Supporting Statement B - CARES Act lab data 5.28.2020 CLEAN
Supporting Statement B - CARES Act lab data 5.28.2020 CLEAN.docx
COVID-19 Pandemic Response, Laboratory Data Reporting
OMB: 0920-1299
COVID-19 Pandemic Response, Laboratory Data Reporting
Request for OMB approval of a New Information Collection
May 28, 2020
Supporting Statement B
Contact:
Lee Samuel
Emergency Operations Center
Centers for Disease Control and Prevention
1600 Clifton Road, NE
Atlanta, Georgia 30333
Phone: (404) 718-1616
Email: [email protected]
Table of Contents
1. Respondent Universe and Sampling Methods 2
2. Procedures for the Collection of Information 2
3. Methods to maximize Response Rates and Deal with No Response 3
4. Tests of Procedures or Methods to be Undertaken 3
5. Individuals Consulted on Statistical Aspects and Individuals Collecting and/or Analyzing Data 4
This collection does not involve statistical methods. The purpose of the collection is not to make statistical generalizations beyond the particular respondents.
Respondent Universe and Sampling Methods
Respondents include public health departments in all fifty states, the District of Columbia, Puerto Rico, U.S. Virgin Islands, and Guam. Public health authorities, including states, territories, and agencies of the Federal government are authorized by law (45 CFR 164.512(b)) “to collect or receive information for the purpose of preventing or controlling disease, injury, or disability, including but not limited to, the reporting of disease, injury, vital events such as birth or death, and the conduct of public health surveillance, public health investigations, and public health interventions; or at the direction of a public health authority, to an official of a foreign government agency that is acting in collaboration with a public health authority;”
Health departments regularly collect reportable data from a myriad of sources within their jurisdictions, including, but not limited to public health departments epidemiology and laboratory services, hospitals, sentinel providers, commercial and private laboratories, academic and NGO partners, pharmacies and drugstores, non-profits, and community-based programs.
The new data collection proposed will formalize and require data elements that have long been part of routine public health surveillance efforts, though historically they have been collected inconsistently.
This new data collection codifies complete data reporting as a national requirement.
