HCP Survey - Main Study
Healthcare Provider Perception of Boxed Warning Information Survey
Appendix C - HCV Survey
HCP Survey - Main Study
OMB: 0910-0890
APPENDIX C
Healthcare Provider Perception of Boxed Warning Information Survey
Survey Questionnaire: Chronic Hepatitis C Scenario
Version: 10/23/20
Prescriber Survey to Assess Boxed Warnings Perceptions
Survey Questionnaire: HCV
(Questions used for pre-testing condition are included at the end)
Introductory Script:
Thank you for your participation in this survey. The first few questions will focus on chronic hepatitis C viral infection, also known as chronic HCV.
// Note: Where [drug] is indicated, respondents in the chronic HCV condition will be randomly assigned to see Harvoni, Mavyret, or Zepatier.//
//HIDDEN FROM PARTICIPANTS, will be used for piping only//
//Assign each HCV participant to one drug randomly, balancing the number assigned to each//
Question Type: Single Punch
Question DAA_Drug
Variable Name: DAA_Drug
Variable Label: DAA Drug Assignment
Question Text: Random DAA Drug Assignment
Value |
Value Label |
1 |
Harvoni |
2 |
Mavyret |
3 |
Zepatier |
//PROGRAMMING NOTES: Display each question on its own separate screen. Do not allow participants to go back on any screen. Include soft prompts for all items. //
Question Type: Single Punch
Question Q1
Variable Name: Q1
Variable Label: Q1 Familiarity with treating HCV
Question Text: How experienced are you with treating chronic HCV?
Value |
Value Label |
1 |
Not at all experienced |
2 |
Slightly experienced |
3 |
Somewhat experienced |
4 |
Experienced |
5 |
Very experienced |
-99 |
Refused |
Question Type: Single Punch
Question Q2
Variable Name: Q2
Variable Label: Q2 Number of patients typically seen
Question Text: How many patients do you typically see for treatment of chronic HCV?
Value |
Value Label |
1 |
One or two patients a week, or fewer |
2 |
Several patients a week |
3 |
Several patients a day |
98 |
Unsure/Don’t know |
--99 |
Refused |
Question Type: Single Punch
Question 3
Variable Name: Q3
Variable Label: Q3 Importance of curing HCV
Question Text: In general, how important is treating chronic HCV with the goal of a curing it?
Value |
Value Label |
1 |
Not at all important |
2 |
Slightly important |
3 |
Somewhat important |
4 |
Important |
5 |
Very important |
98 |
Unsure/Don’t know |
-99 |
Refused |
Question Type: Single Punch
Question Q4A
Variable Name: Q4A
Variable Label: Q4A Experience prescribing DAA
Question Text: Which of the following is true of your prescribing direct-acting antivirals (DAA) for the treatment of chronic HCV?
Value |
Value Label |
1 |
I have written new prescriptions for this product |
2 |
I have only prescribed a refill for this product |
3 |
I have never prescribed this product |
98 |
Unsure/don’t know |
-99 |
Refused |
//SKIP 4B if 4A = 2, 3, 4, or 98//
Question Type: Single Punch
Question Q4B
Variable Name: Q4B
Variable Label: Q4B Frequency of prescribing DAA
Question Text: On average, how often do you prescribe direct-acting antivirals (DAA) for treatment of chronic HCV?
Value |
Value Label |
1 |
I prescribe this product one or more times a week |
2 |
I prescribe this product a few times a month |
3 |
I prescribe this product a few times a year |
98 |
Unsure/Don’t know |
-99 |
Refused |
Question Type: Multi-Punch
Question Q5
Variable Name: Q5
Variable Label: Q5 Which treatments recommend or prescribe in a month
Question Text: Which of the following treatments would you recommend or prescribe in a typical month (Select all that apply)?
