3 HC_Data_Collection_Tool_08_27_2021_Final
Health Center COVID-19 Vaccine Program
FORM 3 - HC_Data_Collection_Tool_08_27_2021_Final
OMB: 0906-0062
|
|
|
COVID-19 Data Collection
Survey Tool Questions
[REVISED 8/17/2021]
As part of COVID-19 (Coronavirus) emergency-response efforts, we are asking health centers to fill out a biweekly survey to help track health center capacity and the impact of COVID-19 on health center operations, patients, and staff. The Health Resources and Services Administration will use the information collected to better understand training and technical assistance, funding, and other health center resource needs.
IMPORTANT:
For questions that ask about initiating a COVID-19 immunization series, only include doses administered that are the first of a two-dose immunization series (for example, Pfizer or Moderna vaccines).
For questions that ask about completing a COVID-19 immunization series, include doses administered as a one-dose vaccine series (for example, Janssen COVID-19 (Johnson & Johnson) vaccine) as well as doses that are the second of a two-dose immunization series (for example, Pfizer or Moderna vaccines).
For Health Center COVID-19 Vaccine Program participants ONLY: If you are a health center participating in this joint HRSA – CDC program, you are required to respond to ALL data reporting elements in this biweekly HRSA Health Center COVID-19 survey AND the addendum by the requested deadline.
Note: Health Centers not
participating in the Health Center COVID-19 Vaccine Program will not
see the addendum questions.
Please refer to the COVID-19 Data Collection Survey Tool User Guide to assist you in completing the survey.
Question Number |
Question Field |
Description |
Answer Field |
Question 1 |
Please enter your email address
|
[text field] |
|
Question 2 |
Please select the State/Territory that your health center is located in: |
[Select an answer choice from the list] |
Pick List of all the States + US Territories |
Question 3 |
Please select your health center name and associated Grant Number: |
[Select an answer choice from the list] |
Pick List of all of the Health Centers + Active H80 Grants |
Question 4 |
On average for this two-week period, how quickly is your health center able to obtain COVID-19 test results for SARS-CoV-2 virus detection (PCR, antigen)? (Do not include test processing times for antibody detection (serology).) |
[This question does not appear if N was selected for Question 3.] [Select answer choices from the list] |
Select one:
|
Question 5 |
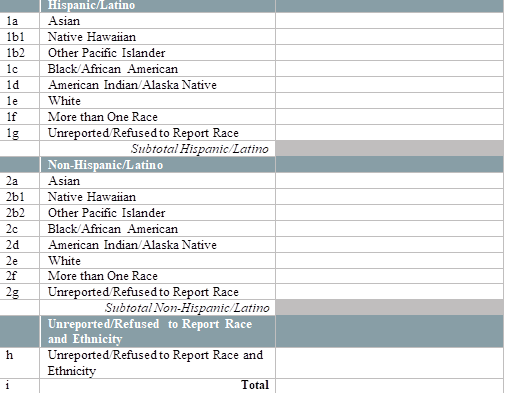
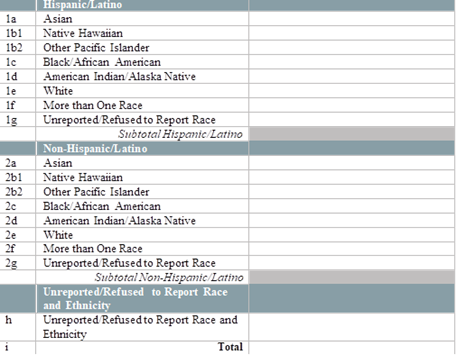
By race and ethnicity, how many of your patients received a test for SARS-CoV-2 virus detection (PCR, antigen) in the last two weeks? (Testing refers to specimen collection regardless of where the specimen is processed. Do not include tests for antibody detection (serology).)
[Enter the number of patients tested by race and ethnicity below]
|
[This question does not appear if N was selected for Question 3. Please enter a numerical value excluding commas (ex. 123123)] |
Number Field |
Question 6 |
By race and ethnicity, how many of your patients have tested positive for SARS-CoV-2 virus detection (PCR, antigen) in the last two weeks? (Report all positive results regardless of where patients were tested. Do not include positive test results for antibody detection (serology).)
