CMS-10185 - Supporting Statement A
CMS-10185 - Supporting Statement A.docx
Medicare Part D Reporting Requirements and Supporting Regulations in MMA Title I, Part 423, §423.514(a) (CMS-10185)
OMB: 0938-0992
Supporting Statement for Paperwork Reduction Act Submissions
Medicare Part D Reporting Requirements and Supporting
Regulations in MMA Title I, Part 423, §423.514(a)
CMS-10185 (OMB 0938-0992)
Background
Section 1860D–12(b)(3)(D) of the Act provides broad authority for the Secretary to add terms to the contracts with Part D sponsors, including terms that require the sponsor to provide the Secretary with information as the Secretary may find necessary and appropriate. Pursuant to our statutory authority, we codified these information collection requirements for Part D sponsors in regulation at 42 CFR §423.514(a).
The Center for Medicare (CM) has identified the appropriate data needed to effectively monitor the Medicare Prescription Drug Benefit through these Part D reporting requirements. Changes to the currently approved data collection instrument reflect new executive orders, legislation, as well as recent changes to Agency policy and guidance.
A. Justification
1. Need and Legal Basis
42 CFR §423.514(a) requires each Part D sponsor to have a procedure to develop, compile, evaluate, and report to CMS, to its enrollees, and to the general public, at the times and in the manner that CMS requires, statistics indicating the following:
The cost of its operations.
The patterns of utilization of its services.
The availability, accessibility, and acceptability of its services.
Information demonstrating that the Part D sponsor has a fiscally sound operation.
Pharmacy performance measures.
Other matters that CMS may require.
42 CFR §423.505 establishes contract provisions that Part D sponsors must comply with the disclosure and reporting requirements in §423.514(a).
2. Information Users
Data collected via the Medicare Part D reporting requirements will be an integral resource for oversight, monitoring, compliance, and auditing activities necessary to ensure quality provision of the Medicare Prescription Drug Benefit to beneficiaries. For all reporting sections (Enrollment and Disenrollment, Medication Therapy Management (MTM)
Programs , Grievances, Improving Drug Utilization Review Controls, Coverage
Determinations and Redeterminations, and Employer/Union Sponsored Sponsors), data are reported electronically to CMS. The data collected via the MTM and Grievances reporting sections are used in the Medicare Part C and D Star Ratings and Display Measures. The other reporting sections’ data are analyzed for program oversight to ensure the availability, accessibility, and acceptability of sponsors’ services, such as coverage determinations and appeals processes, and opioid safety edits at the time of dispensing.
Each reporting section is reported at one of the following levels: Contract (data should be entered at the H#, S#, R#, or E# level) or Plan (data should be entered at the Plan Benefit Package (PBP level, e.g. Plan 001 for contract H#, R#, S#, or E). In accordance with 42 CFR §423.505(d), sponsors should retain documentation and data records related to their data submissions. Data will be validated, analyzed, and utilized for trend reporting by the Division of Clinical and Operational Performance (DCOP) within the Medicare Drug Benefit and C & D Data Group. If outliers or other data anomalies are detected, DCOP will work in collaboration with other Divisions within CMS for follow-up and resolution.
3. Use of Information Technology
Part D sponsors will utilize the Health Plan Management Systems (HPMS) system to submit data for 100% of data elements listed within these reporting requirements. The reporting periods vary for each section of the reporting requirements, on a biannual or annual basis.
HPMS is the current conduit by which Part D sponsors submit many materials (e.g. formulary, transition, exceptions, and bids) and other information to CMS. CMS and its subcontractors, in turn, communicate to sponsors regarding this information, including approval and denial notices and other related communications. HPMS is a familiar tool for Part D sponsors to navigate through the Part D reporting requirements. Additionally, access to HPMS must be granted to each user, and is protected by individual login and password, electronic signatures are unnecessary.
Duplication of Efforts
This collection does not contain duplication of similar information.
Small Businesses
This collection does not impose a significant impact on small businesses and other entities.
6. Less Frequent Collection
In an effort to reduce the burden for Part D sponsors, each reporting section varies its reporting timeline to capture data as frequently as necessary without increasing undue burden for Part D sponsors. All reporting sections are collected on an annual basis, with the exception of one - Enrollment and Disenrollment data are collected bi-annually so that data analysis may be completed, and any issues resolved before enrollment/disenrollment activities begin for the following contract year.