Variable Name |
Value Text |
Variable Label |
Q5_1 |
Direct-acting antivirals (e.g., Harvoni, Epclusa, Mavyret, Vosevi, Zepatier) |
Q5_1 Typical treatment: DAA |
Q5_2 |
Multi-pill regimen(s) (e.g., PEG/Riba therapy) |
Q5_2 Typical treatment: Multi pills |
Q5_3 |
Interferon |
Q5_3 Typical treatment: Interferon |
Q5_4 |
Ribavirin |
Q5_4 Typical treatment: Ribavirin |
Q5_5 |
Other (specify) |
Q5_5 Typical treatment: Other |
Value |
Value Label |
1 |
Selected |
0 |
Not selected |
For the next few questions, we would like you to consider your patients who have been newly diagnosed with chronic HCV or are exhibiting early signs and symptoms of chronic HCV (such as fatigue and mild elevations in liver enzymes).
For this section, also consider the whole class of fixed-dose combination drugs/direct-acting antivirals.
//[SHOW SCENARIO AT THE TOP OF THE SCREEN FOR QUESTION 6]//
Question Type: Single Punch
Question Q6
Variable Name: Q6
Variable Label: Q6 Likelihood of discussing DAA
Question Text: How likely are you to discuss direct-acting antivirals as a potential option for these patients?
Value |
Value Label |
1 |
Very unlikely |
2 |
Unlikely |
3 |
Somewhat likely |
4 |
Likely |
5 |
Very likely |
-99 |
Refused |
Question Type: Single Punch
Question Q7
Variable Name: Q7
Variable Label: Q7 Risk assessment of DAA
Question Text: Which of the following statements describe your assessment of the safety of direct-acting antivirals used to treat HCV?
Value |
Value Label |
1 |
Risks are minimal for almost all patients |
2 |
Risks are minimal for most patients, but are significant for some patients |
3 |
Risks are significant for most patients |
4 |
I am not familiar enough with direct-acting antivirals to make an assessment |
-99 |
Refused |
Question Type: Single Punch
Question Q8
Variable Name: Q8
Variable Label: Q8 Benefits/risks of DAA
Question Text: Which statement best describes your general perspective on the benefits versus risks of direct-acting antivirals for patients with chronic HCV?
Value |
Value Label |
1 |
The benefits of direct-acting antivirals outweigh the risks for most patients |
2 |
The benefits of direct-acting antivirals outweigh the risks for some, but not most, patients |
3 |
The benefits of direct-acting antivirals outweigh the risks for very few patients |
4 |
I am not familiar enough with direct-acting antivirals to make an assessment |
-99 |
Refused |
Question Type: Multi-Punch
Question Q9
Variable Name: Q9
Variable Label: Q9 Factors most important when choosing to prescribe DAA
Question Text: Which of the following factors play the most important role when deciding whether or not to prescribe direct-acting antivirals to a patient with chronic HCV? Please choose the three factors that you consider to be most important.
//PROGRAMMING NOTE: ALLOW PARTICIPANTS TO SELECT UP TO THREE ANSWERS//
[RANDOM ORDER EXCEPT FOR OTHER] [//Multi-punch//]
Variable Name |
Variable Text |
Variable Label |
Q9_1 |
Considerations of this patient’s ability to take the product as prescribed |
Q9_1 Factors: Patient ability to use |
Q9_2 |
This patient’s understanding and comfort with the risks of this medication |
Q9_2 Factors: Understanding and comfort with risks |
Q9_3 |
This patient’s previous experience with treatments |
Q9_3 Factors: Patient’s previous experience |
Q9_4 |
This patient’s medical and health context (e.g., medical history, comorbidities) |
Q9_4 Factors: Medical and health context |
Q9_5 |
Considerations of this patient’s access to the product (e.g., cost, insurance) |
Q9_5 Factors: Patient access |
Q9_6 |
Extent, duration, and severity of patient’s symptoms |
Q9_6 Factors: Symptoms |
Q9_7 |
Patient’s preference for a mode of administration |
Q9_7 Factors: Patient preference for administration |
Q9_8 |
Potential duration of treatment use/course |
Q9_8 Factors: Duration |
Q9_9 |
Other (please specify) |
Q9_9 Factors: Other |
Value |
Value Label |
1 |
Selected |
0 |
Not selected |
Question Type: Open End Essay
Question Q9_ACTIONS
Variable Name: Q9_ACTIONS
Variable Label: Q9_ACTIONS Screening Actions before Starting Treatment

Question Type: Multi-Punch
Question Q10
Variable Name: Q10
Variable Label: Q10 Top three questions from patients about using DAA
Question Text: What are the top three comments or questions you have heard from patients about using direct-acting antivirals to treat chronic HCV? (Select up to three.)
