CMS-10174 - Supporting Statement A
CMS-10174 - Supporting Statement A.docx
Collection of Prescription Drug Data from MA-PD, PDP and Fallout Plans/Sponsors for Medicare Part D Payments (CMS-10174)
OMB: 0938-0982
Supporting Statement (Part A)
Collection of Prescription Drug Event Data
From Contracted Part D Providers for Payment
CMS-10174, OMB 0938-0982
Background
In December 2003, Congress enacted the Medicare Prescription Drug, Improvement, and
Modernization Act of 2003 (P.L. 108-173), referred to as the Medicare Modernization Act (MMA). The Medicare Prescription Drug Benefit program (Part D) was established by section 101 of the MMA and is codified in section 1860D-1 through 1860D-41 of the Social Security Act.
Effective January 1, 2006, the Part D program established an optional prescription drug benefit for individuals who are entitled to Medicare Part A and/or enrolled in Part B.
In general, coverage under the prescription drug benefit is provided predominately through private at-risk Prescription Drug Plans (PDPs) that offer drug-only coverage, Medicare Advantage (MA) plans that offer integrated prescription drug and health care coverage (MA-PD plans), or through Cost Plans that offer prescription drug benefits.
The Patient Protection and Affordable Care Act, as amended by section 1101 of the
Health Care and Education Reconciliation Act of 2010, establishes the Coverage Gap
Discount Program (CGDP) by adding sections 1860D-43 and 1860D-14A of the Social Security Act. Effective January 1, 2011, the CGDP provides manufacturer discounts to applicable Medicare beneficiaries receiving applicable covered Part D drugs in the coverage gap phase of the benefit.
Section 9008 of the Patient Protection and Affordable Care Act (ACA; P.L. 111–148), as amended by section 1404 of the Health Care and Education Reconciliation Act of 2010 (HCERA; P.L. 111–152), imposes an aggregate annual fee on certain manufacturers of branded prescription drugs (please refer to Section 9008 of the ACA for a definition of branded prescription drugs). CMS is required to provide dollar amounts of sales of branded prescription drugs under the Medicare Part D program on a yearly basis to the Secretary of the Treasury in order to determine the fee amount to be paid by each manufacturer.
In order to support these programs, CMS uses the Drug Data Processing System to collect Prescription Drug Event (PDE) data. The PDE data is then used in the Payment Reconciliation System to perform the annual Part D payment reconciliation, any PDE data within the Coverage Gap Phase of the Part D benefit is used for invoicing in the CGDP, and the data are part of the report provided to the Secretary of the Treasury for Section 9008.
The currently approved package is being revised to update burden estimates based on the number of contracts as well as the growth of Medicare beneficiaries enrolled in Part D. We have also added language that reflects the data collected in accordance with section 1860D-12(b)(3)(D) of the Social Security Act (the Act) and 42 C.F.R. § 423.505(f)(3).
History of Program Implementation and Industry Consultation
We requested public comments on the content, format, and frequency for those proposed disclosures, processes and provisions of information in our Notice of Proposed Rulemaking (NPRM) on August 3, 2004. The NPRM may be reviewed in Federal
Register, Vol. 69, No. 148, CMS-4068-P. (46686-46687). In this publication in the
“Collection of Information” section, CMS also requested industry input and discussion, as required under § 3506(c)(2)(A) of the Paperwork Reduction Act of 1995 (PRA). As required under this PRA section, we solicited comments on the following issues:
The need for the information collection and its usefulness in carrying out the proper functions of our agency.
The accuracy of our estimate of the information collection burden.
The quality, utility and clarity of the information to be collected.
Recommendations to minimize the information collection burden on the affected public, including automated collection techniques.
See 69 Fed. Reg. 46631, 46760 (August 3, 2004).
CMS addressed the NPRM and PRA comments on January 28, 2005 in the Final Rule, which may be reviewed in Federal Register, Vol. 70, No. 18, CMS-4068-F. (4307-4308) and (4443, 4447-4448 for the PRA).
On April 27, 2006, CMS issued instructions on Requirements for Submitting Prescription Drug Event Data (PDE) (See Attachment A), which specified the manner, form and frequency of data transmission to CMS. The instructions specified that PDE records must be submitted to CMS electronically at least once a month.
