Supplemental Study Information
Att 13_Supplemental Study Information.docx
Identification of Behavioral and Clinical Predictors of Early HIV Infection (Project DETECT)
Supplemental Study Information
OMB: 0920-1100
Identification of Behavioral and Clinical Predictors of Early HIV Infection
(Project DETECT)
Attachment 13: Supplemental Study Information
Figure A: Project DETECT data flow diagram
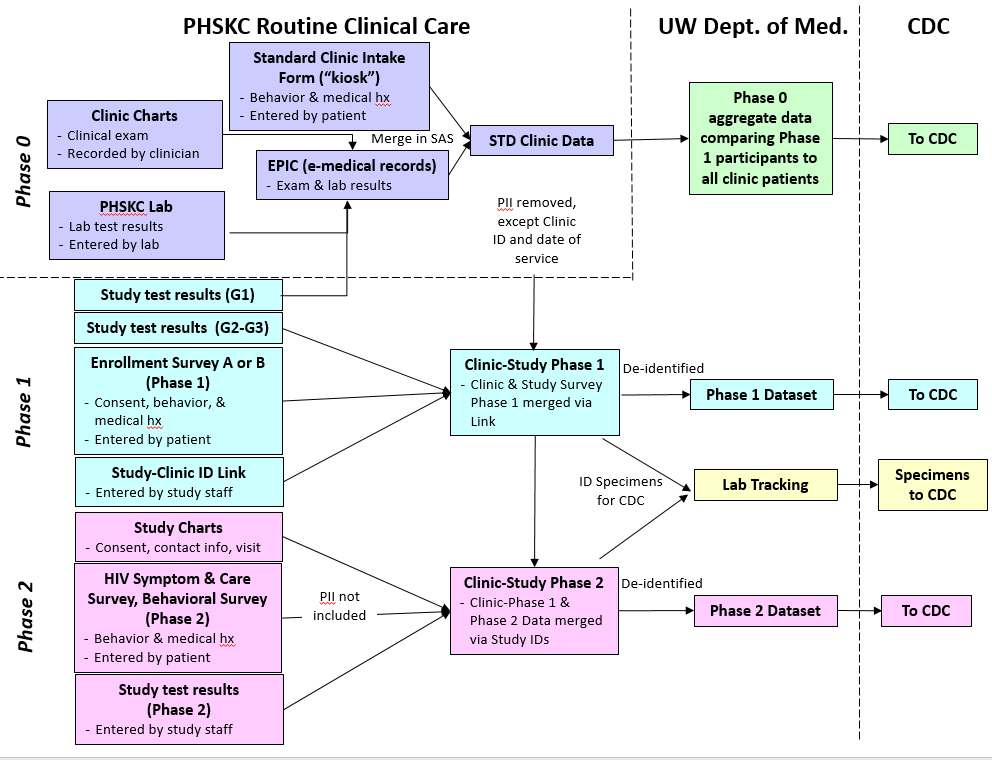
Phase 0 consists of data collected as part of routine medical care from the PHSKC clinic, including clinical exam data, lab results, and behavioral data obtained during clinic intake. Phase 0 data will be de-identified, aggregated and sent to CDC to compare project participants to the overall clinic population. Phase 1 data will be collected as part of study participation and include consent, study test results, and enrollment survey data (enrollment survey A or B, depending upon whether or not the participant was referred to the study or came through the STD clinic). Phase 2 data will be collected as part of study participation among persons with discordant HIV test results. Data collected in this phase include consent, study test results and the HIV symptom and care survey at each visit, and a behavioral survey. All data are de-identified (no PII), linked by study ID, and transmitted securely to CDC in de-identified form.
Table 1: POC and Laboratory Tests Used in Phase 1 and 2
Test technology |
Specimen Type(s) for Phase 1 |
Specimen Type(s) for Phase 2 |
Point-of-Care Screening tests |
|
|
DPP® HIV 1/2 Assay (ChemBio Diagnostic Systems) |
Oral fluid and venous whole blood |
Oral fluid and venous whole blood; fingerstick whole blood |
OraQuick ADVANCE® Rapid HIV-1/2 Antibody (OraSure Technologies) |
Oral fluid and venous whole blood |
Oral fluid and venous whole blood; fingerstick whole blood |
INSTITM HIV-1/HIV-2 Rapid Antibody Test (bioLytical Laboratories) |
Venous whole blood |
Venous whole blood; fingerstick whole blood |
DetermineTM HIV-1/2 Ag/Ab Combo (Alere) |
Venous whole blood |
Venous whole blood; fingerstick whole blood |
Point-of-care supplemental test(s) |
|
|
GeeniusTM HIV 1/2 Confirmatory System (Bio-Rad Laboratories) |
Venous whole blood |
Venous whole blood; fingerstick whole blood |
POC NAAT Confirmatory Test* |
Venous whole blood |
Venous whole blood; fingerstick whole blood |
Laboratory tests to be conducted through PHSKC Laboratory |
|
|
Genetic Systems HIV-1/HIV-2 Plus O EIA (Bio-Rad Laboratories) |
Serum |
Serum |
Multispot HIV-1/2 Rapid Test (Bio-Rad Laboratories) |
Serum |
Serum |
RealTime HIV-1 (Abbott) Pooled testing (27-member pools) if negative Geenius, POC and EIA tests |
Serum |
N/A |
RealTime HIV-1 (Abbott) Individual HIV-1 viral load if at least one reactive result |
Plasma |
Plasma |
* This test may be added to the study later. It is not approved by the FDA at this time.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Bowles, Kristina E. (CDC/OID/NCHHSTP) |
| File Modified | 0000-00-00 |
| File Created | 2021-03-29 |
© 2026 OMB.report | Privacy Policy