//PROGRAMMING NOTE: ALLOW PARTICIPANTS TO SELECT UP TO THREE ANSWERS//
[//Multi-punch//]
Variable Name |
Variable Text |
Variable Label |
Q10_1 |
Concerns about undergoing treatment if the patient is currently asymptomatic |
Q10_1 Comments top 3: Undergoing treatment if asymptomatic |
Q10_2 |
Hearing positive things about how well direct-acting antivirals work |
Q10_2 Comments top 3: Hearing positive things |
Q10_3 |
Concerns about specific side effects |
Q10_3 Comments top 3: Side effects |
Q10_4 |
Concerns about insurance coverage or cost |
Q10_4 Comments top 3: Insurance coverage/cost |
Q10_5 |
Hearing negative things about how well direct-acting antivirals work |
Q10_5 Comments top 3: Hearing negative things |
Q10_6 |
Pros about the mode of administration |
Q10_6 Comments top 3: Pros mode of administration |
Q10_7 |
Cons about the mode of administration |
Q10_7 Comments top 3: Cons mode of administration |
Q10_8 |
Concerns about being able to complete treatment course |
Q10_8 Comments top 3: General concerns re: treatment |
Q10_9 |
Other (specify) |
Q10_9 Comments top 3: Other |
Value |
Value Label |
1 |
Selected |
0 |
Not selected |
Question Type: Multi-Punch
Question Q11
Variable Name: Q11
Variable Label: Q11 Top three topics talk to patients about DAA
Question Text: What are the top three topics that you prioritize when talking to your patients about direct-acting antivirals as a treatment option?
//PROGRAMMING NOTE: ALLOW PARTICIPANT TO SELECT UP TO THREE ANSWERS//
[RANDOM ORDER EXCEPT FOR LAST OPTION] [//Multi-punch//]
Variable Name |
Variable Text |
Variable Label |
Q11_1 |
How the product works |
Q11_1 Talking top 3: How product works |
Q11_2 |
Expected benefits of the product |
Q11_2 Talking top 3: Expected benefits |
Q11_3 |
Common side effects of the product |
Q11_3 Talking top 3: Common side effects |
Q11_4 |
Rare but serious side effects of the product |
Q11_4 Talking top 3: Rare serious effects |
Q11_5 |
Patient’s medical history (e.g., personal history or family history, possible co-morbidities) |
Q11_5 Talking top 3: Medical history |
Q11_6 |
The importance of using the product as directed |
Q11_6 Talking top 3: Importance of use as directed |
Q11_7 |
Other (Specify) |
Q11_7 Talking top 3: Other |
Value |
Value Label |
1 |
Selected |
0 |
Not selected |
//PROGRAMMING NOTE: If Q11D selected, display Q11_RISKS. OTHERWISE, PROCEED TO Q12 //
Question Type: Open End Essay
Question Q11_RISKS
Variable Name: Q11_RISKS
Variable Label: Q11_RISKS Side effects discussed

Question Type: Multi-Punch
Question Q12
Variable Name: Q12
Variable Label: Q12 How monitor for safety risks and side effects after prescribing DAA
Question Text: How do you most commonly monitor your patients for potential safety risks and side effects after prescribing direct-acting antivirals for HCV? Select all that apply.