7. Special Circumstances
Part D records are to be retained for 10 years. CMS could potentially require clarification around submitted data, and therefore CMS may need to contact Part D sponsors within 30 days of data submission. Otherwise, there are no special circumstances that would require an information collection to be conducted in a manner that requires respondents to:
Report information to the agency more often than quarterly;
Prepare a written response to a collection of information in fewer than 30 days after receipt of it;
Submit more than an original and two copies of any document;
Retain records, other than health, medical, government contract, grant-in-aid, or tax records for more than three years;
Collect data in connection with a statistical survey that is not designed to produce valid and reliable results that can be generalized to the universe of study;
Use a statistical data classification that has not been reviewed and approved by OMB;
Include a pledge of confidentiality that is not supported by authority established in statute or regulation that is not supported by disclosure and data security policies that are consistent with the pledge, or which unnecessarily impedes sharing of data with other agencies for compatible confidential use; or
Submit proprietary trade secret, or other confidential information unless the agency can demonstrate that it has instituted procedures to protect the information's confidentiality to the extent permitted by law.
8. Federal Register/Outside Consultation
The 60-day notice published in the Federal Register on March 19, 2021 (86 FR 14926). Twenty-nine public comments were received. They are attached to this package along with our responses.
CM has requested the Part D reporting requirements document be posted in the Federal Registry (86 FR 33301) on June 24, 2021 and the 30-day comment period will end July 27, 2021.
From July 26, 2021 to August 26, 2021 CM staff will review all received comments and questions, and revise the document appropriately. Also, CM staff will prepare a response document summarizing all received comments and questions, and their responses. A final Part D reporting requirements document will be delivered for OMB review by September 24, 2021.
9. Payments/Gifts to Respondents
There are no payments/gifts to respondents associated with this information collection request.
10. Confidentiality
CMS will adhere to all statutes, regulations, and agency policies.
11. Sensitive Questions
There are no sensitive questions associated with this collection. Specifically, the collection does not solicit questions of a sensitive nature, such as sexual behavior and attitudes, religious beliefs, and other matters that are commonly considered private.
12. Burden Estimates (Hours & Wages)
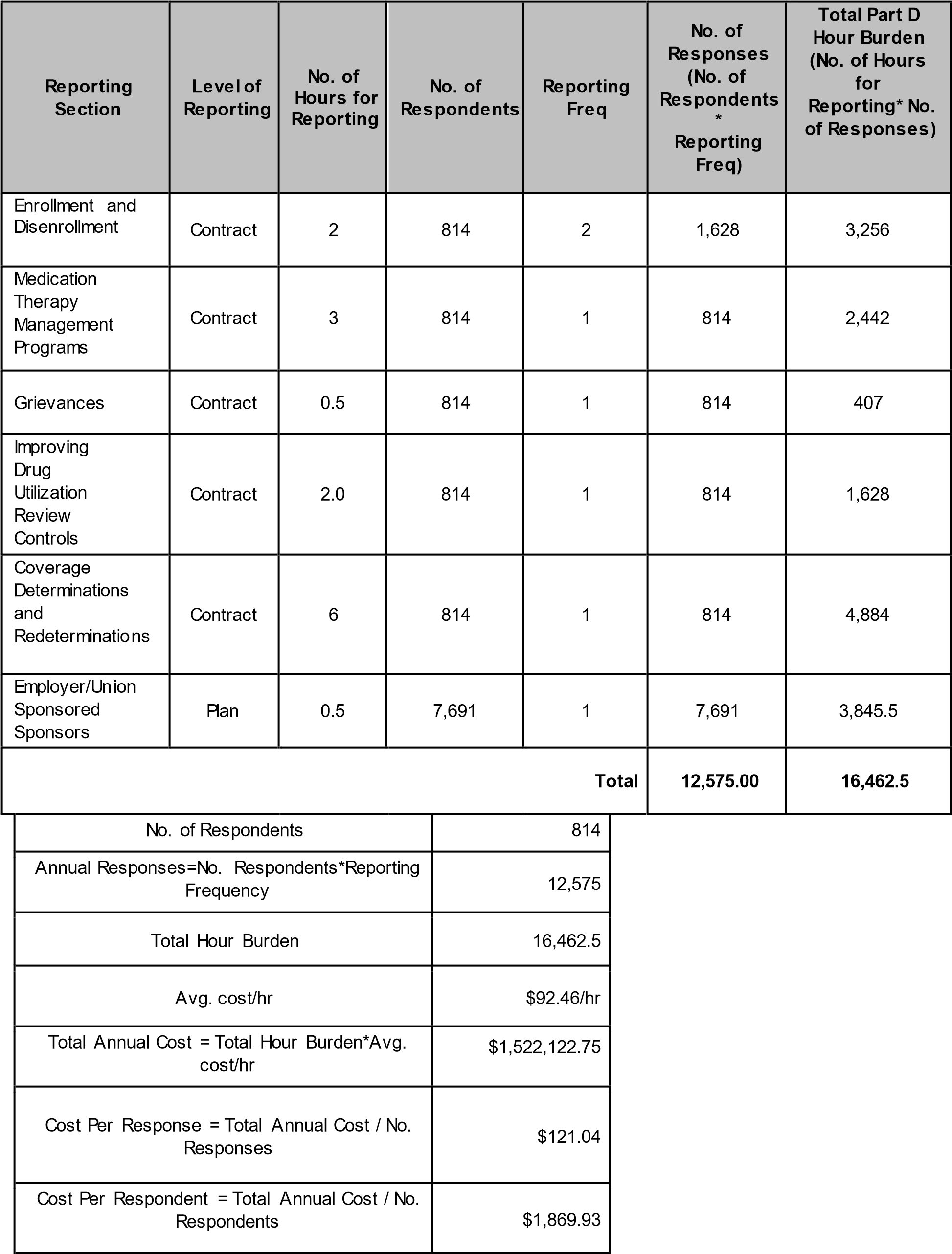
For CY 2022 Medicare Part D reporting requirements, the following 6 reporting sections will be reported and collected at the Contract-level or Plan-level:
Enrollment and Disenrollment – to evaluate sponsors’ processing of enrollment, disenrollment, and reinstatement requests in accordance with CMS requirements.