We requested public comments on the approved data collection, Collection of Drug Event Data From Contracted Part D Providers For Payment, on June 5, 2009, August 21, 2009, June 21, 2013, June 28, 2013, August 30, 2013, March 21, 2017, and July 7, 2017. See 74 Fed Reg 27040 (June 5, 2009), 74 Fed Reg 42307 (August 21, 2009), 78 Fed Reg 37542 (June 21, 2013), 78 Fed Reg 38986 (June 28, 2013), 78 Fed Reg 53766 (August 30, 2013), 82 Fed Reg 14517 (March 21, 2017), and 82 Fed Reg 31609 (July 7, 2017). When necessary, we made updates to the data collection requirements to improve oversight and support the acceptance of accurate payment data submitted by Part D sponsors.
A. JUSTIFICATION
1. Need and Legal Basis
Need
Our fundamental goal is to have the least burdensome data submission requirements necessary to acquire the data needed for accurate payment and appropriate program oversight. We believe that claims data provide the most reliable approach to ensuring that payment calculations are accurate. In the absence of claims level data, we would not be able to determine that reinsurance and risk corridor payments are accurate, that fallback plans have been paid accurately, or that low income subsidies are appropriate. The prescription drug industry commonly uses the NCPDP claim format for data transmissions. This format includes over 450 data elements. We require only 57 data elements, and 20 of those elements are NCPDP elements. We believe that our limited data element requirement will greatly reduce the burden of data collection and management, while maintaining the accuracy of payment related calculations. Also, by focusing on a small number of critical data elements the ability of plans to collect and submit accurate and complete data for the purpose of payment calculations will be optimized. Our view is that in order to fulfill the statutory requirements of the Act, we will need the following data categories:
Entity identification (for example, submitter ID, contract number and PBP ID)
Beneficiary identification (for example, Medicare beneficiary identifier (HICN or MBI), date of birth, gender)
Event identification information (for example, claim control number, and adjustment/deletion code)
Drug and Quantity identification information (for example, date of service, fill number, and Compound Code)
Cost information (for example, paid date, ingredient cost, dispensing fee, vaccine administration fee, and sales tax)
Payment Breakout information (for example, catastrophic coverage code, Total Gross Covered Drug Cost Accumulator, True Out-of-Pocket Accumulator, beneficiary amount paid and low-income cost sharing subsidy amount)
Prescriber information (for example, prescriber ID, prescriber ID qualifier and DAW/product selection code)
Service Provider Information (for example service provider ID and pharmacy service type)
Benefit Design information (for example, beginning benefit phase, ending benefit phase, brand/generic code)
In addition to data for interim payments (i.e., Direct subsidies), we will need these data on 100 percent of prescription drug claims for appropriate risk adjustment, reconciliation of reinsurance and low-income subsidies, calculation of risk sharing payments or savings, and program auditing. The PDEs submitted for claims falling in the coverage gap phase are used for the Coverage Gap Discount Program. Data will also be required for assessing and improving quality of care.
Legal Basis
The sections of the Act that provide the statutory authority for data submission in the prescription drug benefit program are the following:
Payments 1860D-11(g)(5), 1860D–14, 1860D-15, 1860D-22
Data Submission 1860D-12(b)(3)(D), 1860D–15(c)(1)(C), 1860D–15(c)(2)(C), 1860D–15(d)(2), 1860D–15(f), 1860D-14A(c)(1)(C)
The regulations set forth in this requirement for submitting Prescription Drug Event Data are codified in 42 CFR Part 423–Voluntary Medicare Prescription Drug Benefit. There are a number of places in which statutory provisions in Part D reference specific sections in Part C of Medicare (the MA program). The MA regulations appear at 42 CFR Part 422− Medicare Advantage Program. The major subjects applicable to the Prescription Drug data submission in Parts 423 Subparts are as follows:
Subpart G of part 423 (§ 423.301) implements section 1860D-15 of the Act and the deductible and cost sharing provisions are addressed in section 1860D–14(a) of the Act. This section sets forth rules for the calculation and payment of our direct and reinsurance subsidies for Part D plans; the application of risk corridors and risk sharing adjustments to payments; and retroactive adjustments and reconciliations to actual enrollment and interim payments. References to § 422 of our regulations are provided in the new MA rules found in Part 422.
Requirements for Disclosure of Information (§ 423.322): Payments to a Part D sponsor are conditioned upon provision of information to CMS that is necessary to carry out this subpart, as provided under sections 1860D-15(c)(1)(C), 1860D-15(c)(2)(C), 1860D15(d)(2) and 1860D–15(f) of the Act and in § 423.322 and § 423.329(b)(3)(i) – (ii) of our regulations.