Lab data are collected by jurisdictional health departments in a variety of methods, depending on the testing entity. Most data are transmitted electronically, including HL7-based electronic laboratory reporting (ELR) and CSV files. Some jurisdictions grapple with more manual reporting methods, such as through spreadsheets, or paper-, fax-, and email-based. Data are compiled and analyzed locally, to inform the state and local public health operations and interventions and then reported to CDC using a CDC-provided CSV file or by forwarding HL7 messages (attachments 3 and 4).
The diagram below presents a simplified flow chart of how COVID-19 surveillance data currently flows from health departments to CDC and HHS.
Diagram A: COVID-19 Testing Results Data Flow from Jursidictional Health Departments
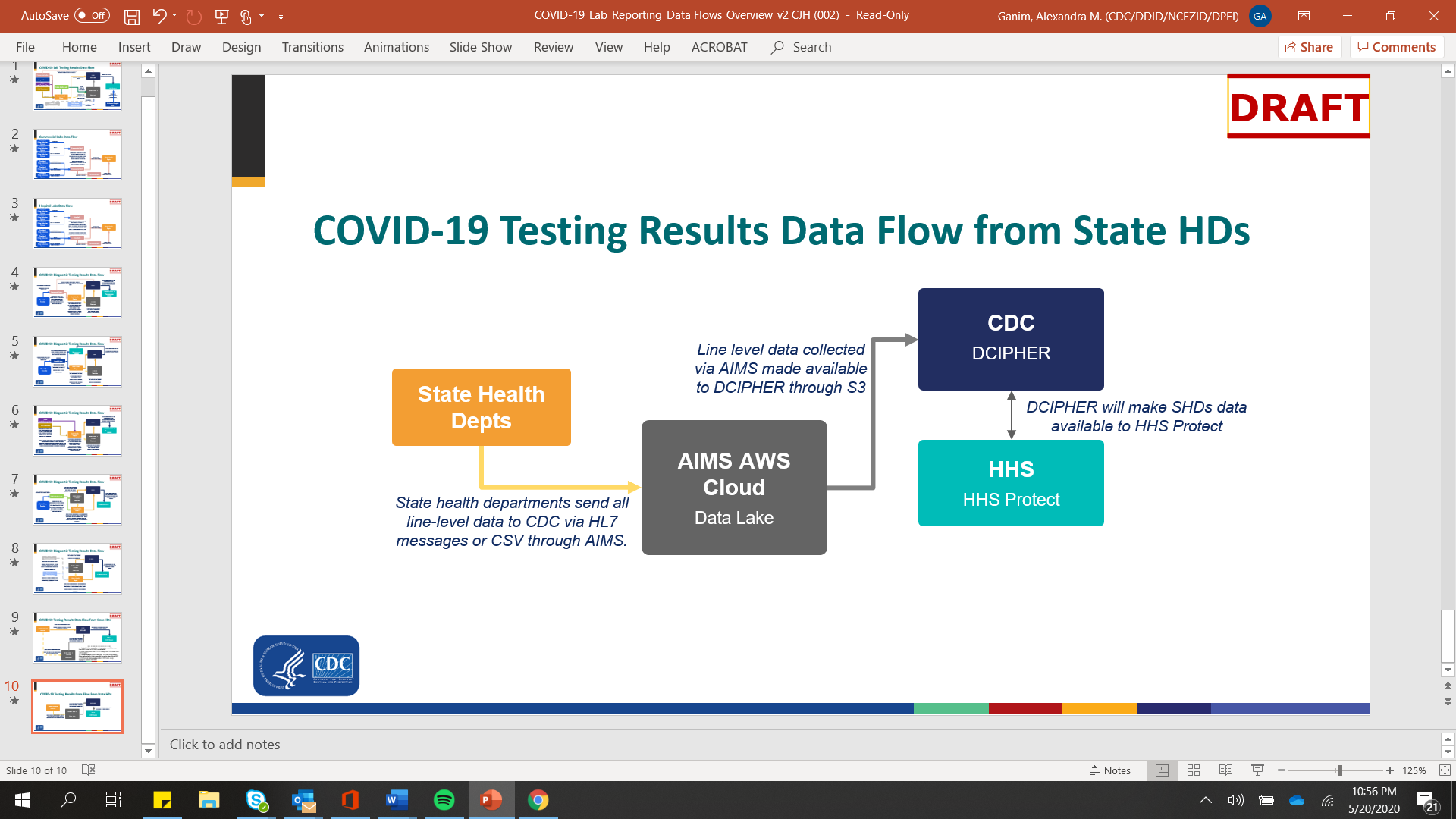
Given the high-profile and urgent priority of this new data collection, CDC is increasing the monitoring, technical assistance, and benchmarking with jurisdictional health departments. Monitoring efforts will include, but won’t be limited to: daily reminders to report, immediate follow-up on data anomalies, weekly outreach, multi-weekly calls, outreach and assistance through public health partner organizations such as CSTE and APHL, and progress tracking through a web-based monitoring dashboard.
Jurisdictions are currently voluntarily sharing laboratory and case reporting data with the CDC through the methods described above. When the data flow, formats or systems change, CDC works closely with jurisdictions to ensure test data are sent and verified in test environments before any reporting is made public.
The foundations of this data collection and reporting have advanced through legacy cooperative agreement programs at CDC, and across many diseases, syndromes, and conditions, both infectious and non-infectious. The core infrastructure and expertise needed to conduct the surveillance and report is largely in place and operational.
The surveillance and reporting work is quickly accelerating at every level, and with this acceleration CDC and HHS will increase active outreach, offers of technical assistance with onboarding, monitoring efforts, and sharing best practices nationwide.
Jason Hall, Information Technology Specialist, Division of Preparedness and Emerging Infections. [email protected]. 404.639.7884
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Modified | 0000-00-00 |
| File Created | 2021-01-14 |
© 2026 OMB.report | Privacy Policy