequency |
Burden per response (in hours) |
Total burden (in hours) |
Number of respondents |
Frequency |
Burden per response (in hours) |
Total burden (in hours) |
||
Data Elements for All CPC Programs: Initial MIS Population |
22 |
1 |
2 |
44 |
0 |
0 |
0 |
0 |
-44 |
Data Elements for All CPC Programs: Annual Reporting |
65 |
2 |
2 |
260 |
66 |
1 |
1 |
66 |
-194 |
TOTAL |
304 |
TOTAL |
66 |
-238 |
|||||
16. Plans for Tabulation and Publication and Project Time Schedule
A. Time schedule for the entire project
The cooperative agreement cycle for DP17-1701 is 5 years. OMB approval is being requested for the final three years of this NOFO. Tables including beginning and ending dates for the collection of information for the FOA and other actions are provided below.
-
Table 16-1 Project Time Schedule for NCCCP Reporting
Activity
Time Schedule
Notify respondents
Within 2 weeks after OMB approval
Training
1 month after OMB approval
Ongoing support (as needed)
1 month after OMB approval
Analyses and Validation
2 months after OMB approval
B. Publication plan
DP17-1701-related information collected through the MIS will be reported in internal CDC documents and shared with CCC programs.
C. Analysis plan
CDC will not use complex statistical methods for analyzing progress report-related information. All information will be aggregated. Most statistical analyses will be descriptive. Statistical modeling may be included to examine predictors of specified outcomes.
17. Reason(s) Display of OMB Expiration Date is Inappropriate
The CCC MIS program will display the OMB Control number and expiration date of the MIS data collection at the bottom of on its Internet home page as seen in the screenshot below.
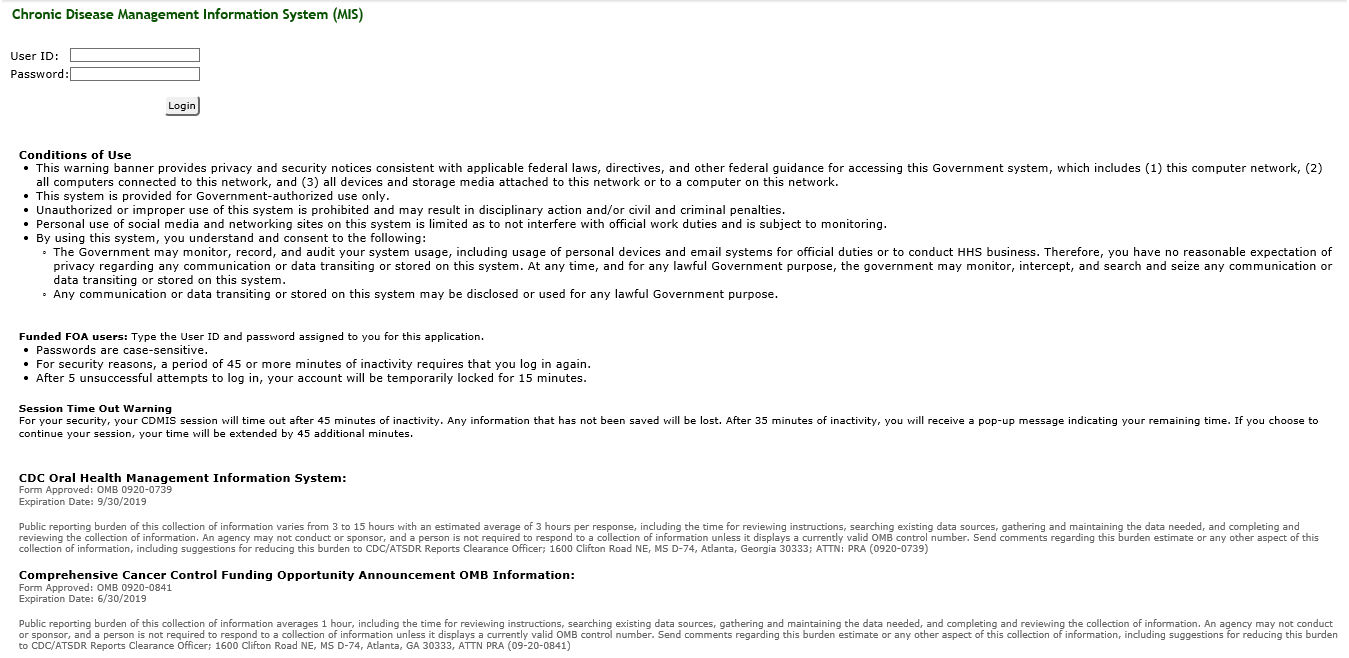
18. Exceptions to Certification for Paperwork Reduction Act Submissions
There are no exceptions to the certification statement.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | CDC User |
| File Modified | 0000-00-00 |
| File Created | 2021-01-14 |
© 2026 OMB.report | Privacy Policy