Subpart P of part 423 (§ 423.771, § 423.772, § 423.780, § 423.782, and § 423.800) implements section 1860D-14. This section sets forth rules for Premiums and Cost Sharing Subsidies for Low-Income beneficiaries. References to § 422 of our regulations are provided in the new MA rules found in Part 422 (§ 422.1, § 422.2, §422.4(c), and § 422.304(b)).
Subpart Q of part 423 (§ 423.875) implements section 1860D-11(g) of the Act, and sets forth, but not limited to, the amount payable for a fallback prescription drug plan in accordance with § 423.871(e).
Subpart R of part 423 (§ 423.888) provides payment methods, including provision of necessary information, and implements section 1860D-22(a) of the Act, as amended by section 101 of the MMA. This section implements the statutory requirement that a subsidy payment be made to sponsors of qualified retiree prescription drug plans.
Subpart W of part 423 implements sections 1860D-14A and 1860D-43 of the Act. Provision of applicable discounts (§ 423.2325), describes collection of data in which sponsor must provide CMS with appropriate data on the applicable discounts provide by the Part D sponsor in a manner specified by CMS.
Part 422 (§ 422.310) sets forth rules for submitting data that can be linked at the individual level to Part A and Part B data.
Subpart K of part 423 (§ 423.505(f)(3)) implements section 1860D-12(b)(3)(D) of the Act to allow the Secretary to collect the same claims information collected under the authority of section 1860D–15 of the Act for purposes including reporting to the Congress and the public, conducting evaluations of the overall Medicare program, making legislative proposals to Congress, and conducting demonstration projects.
2. Information Users
The information users will be Pharmacy Benefit Managers (PBMs), third party administrators and pharmacies, and the PDPs, MA-PDs, Fallbacks and other plans that offer coverage of outpatient prescription drugs under the Medicare Part D benefit to Medicare beneficiaries. The statutorily required data is used primarily for payment and is used for claim validation as well as for other legislated functions such as quality monitoring, program integrity and oversight.
The PDE data are also used to support operations and program development.
Annually, CMS publishes a Public Use File that summarizes annual the Low-Income reconciliation amount, the Reinsurance reconciliation amount, the Risk-Sharing amount, and the Reconciliation amount (which is a sum of the 3 previously stated reconciliation amounts). The PDEs are one of the inputs used to determine these reconciliation amounts.
CMS has used PDE data to create summarized dashboards and tools, including the
Medicare Part D Drug Spending Dashboard & Data, the Part D Manufacturer Rebate Summary Report, and the Medicare Part D Opioid Prescribing Mapping Tool. The data are also used in the Medicare Trustees Report. Due to the market sensitive nature of PDE data, external uses of the data are subject to significant limitations. However, CMS does analyze the data on a regular basis to determine drug cost and utilization patterns in order to inform programmatic patterns and to develop informed policy in the Part D program.
3. Use of Information Technology
The Drug Data Processing System (DDPS) is the information system that collects, validates and stores PDE data received from PDPs, MA-PDs, Fallback and other plans offering coverage of outpatient prescription drugs under the Medicare Part D benefit. PDE records enter the DDPS through the Prescription Drug Front-End System (PDFS). The PDFS receive the PDE records at least monthly, once the plans’, organizations’, or sponsoring entities’ PBMs or other third-party administrators have received drug claims from pharmacies and completed their “in-cycle” events. Plans or their third-party submitters must submit PDE records electronically. The PDFS perform file format and face validity checks. Once the file has passed the front-end checks, it moves through the DDPS where detail level edits are performed and the data are stored. The DDPS also receives corrected and adjusted PDE records.
The PDFS and DDPS consists of two levels of edits, front-end (face validity and file format) and detail (validity and verification) edits. CMS provides a processing system that allows critical data fields to be edited before rejecting or returning a file for more information to allow, at a minimum, a one-time resubmission for corrected data.
The system also provides simplified reporting. Plan sponsor organizations will receive one complete report with all transactions and statuses listed. The report identifies which PDEs were accepted and which were not, along with reason codes. Data tracking is improved by reporting complete results daily. Plan sponsor organizations also receive periodic summary reports that outline the number of drug events submitted, rejected and accepted.
