3 FORM 3 - Health Center COVID-19 Vaccine Program Addendum
Health Center COVID-19 Vaccine Program
FORM 3 - Health Center COVID-19 Vaccine Program Addendum
OMB: 0906-0062

Health Center COVID-19 Vaccine Program Addendum
[REVISED 2/13/2021]
To ensure our nation's underserved communities and those disproportionately affected by COVID-19 are equitably vaccinated against COVID-19, HRSA and CDC launched the Health Center COVID-19 Vaccine Program. As a condition of participation in this program, participating health centers must complete the Health Center COVID-19 Weekly Survey and the Health Center COVID-19 Vaccine Program Addendum.1 The additional questions in the Addendum will track vaccine administration progress and assist in identifying technical assistance needs. HRSA will use the information collected to track health center progress in administering allocated doses, evaluate the impact of the Program, and inform subsequent vaccine allocations.
Please refer to the COVID-19 Data Collection Survey Tool User Guide for the Health Center COVID-19 Vaccine Program to assist you in completing the Addendum.
Question 1 |
How many health center staff members have initiated (1st dose received) their COVID-19 immunization series in the last week? [Enter the number of staff who initiated an FDA-approved vaccine series in the last week below.] [Note: Exclude vaccines administered to health center staff while participating in clinical trials. Only include vaccines that are given from the Health Center COVID-19 Vaccine Program allocation.] |
[Please enter a numerical value excluding commas ( ex. 123123)] |
Number Field |
Question 2 |
How many health center staff members have completed (2nd dose received) their COVID-19 immunization series in the last week? [Enter the number of staff who completed an FDA-approved vaccine series in the last week below.] [Note: Exclude vaccines administered to health center staff while participating in clinical trials. Only include vaccines that are given from the Health Center COVID-19 Vaccine Program allocation] |
[Please enter a numerical value excluding commas ( ex. 123123)] |
Number Field |
Question 3 |
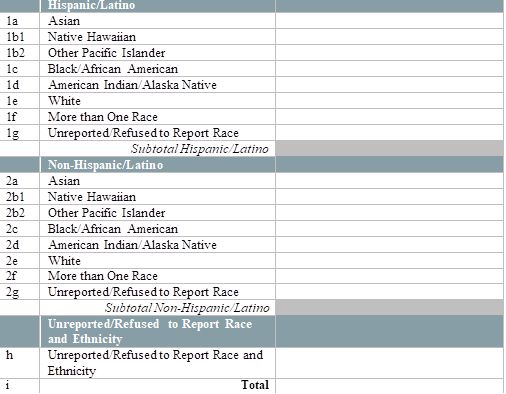
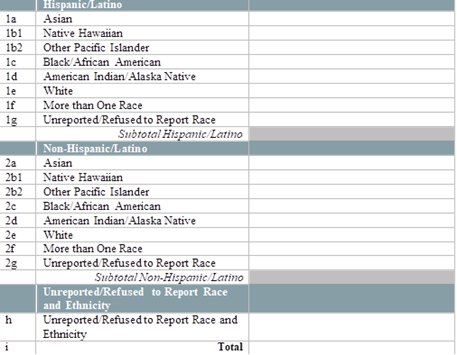
By race and ethnicity, how many patients have initiated (1st dose received) their COVID-19 immunization series in the last week? [Enter the number of patients who initiated an FDA-approved vaccine series in the last week, by race and ethnicity below.] [Note: Exclude vaccines administered to health center patients while participating in clinical trials. Only include vaccines that are given from the Health Center COVID-19 Vaccine Program allocation.] [Enter the number of patients tested by race and ethnicity below]
|
[Please enter a numerical value excluding commas ( ex. 123123)] |
Number Field |
Question 4 |
By race and ethnicity, how many patients have completed (2nd dose received) their COVID-19 immunization series in the last week? [Enter the number of patients who completed an FDA-approved vaccine series in the last week, by race and ethnicity below.] [Note: Exclude vaccines administered to health center patients while participating in clinical trials. Only include vaccines that are given from the Health Center COVID-19 Vaccine Program allocation]
|
[Please enter a numerical value excluding commas ( ex. 123123)] |
Number Field |
Question 5 |
By special population type, how many patients have initiated (1st dose received) their COVID-19 immunization series in the last week? [Enter the number of patients who initiated an FDA-approved vaccine series in the last week, by race and ethnicity below.] [Note: Exclude vaccines administered to health center patients while participating in clinical trials. Only include vaccines that are given from the Health Center COVID-19 Vaccine Program allocation]
|
[Please enter a numerical value excluding commas ( ex. 123123)] |
Number Field |
Question 6 |
By special population type, how many patients have completed (2nd dose received) their COVID-19 immunization series in the last week? [Enter the number of patients who completed an FDA-approved vaccine series in the last week, by race and ethnicity below.] [Note: Exclude vaccines administered to health center patients while participating in clinical trials. Only include vaccines that are given from the Health Center COVID-19 Vaccine Program allocation]
|
[Please enter a numerical value excluding commas ( ex. 123123)] |
Number Field |
Question 7 |
In the past week, has your health center been able to administer all COVID-19 vaccines received from the Health Center COVID-19 Vaccine Program? |
[Select an answer choice from the list] |
Pick List Y/N |
Public Burden Statement: Health centers (section 330 grant funded and Federally Qualified Health Center look-alikes) deliver comprehensive, high quality, cost-effective primary health care to patients regardless of their ability to pay. The Health Center COVID-19 Vaccine Program is part of a White House Initiative with the goal of administering 100 million shots in 100 days, with a focus on equity. In a collaboration between HRSA and the Centers for Disease Control and Prevention (CDC), this program will directly allocate a limited supply of COVID-19 vaccines to select HRSA-funded health centers. These forms provide HRSA with the information essential for Health Center COVID-19 Vaccine Program evaluation and determination of future vaccination allocations. The OMB control number for this information collection is 0906-xxxx and it is valid through XX/XX/202X. This information collection is mandatory under the Health Center Program authorized by section 330 of the Public Health Service (PHS) Act (42 U.S.C. 254b). Public reporting burden for this collection of information is estimated to average 1 hour per response, including the time for reviewing instructions, searching existing data sources, and completing and reviewing the collection of information. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden, to HRSA Reports Clearance Officer, 5600 Fishers Lane, Room 14N136B, Rockville, Maryland, 20857 or [email protected].
1 Per the Health Center COVID-19 Vaccine Program Conditions of Participation Agreement
OMB # 0906-xxxx
Expires: xx/xx/20xx
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Elyana N. Bowman |
| File Modified | 0000-00-00 |
| File Created | 2021-04-06 |
© 2026 OMB.report | Privacy Policy