[Enter the number of patients who tested positive for SARS-CoV-2 virus detection (PCR, antigen) by race and ethnicity below.]
|
[Please enter a numerical value excluding commas (ex. 123123)] |
Number Field |
Question 7 |
How does this last two weeks’ number of visits compare to your average number of weekly visits pre-COVID-19? (Consider all visits regardless of service type (e.g., medical, dental, behavioral health, etc.), including virtual visits.) |
[With 100% being average, <100% being below average, >100% being above average] |
Slider - Range 10-150 Interval of 5 |
Question 8 |
What percentage of your health center’s visits in the last two weeks were virtual (e.g., telehealth/telephonic)? (Consider all visits regardless of service type (e.g., medical, dental, behavioral health, etc.).) |
[Select an answer choice] |
Slider – Range 0-100 Interval of 5 |
Question 9 |
By race and ethnicity, how many patients have initiated (1st of two doses received) their COVID-19 immunization series in the last two weeks? [Enter the number of patients who initiated an FDA-approved vaccine series in the last two weeks, by race and ethnicity below.] [Note: Exclude vaccines administered to health center patients while participating in clinical trials.] [Note: If applicable, please include vaccine doses received under the Health Center COVID-19 Vaccine Program.] [Note: If you are administering a one-dose vaccine series, ONLY report those in the completed dose question.] [Enter the number of patients vaccinated by race and ethnicity below]
|
[Please enter a numerical value excluding commas (ex. 123123)] |
Number Field |
Question 10 |
By race and ethnicity, how many patients have completed (2nd, or only, dose received) their COVID-19 immunization series in the last two weeks? [Enter the number of patients who completed an FDA-approved vaccine series in the last two weeks, by race and ethnicity below.] [Note: Exclude vaccines administered to health center patients while participating in clinical trials.] [Note: If applicable, please include vaccine doses received under the Health Center COVID-19 Vaccine Program.] [Note: If you are
administering a one-dose vaccine series, report those in this
question as completed.] |
[Please enter a numerical value excluding commas (ex. 123123)] |
Number Field |
Question 11 |
Did your health center utilize mobile vans, host pop-up clinics, and/or host school-based vaccination clinics to enhance access to COVID-19 vaccination sites in the last two weeks? |
[Select from the list] |
Pick List:
|
Question 11a
[Required if response to Question 11 is ‘Yes’]
[Skip if response to Question 11 is ‘No’] |
How many mobile van clinics, pop-up clinics, and/or school-based vaccination clinics did you host in the last two weeks for COVID-19 vaccinations?
Mobile van clinics Pop-up clinics School-based vaccination clinics |
[Please enter a numerical value for each type excluding commas (ex. 123123)] |
Three Number Fields
|
Question 12 |
What challenges does your health center face in deploying the COVID-19 vaccine?
|
[Select all answers that apply from the list]
[Please briefly describe the challenges] |
Pick List Multi-select (subcategory choices)
[Free text is optional]
|
Question 13 |
Does your health center provide access to monoclonal antibody therapies? |
[Select from the list] |
Pick List:
|
Question 14a
[Skip if response to Question 13 is No; use this only if response to Question 13 is Yes] |
Which method of monoclonal antibody therapy do you provide access to? |
[Select from the list] |
Pick List:
|
Question 14aa
[Use this only if response to 14a is “Direct provision …”] |
How many doses of monoclonal antibody therapy have you administered in the last two weeks? |
[Please enter a numerical value excluding commas (ex. 123123)] |
Number field (must be 0 or greater) |
Question 14b
[Skip if response to Question 13 is Yes; use this only if response to Question 13 is No] |
What are your top barriers/challenges related to providing access to monoclonal antibody therapy?
|
[Select all answers that apply from the list]
[Please briefly describe the challenges] |
Pick List Multi-select (subcategory choices)
[Free text is optional]
|
Question 15 |
Please provide any additional information, comments, or challenges you are experiencing due to COVID-19. |
|
[Free text] |
Health Center COVID-19 Vaccine Program Addendum
A condition of participation in this program is to complete both the Health Center COVID-19 Biweekly Survey and additional questions outlined in the addendum below. Only health centers identified for participation in the Health Center COVID-19 Vaccine Program to receive a direction allocation of the COVID-19 vaccine are required to respond to these additional questions.
The information collected from these additional questions will assist HRSA and CDC to:
Assess COVID-19 vaccine administration capacity;
Monitor COVID-19 vaccine administration progress;
Evaluate the impact of the program to inform subsequent vaccine allocations; and
Identify training and technical assistance needs of participating health centers and their service delivery sites.
Please refer to the COVID-19 Data Collection Survey Tool User Guide, Addendum for Participants of the Health Center COVID-19 Vaccine Program, to assist you in completing the additional questions outlined below.