Drug event data can be submitted via the Medicare Data Communications Network
(MDCN) utilizing Internet Protocol (IP) and Secure File Transfer protocol (SFTP) or Systems Network Architecture (SNA) and Connect: Direct, or through the CMS TIBCO mail boxing system. Production PDE data are submitted into the Prescription Drug Front-End System (PDFS) and the Drug Data Processing System (DDPS). When data are submitted, each system runs various edit checks of the data and issues response reports to submitters describing data that was accepted, rejected and any errors that were or may be present in the data so that plans can manage, correct and resubmit their data as necessary.
The DDPS edits records at the detail level, checking event-level information on the beneficiary, drug, costs, and other items. CMS maintains a complete, up-to-date listing of edits at
https://www.csscoperations.com/internet/csscw3.nsf/DIDC/FGSMOX8LWK~Prescriptio n%20Drug%20Program%20(Part%20D)~References.
Once DDPS performs all necessary edits, the accepted PDE records are forwarded to the Integrated Data Repository (IDR). The IDR stores PDE records and accumulates summary data for payment reconciliation. The Payment Reconciliation System (PRS) creates a beneficiary/plan record for each beneficiary enrolled in a plan during the payment year and calculates reconciliation payments at the beneficiary and plan level. Specific reports issued from the IDR inform plans of their year-to-date financial values in preparation for reconciliation. CMS calls these management reports.
An outside contractor, Palmetto GBA, manages the data submission process for CMS and maintains a Customer Service and Support Center (CSSC) that provides customer service and support to data submitters.
All data (100%) is collected by CMS electronically.
See section 12 of this Supporting Statement under Collection of Prescription Drug Event Data for an illustration of the dataflow.
4. Duplication of Efforts
This information collection does not duplicate any other effort and the information cannot be obtained from any other source.
5. Small Businesses
The collection of information will have a minimal impact on small businesses or other small organizational entities since the applicants must possess an insurance license and be able to accept risk. Generally, state statutory licensure requirements effectively prevent small organizations from accepting the level of risk needed to provide the pharmacy benefits required in the Medicare Prescription Drug Benefit program.
6. Less Frequent Collection
In the April 27, 2006, Instructions on Requirements for Submitting Prescription Drug Event Data (PDE), CMS indicated that PDE records must be submitted to CMS electronically at least once a month. In addition to this guidance, CMS has issued more specific guidance on submission of original PDEs and corrections. In the October 6, 2011 Health Plan Management System (HPMS) memorandum titled, Revision to Previous Guidance Titled “Timely Submission of Prescription Drug Event (PDE) Records and Resolution of Rejected PDEs”, it states original PDEs must be submitted within 30 days following Date Claim Received or Date of Service (whichever is greater); resolve rejected records and re-submit within 90 days following receipt of rejected record status from CMS; submit adjustments within 90 days of discovery; and submit adjustments and deletions within 90 days following discovery of issue requiring change.
CMS believes this frequency minimizes burden yet allows for reimbursement to proceed in a timely and accurate manner. Less frequent collection would delay payment. CMS will conduct an initial reconciliation after 6 months of the end of a coverage year and will perform a global reopening approximately four years after sending the reports and/or payments associated with the initial reconciliation for a contract year.
7. Special Circumstances
There are no special circumstances that would require an information collection to be conducted in a manner that requires respondents to:
Report information to the agency more often than monthly;
Prepare a written response to a collection of information in fewer than 30 days after receipt of it;
Submit more than an original and two copies of any document;
Retain records, other than health, medical, government contract, grant-in-aid, or tax records for more than three years;
Collect data in connection with a statistical survey that is not designed to produce valid and reliable results that can be generalized to the universe of study,
Use a statistical data classification that has not been reviewed and approved by OMB; or
Include a pledge of confidentiality that is not supported by authority established in statute or regulation that is not supported by disclosure and data security policies that are consistent with the pledge, or which unnecessarily impedes sharing of data with other agencies for compatible confidential use.
8. Federal Register Notice/Outside Consultation
Federal Register
The 60-day notice published in the Federal Register on 06/23/2021 (86 FR 32935).
No comments were received.
The 30-day notice published in the Federal Register on 09/02/2021 (86 FR 49332).
Outside Consultation
There has not been any outside consultation since January 28, 2005.
Ongoing Communication
The table below is a summary of our specific communications and consultations with the industry since the package was last approved. The table breaks out events by Type, Date and Status.