Variable Name |
Variable Text |
Variable Label |
Q12_1 |
I schedule routine follow-up appointments with patients. |
Q12_1 Monitor: Follow-up appointment |
Q12_2 |
I instruct patients to get bloodwork before follow-up appointment. |
Q12_2 Monitor: Bloodwork |
Q12_3 |
I instruct patients to call and schedule an appointment if they experience side effects. |
Q12_3 Monitor: Appointment if side effects |
Q12_4 |
I leave it to the patient to follow up if they feel a need to. |
Q12_4 Monitor: Leave up to patient |
Q12_5 |
A follow-up appointment is not necessary. |
Q12_5 Monitor: Follow-up not necessary |
Q12_6 |
Other (specify) |
Q12_6 Monitor: Other |
Value |
Value Label |
1 |
Selected |
0 |
Not selected |
Question Type: Grid
Question Q13
Variable Name: Q13
Variable Label: Q13 How often look for information on DAA
Question Text: How often do you look for information about direct-acting antivirals from the following sources:
Variable Name |
Variable Text |
Variable Label |
Q13_ 1 |
Medical journals |
Q13_1 Info: Medical journals |
Q13_2 |
Medical websites or software (e.g. UpToDate, Epocrates, Medscape) |
Q13_2 Info: Medical websites or software |
Q13_3 |
Drug company/pharmaceutical representatives or their website |
Q13_3 Info: Drug company rep or website |
Q13_4 |
Go online/use a search engine (e.g., Google) |
Q13_4 Info: Online search |
Q13_5 |
Professional medical societies (e.g., publications, guidelines) |
Q13_5 Info: Professional medical societies |
Q13_6 |
Conferences |
Q13_6 Info: Conferences |
Q13_7 |
Discussion with colleagues (e.g., in person, email) |
Q13_7 Info: Discussion w/ colleagues |
Q13_8 |
FDA website or other FDA sources of information (e.g., email, alerts) |
Q13_8 Info: FDA |
Q13_9 |
Other government agencies (e.g., NIH, CDC) |
Q13_9 Info: Other government agencies |
Value |
Value Label |
1 |
Never |
2 |
Rarely |
3 |
Sometimes |
4 |
Often |
//[SHOW QUESTION AT THE TOP OF THE SCREEN FOR QUESTION 14]//
The next question refers to boxed warnings, commonly referred to as black box warnings, on the product labeling for direct-acting antivirals.
Question Type: Multi-Punch
Question 14
Variable Name: Q14
Variable Label: Q14 Primary role of boxed warning
Question Text: In your opinion, what is the primary role of a boxed warning? Choose up to three options.
//Random Order, except last three//
//PROGRAMMING NOTE: ALLOW PARTICIPANT TO UP TO SELECT THREE ANSWERS//
Variable Name |
Variable Text |
Variable Label |
Q14_1 |
To highlight if the product has risk of serious or life-threatening adverse effects |
Q14_1 BW Primary Role: Highlight most serious risks |
Q14_2 |
To provide information that should be factored into a decision to prescribe the product |
Q14_2 BW Primary Role: Provide prescribing information |
Q14_3 |
To provide an overview of the safety profile of the product |
Q14_3 BW Primary Role: Provide safety profile overview |
Q14_4 |
To disclose clinical trial and other product safety testing information |
Q14_4 BW Primary Role: Clinical trial and safety testing |
Q14_5 |
To provide information that prescribers should be communicating to patients |
Q14_5 BW Primary Role: Info to communicate to patients |
Q14_6 |
To provide information on how to safely use the product |
Q14_6 BW Primary Role: Info on how to safely use product |
Q14_7 |
I do not see a role for them |
Q14_7 BW Primary Role: No role for BW |
Q14_8 |
Other (specify) |
Q14_8 BW Primary Role: Other |
Q14_9 |
Unsure/Don’t know |
Q14_9 BW Primary Role: Unsure/Don’t know |
Value |
Value Label |
1 |
Included |
0 |
Not included |
//PROGRAMMING NOTE: SHOW PARAGRAPH ON ITS OWN PAGE//
The next set of questions will ask you more specifically about the boxed warning and the prescribing information for direct-acting antivirals to treat chronic HCV. This boxed warning appears on all prescription direct-acting antiviral products.