Medication Therapy Management (MTM) Programs – to evaluate Part D MTM programs, and sponsors’ adherence to CMS requirements.
Grievances – to assess sponsors’ compliance with timely and appropriate resolution of grievances filed by their enrollees.
Improving Drug Utilization Review Controls – to determine the impact of formulary-level safety edits at point of sale in sponsors’ processing of opioid prescriptions.
Coverage Determinations and Redeterminations - to assess sponsors’ compliance with appropriate resolution of coverage determinations and redeterminations requested by their enrollees.
Employer/Union Sponsored Sponsors - to ensure PDPs and the employer groups that contract with the PDPs properly utilize appropriate waivers and modifications.
Wage Estimates
To derive average costs, we used data from the U.S. Bureau of Labor Statistics’
2019 National Occupational Employment and Wage Estimates for all salary estimates (http://www.bls.gov/oes/current/oes_nat.htm). In this regard, the following table presents the mean hourly wage, the cost of fringe benefits (calculated at 100 percent of salary), and the
adjusted hourly wage.
Occupation Title |
Occupation Code |
Mean Hourly Wage ($/hr) |
Fringe Benefit ($/hr) |
Adjusted Hourly Wage ($/hr) |
Computer Systems Analyst |
15-1211 |
46.23 |
46.23 |
92.46 |
As indicated, we are adjusting our employee hourly wage estimates by a factor of 100 percent. This is necessarily a rough adjustment, both because fringe benefits and overhead costs vary significantly from employer to employer, and because methods of estimating these costs vary widely from study to study. We believe that doubling the hourly wage to estimate total cost is a reasonably accurate estimation method.
Burden Estimates
The tables below illustrate the estimated hours and costs associated with each reporting section of the CY 2022 Medicare Part D reporting requirements. Please note that the level of each section’s reporting (contract or plan level) determines the number of respondents used to base the reporting section’s burden estimate.
Information Collection Instruments/Instructions
• Medicare Part D reporting requirements (Effective January 1, 2022)
13. Capital Costs
There is no capital costs associated with this collection.
14. Cost to Federal Government
The cost to the Federal Government will be $300,000 to support electronic data collection through HPMS performed by a contractor.
15. Changes to Burden
There was an overall increase in contract respondents (from 744 to 814) and plan respondents (from 6,680 to 7,691) due to an increase in the total number of Part D contracts.
We are not changing the frequency of any reporting requirements.
For CY 2022, to determine the total number of annual responses, we summed the number of responses for each reporting section.
We are proposing revisions to several sections of the CY 2022 Medicare Part D reporting requirements:
We are revising several data elements in the Improving Drug Utilization Review Controls section. These revisions include modifying existing elements, adding new elements, and removing elements. The changes improve the quality and reliability of the data collected for Part D opioid point of sale safety edits, including the care coordination edit, the morphine milligram equivalent (MME) hard edit and the opioid naïve days supply edit. For example, we will collect more specific data about the disposition of affected claims at the pharmacy counter while reducing or removing data related to the outcomes of subsequent coverage determinations and appeals. Additionally, we have tailored the data elements to collect information in a manner that is most appropriate to the specific edit. For example, for the MME hard edit we have removed most of the claim level data elements to focus on beneficiary level data for this edit, which impacts chronic opioid users. Lastly, we changed the data reporting level for this section from plan level reporting to contract level reporting because we identified no significant difference in data submitted (reduced burden hours).