Type of Communication |
Dates |
Status |
Weekly Part D User Group Teleconferences |
Began February 9, 2005 |
Active (now held as-needed) |
NCPDP Workgroup |
August 2006 November 2006 November 2007 August 2008 May 2009 August 2012 – present
|
Active |
PDE Training- Webinars |
June 2014 August 2014 February 2015 |
|
PDE Training- Computer Based Training |
November 2015 December 2015 July 2016 December 2018 May 2021 |
|
9. Payments/Gifts to Respondents
Filing a prescription drug benefit claim does not result in gifts to respondents; many conditions must be met before payment can be made to a plan sponsor. Submitting information using the prescribed CMS format is one of the requirements for participation in the Medicare Part D drug benefit program.
10. Confidentiality
The information provided by the plan or sponsor organizations regarding prescription drug events are protected and held confidential in accordance with 20 CFR 401.30. The information provided electronically and, on the forms, will become part of the contracted organization’s computer history, microfilm, and hard copy records retention system as published in the Federal Register, Part VI, "Privacy Act of 1974 System of Records" on September 20, 1976 (HI CAR 0175.04).
All electronic claims or drug events sent from pharmacies to PBMs or other third-party administrators and from them to plans constitute HIPAA-covered transactions. Any plan or sponsor organizations that utilize an electronic format for their drug data collection will need to convert to ANSI X12.
11. Sensitive Questions
Other than the labeled information noted above in section 10 above, there are no questions of a sensitive nature. Specifically, the collection does not solicit questions of a sensitive nature, such as sexual behavior and attitudes, religious beliefs, and other matters that are commonly considered private.
12. Burden Estimate (Hours & Wages)
Burden Estimates
The burden placed on Part D plans (contracts) associated with submitted PDE data is predicated upon the following factors: (a) the amount of data that must be submitted; (b) the number of plans submitting data; and (c) the time required to complete the data processing and transmission transactions.
PDE Data Submission: The amount of data that must be submitted is a function of the number of prescription drug events per beneficiary and the number of data elements per event (57). Based on three years of enrollment data (2017, 2018, and
2019), CMS estimates that an annual average of 46,793,272 Medicare beneficiaries enroll in Part D prescription drug coverage. The average number of PDEs per year is1,499,238,090 based on data from 2017, 2018, and 2019. To compute the average number of PDEs per beneficiary, we divide the average number of PDEs per year by the average number of beneficiaries enrolled per year. This computation leads to an average of 32 PDEs per beneficiary per year.
Number of Part D Contracts (Respondents): The average number of Part D contracts per year is 739 (based on 2017, 2018, and 2019 data).
Time Required to Process Data: The third factor that contributes to the burden estimate for submitting PDE data depends upon the time and effort necessary to complete data transaction activities. Since our regulations require Part D plans/sponsors to submit drug event data to CMS that can be linked at the individual level to Part A and Part B data in a form and manner similar to the process provided under § 422.310 (Part C), the data transaction timeframes will be based on risk adjustment (Part C) and prescription drug industry experiences. Moreover, our PDE data submission format (as well as the drug industry’s) will only support electronic formats. The drug industry’s estimated average processing time for electronic data submission is 1 hour for 500,000 records. The risk adjustment estimated average annual electronic processing time cost per hour is $17.75.
All three factors are reflected in Table 1 and illustrate the relationship between these results and the burden estimate.