//PROGRAMMING NOTE: “NEXT” BUTTON TO PROCEED TO Q15//
Question Type: Single Punch
Question Q15
Variable Name: Q15
Variable Label: Q15 Familiarity with BW for DAA
Question Text: How would you rate your familiarity with the boxed warning for direct-acting antivirals?
Value |
Value Label |
1 |
Not at all familiar |
2 |
Slightly familiar |
3 |
Somewhat familiar |
4 |
Familiar |
5 |
Very familiar |
-99 |
Refused |
Question Type: Single Select Grid
Question Q16
Variable Name: Q16
Variable Label: Q16 Risks recalled from DAA boxed warning
Question Text: Which of the following risks do you recall being included in the boxed warning for direct-acting antivirals? Please indicate a response for each risk.
[RANDOM ORDER EXCEPT FOR LAST OPTIONS]
Variable Name |
Variable Text |
Variable Label |
Q16_1 |
Hepatitis B reactivation in coinfected patients |
Q16_1 Risks: Hepatitis B |
Q16_2 |
Bradycardia |
Q16_2 Risks: Bradycardia |
Q16_3 |
Interaction with beta blockers |
Q16_3 Risks: Interaction with beta blockers |
Q16_4 |
Asthenia |
Q16_4 Risks: Asthenia |
Q16_5 |
Visual abnormalities |
Q16_5 Risks: Visual abnormalities |
Q16_6 |
Elevated blood pressure |
Q16_6 Risks: Elevated blood pressure |
Q16_7 |
Stroke |
Q16_7 Risks: Stroke |
Q16_8 |
Other (specify) |
Q16_9 Risks: Other |
Value |
Value Label |
1 |
Included |
2 |
Not included |
98 |
Don’t know |
//PROGRAMMING NOTE: SHOW PARAGRAPH ON ITS OWN PAGE, THEN CLICK NEXT TO VIEW BOXED WARNING//
We would like to show you some of the boxed warning information for [DAA drug], which is available in the FDA-approved prescribing information. The boxed warning information appears on the labeling for all direct-acting antivirals. Please consider [DAA drug] to serve as an example that represents the direct-acting antiviral drug class as a whole. Please take a few moments to review this information.
//PROGRAMMING NOTE: SHOW THE BOXED WARNING ON ITS OWN PAGE , CAPTURE TIME SPENT ON BOXED WARNING //
[View boxed warning information]123
/
/DISPLAY
IF DAA_DRUG = Harvoni//
/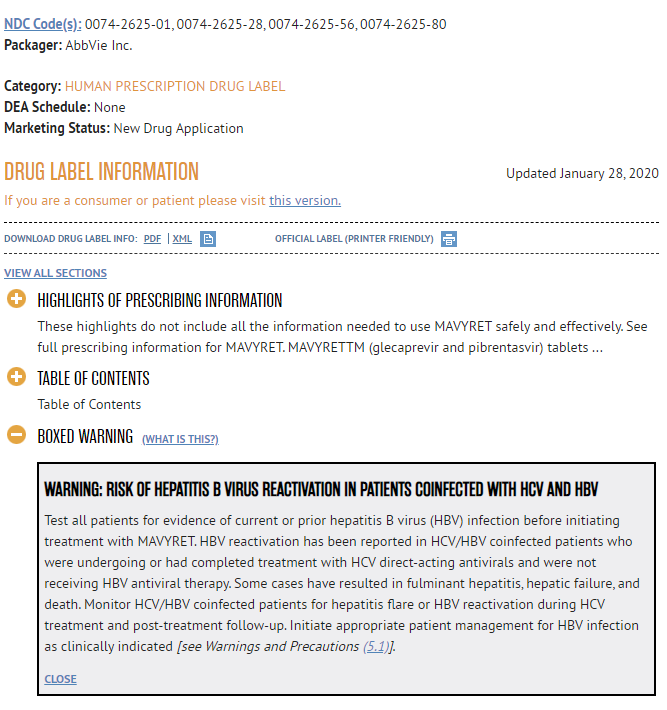
/DISPLAY
IF DAA_DRUG = Mavyret//
/
/DISPLAY
IF DAA_DRUG = Zepatier//
//PROGRAMMING NOTE: HAVE A “NEXT” BUTTON AT THE BOTTOM OF THE BOXED WARNING PAGE TO PROCEED TO QUESTION 17//
Question Type: Single Punch
Question 17
Variable Name: Q17
Variable Label: Q17 Usefulness of BW for DAA
Question Text: How useful is the information in the boxed warning for [DAA drug]?