In the Medication Therapy Management (MTM) Program section, we made revisions to remove unnecessary data elements and added elements that reflect new MTM program requirements under the SUPPORT (Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities) Act (no net burden increase). On January 19, 2021 in the Federal Register, CMS published a final rule titled “Medicare and Medicaid Programs; Contract Year 2021 and 2022 Policy and Technical
Changes to the Medicare Advantage Program, Medicare Prescription Drug Benefit
Program, Medicaid Program, Medicare Cost Plan Program, and Programs of All-Inclusive
Care for the Elderly (CMS-4190-F2)” (https://www.federalregister.gov/publicinspection/2021-00538/medicare-and-medicaid-programs-contract-year-2022-policy-andtechnical-changes-to-the-medicare) that implements changes to MTM programs for CY 2022. This rule changes the definition of “targeted beneficiaries” for the purposes of MTM programs to include at-risk beneficiaries in a drug management program (42 CFR
§423.153(d)(2)(ii)), and requires sponsors to provide MTM enrollees with information on the safe disposal of controlled substances (42 CFR. §423.153(d)(1)(vii)(E)).
Additional data elements were added to the Coverage Determination/Redeterminations section to increase the detail in Part D reporting requirements to better analyze plan appeal and overturn rates. Due to the addition of the new elements, as well as an increase in the number of respondents from 744 in CY 2021 to 814 in CY 2022, CMS increased our response estimate. Consequently, we increased our per response estimate for this section from 4 hr/response to 6 hr/response.
Finally, we removed four data elements from the Enrollment/Disenrollment section because this data will be available to CMS from March 2021 via updates to the Medicare Advantage Prescription Drug (MARx) System. These changes will be submitted to OMB for approval under control number 0938–0964 (CMS– 10141) for Part D. The changes to this section will remove duplicative reporting.
The following table illustrates the section changes in burden hours per response from CY 2021 to CY 2022:
Reporting Section |
Hours Per Response for CY 2021 Reporting |
Hours Per Response for CY 2022 Reporting |
Increase/(Decrease)
|
Enrollment and Disenrollment |
2 |
2 |
No change |
Medication Therapy Management Programs |
3.0 |
3.0 |
No change |
Grievances |
0.5 |
0.5 |
No change |
Improving Drug Utilization Review Controls |
2 |
2 |
No change |
Coverage Determinations and Redeterminations |
4 |
6 |
2 |
Employer/Union Sponsored Sponsors |
0.5 |
0.5 |
No change |
*Removed in its entirety.
The following table illustrates the change in burden hours per reporting section from CY 2021 to CY 2022:
Reporting Section |
No. of Hours for CY2021Reporting* |
No. of Hours for CY2022
Reporting** |
Increase/(Decrease) |
Enrollment and Disenrollment |
2,976 |
3,256 |
280 |
Medication Therapy Management Programs |
2,232 |
2,442 |
210 |
Grievances |
372 |
407 |
35 |
Improving Drug Utilization Review Controls |
13,360 |
1,628 |
(11,732) |
Coverage Determinations and Redeterminations |
2,976 |
4,884 |
1,908 |
Employer/Union Sponsored Sponsors |
3,340 |
3,845.5 |
505.5 |
TOTAL |
25,256 |
16,462.5 |
(8,793.5) |
*Based on the per response changes cited in the preceding table and 744 contract respondents and 6,680 plan respondents.
**Based on the per response changes cited in the preceding table and 814 contract respondents and 7,691 plan respondents.
Overall, there was a significant decrease in responses and burden hours associated with this revised data collection; however, annualized burden per respondent increased by 2. These changes are reflected in the revised Part D reporting requirements document. The following table illustrates the changes in burden from CY 2021 to CY 2022:
|
CY 2021 |
CY 2022 |
Differential |
Annual Responses |
17,080 |
12,575 |
4,505 |
Annual Hour Burden |
25,256 |
16,462.5 |
8,793.5 |
Annualized Burden per Respondent |
14 |
16 |
2 |
Data included in Part D reporting requirements are already available to Part D sponsors. CMS does not expect compliance with these reporting requirements would result in additional start-up costs.
Anticipated staff performing these data collection would be data analysts, and/or IT analysts. An adjusted hourly wage of $92.46/hr for a Computer Systems Analyst was used to calculate our cost estimates. The previous hourly wage rate was $90.02/hr for the same position.
16. Publication/Tabulation Dates
Following final submission of these data in the spring 2023, and independent data validation in summer 2023, CMS will release a public use file (PUF) of validated plan reported data.
17. Expiration Date
The expiration date is set out in the reporting requirements document. (Note the effective date is upon approval by OMB).
18. Certification Statement
There are no exceptions.
B. Collections of Information Employing Statistical Methods
This information collection does not employ any statistical analyses.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | Supporting Statement For Paperwork Reduction Act Submissions: Medicare Part D Reporting Requirements and |
| Author | CMS |
| File Modified | 0000-00-00 |
| File Created | 2021-06-28 |
© 2026 OMB.report | Privacy Policy