|
|
TABLE 1 |
|
|
|
|
NOTES |
A |
NUMBER OF RESPONDENTS |
739 |
739 is the annual average number of Part D contracts from 2017, 2018, and 2019 |
B |
NUMBER OF MEDICARE BENEFICIARIES ENROLLED IN PART D PER YEAR |
46,793,272 |
Average number of Medicare beneficiaries enrolled in Part D |
C |
AVERAGE NUMBER OF PART D BENEFICIARIES PER CONTRACT |
63,320 |
(B) divided by (A) |
D |
AVERAGE NUMBER OF PDES PER YEAR |
1,499,238,090 |
The average is based on annual average PDEs from 2017, 2018, and 2019 |
E |
FREQUENCY OF RESPONSE |
32 PDEs/per beneficiary per year |
average PDEs per beneficiary per year |
F |
NUMBER OF TRANSACTIONS PER HOUR |
500,000 |
Drug industry's estimated average processing volume per hour |
G |
TOTAL ANNUAL TRANSACTION HOURS |
2,998 |
(D) divided by (F) |
H |
AVERAGE ELECTRONIC COST PER HOUR |
$17.75 |
Based on $17.75 per hour, the risk adjustment estimated average annual electronic processing cost per hour |
I |
COST OF ANNUAL TRANSACTION HOURS |
$53,215
|
(H) multiplied by (G) |
J |
AVERAGE COST PER PART D BENEFICIARY |
$0.0011 |
(I) divided by (B) |
K |
ANNUAL COST TO RESPONDENTS |
$69.66 |
(J) multiplied by (C) |
Information Collection/Reporting Instruments and Instruction/Guidance Documents
• Requirements for Submitting Prescription Drug Event Data (PDE) [Available at: https://csscoperations.com/internet/csscw3.nsf/DIDC/GZEB9OUQJ9~Prescriptio n%20Drug%20Program%20(Part%20D)~References
Submission Requirements
The MMA requires Medicare payment to MA organizations, PDP plans, Fallbacks, and other plans offering coverage of outpatient prescription drugs under the new Medicare Part D benefit. The Act provided four summary mechanisms for paying plans:
direct subsidies
subsidized coverage for qualifying low-income individuals
federal reinsurance subsidies
risk corridor payments
In order to make payment in accordance with these provisions, CMS has determined to collect a limited set of data elements for 100 percent of prescription drug claims or events from plans offering Part D coverage. In determining these requirements, we have incorporated feedback from industry and other stakeholders obtained by formal and informal means including the rulemaking process, Open Door Forums and other consultation. We used four criteria in selecting the required data elements:
ability to pay plans timely and accurately using the four legislated payment mechanisms (direct subsidy, reinsurance, risk corridors, and low-income subsidy);
minimal administrative burden on CMS, plans, and other entities including MA-
PDs, PDPs, fallback plans, pharmacy benefit managers, pharmacies, and others;
legislative authority; and
validity and reliability of the data elements requested, to ensure that the information will be useful.
The requirements for submitting PDE data provide that much of the data, especially financial fields, will be used primarily for payment. However, other data elements will be used for validation of the claims as well as for other legislated functions such as quality monitoring, program integrity, and oversight.
Our instructions for submitting PDE data require that plans must submit a PDE record for each dispensing event. The PDE record is a summary record that documents the final adjudication of the dispensing event. Since the pharmacy industry has an effective drug claims submission standard, which is electronically automated, we will use the National Council of Prescription Drug Programs (NCPDP) version D.0 as the data format for PDE submissions. Thus, our 57 required PDE elements include 15 data elements from the NCPDP billing transaction, 5 data elements from the NCPDP billing response transaction and 37 CMS defined data elements. Although a number of the statutory requirements of the Act were not available in the NCPDP data elements, we utilized the NCPDP format to construct the CMS defined data elements to ensure minimal burden on plans. The
Prescription Drug Event Record Layout is available at https://csscoperations.com/internet/csscw3.nsf/DIDC/ETTDDMFAAP~Prescription%20 Drug%20Program%20(Part%20D)~File%20and%20Report%20Layouts.
Collection of Prescription Drug Event Data
As a condition of payment, all Part D plans must submit data and information necessary for CMS to carry out payment provisions. Much of the data, especially dollar fields, will be used primarily for payment. However, some of the other data elements such as pharmacy and prescriber identifiers will be used for other legislated functions such as quality monitoring, program integrity, and oversight.
Every time a beneficiary fills a Part D prescription, plans must submit a summary record called the Prescription Drug Event (PDE) record to CMS. The PDE data is an extract of information from claims made by beneficiaries purchasing prescription drugs that are covered under Part D. The PDE record contains prescription drug cost and payment data that will enable CMS to make payment to plans and otherwise administer the Part D benefit. Specifically, the PDE record will include covered Part D drug costs above and below the out-of-pocket threshold; distinguish supplemental benefits from benefits provided under basic prescription drug coverage; and will record payments made by Part D plans, other payers, and by or on behalf of beneficiaries. Plans must also identify costs that contribute towards a beneficiary’s True-Out-Of-Pocket (TrOOP) costs, separated into four categories: low-income cost-sharing subsidy amounts paid by the plan at the Point of Sale (POS), beneficiary payments, reported gap discount amount, and all TrOOP eligible payments made by qualified entities on behalf of a beneficiary. PDE data also reflect how a plan has administered its Part D benefit package. CMS uses the data to reconcile low-income cost-sharing subsidy and reinsurance payments and to implement risk sharing between the plan and the Federal government.