Value |
Value Label |
1 |
Not at all useful |
2 |
Slightly useful |
3 |
Somewhat useful |
4 |
Useful |
5 |
Very useful |
-99 |
Refused |
Question Type: Single Punch
Question Q18
Variable Name: Q18
Variable Label: Q18 Assessment of risk framing in DAA BW
Question Text: What is your assessment of the way the risks of [DAA drug] are framed in the boxed warning?
Value |
Value Label |
1 |
Strongly understates risk |
2 |
Somewhat understates risk |
3 |
Provides appropriate assessment of risk information |
4 |
Somewhat overstates risk |
5 |
Strongly overstates risk |
6 |
Other (specify) |
-99 |
Refused |
Question Type: Single Punch
Question Q19
Variable Name: Q19
Variable Label: Q19 Assessment of benefits versus risks Post-BW
Question Text: Which statement best reflects your opinion on the relative benefits versus risks of direct-acting antivirals for patients with chronic HCV?
Value |
Value Label |
1 |
The benefits of direct-acting antivirals outweigh the risks for most patients |
2 |
The benefits of direct-acting antivirals outweigh the risks for some, but not most, patients |
3 |
The benefits of direct-acting antivirals outweigh the risks for very few patients |
4 |
I am not familiar enough with direct-acting antivirals to make an assessment |
-99 |
Refused |
//[SHOW SCENARIO AT THE TOP OF THE SCREEN FOR QUESTION 19_HBV]//
Imagine this scenario when answering the following question:
In prescreening, you determine that your patient with HCV has previously received treatment for an HBV infection. The patient tests positive for HBV antibodies, but has no signs of currently active infection.
Question Type: Single Punch
Question Q19_HBV
Variable Name: Q19_HBV
Variable Label: Q19_HBV Treating patient using DAA
Question Text: Which of the following best describes your course of action for treating this patient?
Value |
Value Label |
1 |
I would not prescribe [DAA drug] to this patient |
2 |
I might still prescribe [DAA drug] to this patient, but would consider other options first |
3 |
I would prescribe [DAA drug] to this patient but monitor her more carefully than other patients |
4 |
I would refer this patient to someone else for treatment |
5 |
Other (specify) |
98 |
Unsure/Don’t know |
-99 |
Refused |
[PIPE ALL RESPONSES TO Q19_HBV_OTH]
Question Type: Open End Essay
Question Q19_HBV_OE
Variable Name: Q19_HBV_OE
Variable Label: Q19_HBV_OE Reason for treatment option
Question Text: You said you would take the following course of action for treating this patient:
PIPE SELECTED RESPONSE FROM Q19_HBV]
//BOLD PIPED SELECTED RESPONSE//
Please briefly elaborate on the reason(s) for your response.
[SOFT PROMPT]

Question Type: Multi-Punch
Question 20
Variable Name: Q20
Variable Label: Q20 How improve DAA boxed warning
Question Text: What would improve the boxed warning for [DAA drug] in your opinion? (Select all that apply.)