Question 16 |
In the past two weeks, has your health center been able to administer all COVID-19 vaccines allocated from the Health Center COVID-19 Vaccine Program? |
Pick List Y/N |
|
Question 16a
[Required if response to Question 20 is ‘No’]
[Skip if response to Question 20 is ‘Yes’] |
Please briefly explain why your health center has not been able to administer all the vaccines received from the Health Center COVID-19 Vaccine Program. |
|
[Free text] |
Question 17 |
How many health center staff members have initiated (1st of two doses received) their COVID-19 immunization series in the last two weeks from vaccines allocated under the Health Center COVID-19 Vaccine Program? [Enter the number of staff who initiated an FDA-approved vaccine series in the last two weeks below. Only report on vaccines allocated from the Health Center COVID-19 Vaccine Program.] [Note: If you are administering a one-dose vaccine series, ONLY report those in the completed dose question.] |
[Please enter a numerical value excluding commas (ex. 123123)] |
Number Field |
Question 18 |
How many health center staff members have completed (2nd, or only, dose received) their COVID-19 immunization series in the last two weeks from vaccines allocated under the Health Center COVID-19 Vaccine Program? [Enter the number of staff who completed an FDA-approved vaccine series in the last two weeks below. Only report on vaccines allocated from the Health Center COVID-19 Vaccine Program.] [Note: If you are administering a one-dose vaccine series, report those in this question as completed.] |
[Please enter a numerical value excluding commas (ex. 123123)] |
Number Field |
Question 19 |
By race and ethnicity, how many patients have initiated (1st of two doses received) their COVID-19 immunization series in the last two weeks from vaccines allocated under the Health Center COVID-19 Vaccine Program? [Enter the number of patients who initiated an FDA-approved vaccine series in the last two weeks, by race and ethnicity below. Only report on vaccines allocated from the Health Center COVID-19 Vaccine Program.] [Note: If you are administering a one-dose vaccine series, ONLY report those in the completed dose question.] [Enter the number of patients vaccinated by race and ethnicity below]
|
[Please enter a numerical value excluding commas (ex. 123123)] |
Number Field |
Question 20 |
By race and ethnicity, how many patients have completed (2nd, or only, dose received) their COVID-19 immunization series in the last two weeks from vaccines allocated under the Health Center COVID-19 Vaccine Program? [Enter the number of patients who completed an FDA-approved vaccine series in the last two weeks, by race and ethnicity below. Only report on vaccines allocated from the Health Center COVID-19 Vaccine Program.] [Note: If you are administering a one-dose vaccine series, report those in this question as completed.]
|
[Please enter a numerical value excluding commas (ex. 123123)] |
Number Field |
Question 21 |
By population type, how many patients have initiated (1st of two doses received) their COVID-19 immunization series in the last two weeks from vaccines allocated under the Health Center COVID-19 Vaccine Program? [Enter the number of patients who initiated an FDA-approved vaccine series in the last two weeks, by disproportionately affected populations below. Only report on vaccines allocated from the Health Center COVID-19 Vaccine Program.] [Note: If you are administering a one-dose vaccine series, ONLY report those in the completed dose question.]
|
[Please enter a numerical value excluding commas (ex. 123123)] |
Number Field |
Question 22 |
By population type, how many patients have completed (2nd, or only, dose received) their COVID-19 immunization series in the last two weeks from vaccines allocated under the Health Center COVID-19 Vaccine Program? [Enter the number of patients who completed an FDA-approved vaccine series in the last two weeks, by disproportionately affected populations below. Only report on vaccines that are allocated from the Health Center COVID-19 Vaccine Program.] [Note: If you are administering a one-dose vaccine series, report those in this question as completed.]
|
[Please enter a numerical value excluding commas (ex. 123123)] |
Number Field |
Public Burden Statement: Health centers (section 330 grant funded and Federally Qualified Health Center look-alikes) deliver comprehensive, high quality, cost-effective primary health care to patients regardless of their ability to pay and are critical in the national response to COVID-19. These forms provide HRSA with the information essential for analyzing health center progress, challenges, and needed technical assistance around COVID-19. The OMB control number for this information collection is 0906-0062 and it is valid through XX/XX/202X. This information collection is mandatory under the Health Center Program authorized by section 330 of the Public Health Service (PHS) Act (42 U.S.C. 254b). Public reporting burden for this collection of information is estimated to average 1 hour per response, including the time for reviewing instructions, searching existing data sources, and completing and reviewing the collection of information. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden, to HRSA Reports Clearance Officer, 5600 Fishers Lane, Room 14N136B, Rockville, Maryland, 20857 or [email protected].
|
|
|
OMB # 0906-0062
Expires: XX/XX/202X
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | HC Data Collection Tool 10-23-2020 |
| Author | Mitchell, Kathryn (Kate) (HRSA) |
| File Modified | 0000-00-00 |
| File Created | 2021-10-13 |
© 2025 OMB.report | Privacy Policy