Variable Name |
Variable Text |
Variable Label |
Q20_1 |
Nothing, the warning is fine the way it is |
Q20_1 Improve BW: Nothing |
Q20_2 |
Better evidence to support risk information |
Q20_2 Improve BW: Better risk info evidence |
Q20_3 |
Decrease the amount of information given/fewer words |
Q20_3 Improve BW: Decrease amount of info/shorten |
Q20_4 |
Simpler language |
Q20_4 Improve BW: Simpler language |
Q20_5 |
Formatting improvements (e.g., use of boldface) |
Q20_5 Improve BW: Formatting |
Q20_6 |
Increase the amount of information (e.g., statistics, more details) |
Q20_6 Improve BW: Increase amount of info |
Q20_7 |
Other (Specify) |
Q20_7 Improve BW: Other (specify) |
Q20_8 |
Unsure/Don’t know |
Q20_8 Improve BW: Unsure/Don’t know |
//Q20_8: Make exclusive//
Value |
Value Label |
1 |
Included |
0 |
Not included |
Question Type: Open End Essay
Question Q21
Variable Name: Q21
Variable Label: Q21 Specific feedback on DAA BW information
Question Text: Please provide any specific feedback on the boxed warning information for [DAA drug].

//PROGRAMMING NOTE: SHOW SENTENCE ON ITS OWN PAGE, THEN CLICK NEXT TO VIEW Q22//
Please now consider boxed warnings in general, not only the one you saw for [DAA drug].
Question Type: Grid
Question Q22
Variable Name: Q22
Variable Label: Q22 Degree agree or disagree with BW statements
Question Text: To what degree do you agree or disagree with the following statements about boxed warnings in general (in other words, across all classes of prescription drugs)?
[RANDOM ORDER for 1-6]
Value |
Value Label |
1 |
Strongly disagree |
2 |
Disagree |
3 |
Neither agree nor disagree |
4 |
Agree |
5 |
Strongly agree |
98 |
Unsure/Don’t know |
Question Type: Single Punch
Question 23
Variable Name: Q23
Variable Label: Q23 General favorability of BW
Question Text: How favorable is your opinion of boxed warnings in general (across all classes of prescription drugs)?
Value |
Value Label |
1 |
Very unfavorable |
2 |
Somewhat unfavorable |
3 |
Neither unfavorable nor favorable |
4 |
Somewhat favorable |
5 |
Very favorable |
98 |
Unsure/Don’t know |
-99 |
Refused |
Question Type: Open End Essay
Question Q24
Variable Name: Q24
Variable Label: Q24 Suggestions for better using BW to highlight safety info
Question Text: In general, what suggestions do you have on how to better use boxed warnings as a tool to highlight important safety information?

//PRETEST ONLY//
//DISPLAY TEXT//
We would like your feedback on the survey that you completed. This will help FDA improve the survey for future use with healthcare providers.
Question Type: Single Punch
Question P1
Variable Name: P1
Variable Label: P1 One session completion
Question Text: Were you able to complete the survey in one session?
Value |
Value Label |
1 |
Yes |
0 |
No |
Question Type: Single Punch
Question P2
Variable Name: P2
Variable Label: P2 Participant Experience with Survey
Question Text: How would you rate your experience taking the survey?
Value |
Value Label |
1 |
Poor |
2 |
Fair |
3 |
Good |
4 |
Very good |
5 |
Excellent |
-99 |
Refused |
Question Type: Open End Essay
Question P3
Variable Name: P3
Variable Label: P3 Difficulty answering question
Question Text: Were there any questions you had difficulty answering? If yes, please explain.

Question Type: Open End Essay
Question P4
Variable Name: P4
Variable Label: P4 Other feedback
Question Text: What other suggestions of feedback about this survey would you like to share?

//DISPLAY FOR BOTH PRETEST AND MAIN STUDY//
Thank you for taking this survey. Your time is greatly appreciated.
1 NIH NLM (2019). LABEL: HARVONI- ledipasvir and sofosbuvir tablet, film coated. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f4ec77e4-bae8-4db0-b3d5-bde09c5fa075
2 NIH NLM (2020). LABEL: MAVYRET- glecaprevir and pibrentasvir tablet, film coated. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7bf99777-0401-9095-8645-16c6e907fcc0
3 NIH NLM (2018). LABEL: ZEPATIER- elbasvir and grazoprevir tablet, film coated. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=164dc02a-9180-426a-b8b5-04ab39d2bbd4
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Lauren Burr |
| File Modified | 0000-00-00 |
| File Created | 2021-01-12 |
© 2026 OMB.report | Privacy Policy