In most situations, the MA-PD/PDP or a designated third-party processor on its behalf processes the claim that has been submitted electronically by the network provider (e.g., pharmacy or physician office) and determines the applicable cost sharing to be made by the beneficiary. Typically, the network providers provide billing transactions to MAPD/PDP in real-time and the claims processor can file the information to CMS promptly. In a limited number of situations, a beneficiary or other entity may submit a non-standard format claim such as a paper claim to a plan or its third-party processor. The plan/processor then creates a PDE record from the claim to submit electronically to CMS in a non-standard format (for example, there are special rules and exceptions for populating certain non-financial data elements).
The PDE record contains prescription drug cost and payment data that enable CMS to make payment to plans and otherwise administer the Part D benefit. Specifically, the PDE record includes covered drug costs above and below the Out-of-Pocket (OOP) threshold; distinguishes enhanced alternative costs from the costs of drugs provided under the Basic Benefit; and records payments made by Part D plans, other payers, beneficiaries, or individuals on behalf of a beneficiary. Plans must also identify costs that contribute toward a beneficiary’s TrOOP limit.
Many electronic transactions take place between plans, pharmacies, and intermediaries when an enrollee fills a prescription. This process allows determination of patient cost-sharing at the POS by plan adjudication of the claim, and drives eventual plan payment to the pharmacy. The PDE record contains information that is vital for payment, quality oversight, and program integrity.
For each dispensing event, the plan must submit a PDE record. Most organizations use a Pharmacy Benefit Manager (PBM) or other third-party administrator to process incoming claims from pharmacies. Claims typically undergo several rounds of transactions between these parties before the plan finally adjudicates a claim for payment. The PDE is a summary record that documents the final adjudication of a dispensing event.
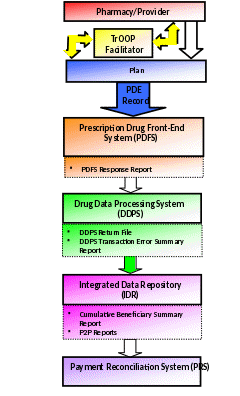
The following is an illustration of the prescription drug event dataflow:
The pharmacy, physician, or other provider submits a claim to the Part D Plan.
If necessary, the pharmacy generates a secondary claim to any other payers via the TrOOP facilitator.
The Part D Plan submits data to CMS via the PDE record.
The Part D Plan successfully submits PDE records at least once a month to PDFS.
The PDE records are sent to PDFS where front-end edits are applied.
The PDFS response report indicates file acceptance or rejection. If any PDE records fail front-end edits, PDFS report the failure on the PDFS Response Report.
After passing the PDFS checks, the file is submitted to DDPS where detail editing is performed.
The DDPS Return File is returned daily and shows the disposition of all DET records and where errors occurred.
The DDPS Transaction Error Summary displays the count and rate for each error code found in the submitted data.
The IDR sums LICS and calculates unadjusted reinsurance and risk corridor costs.
Management reports are generated in the IDR and provide a summary of net accumulated totals for all dollar fields.
PRS creates a beneficiary/plan record for each beneficiary enrolled in a plan during the payment year and calculates reconciliation payments at the beneficiary and plan level.
Prescription Drug Event (PDE) Record Fields
The record contains 60 fields. Included in these fields are 57 data elements that plans must populate for CMS to reconcile payment and provide program oversight and three filler fields, which are populated with spaces. Plans must sort Detail (DET) records within each batch by the Medicare beneficiary identifier. This section reviews data elements within the DET record, with emphasis on data used for payment reconciliation.
Beneficiary Identifiers: The following data elements identify the beneficiary:
Medicare beneficiary identifier
Cardholder ID
Patient Date of Birth (DOB)
Patient Gender
The Medicare beneficiary identifier is the only data element used to identify a beneficiary that is not available in NCPDP standard format. The Medicare beneficiary identifier (Mbi) can be the Health Insurance Claim Number (HICN), the Medicare Beneficiary Identifier (MBI), or the Railroad Retirement Board (RRB) number.
Prescription Drug Event Identifiers: Fourteen data elements, which are standard throughout the industry, describe both the drug and the method in which the drug was dispensed.
Date of Service (DOS)
Prescription Service Reference Number
Product Service ID
Service Provider ID Qualifier
Service Provider ID
Fill Number
Dispensing Status
Compound Code
Dispense as Written (DAW) Product Selection Code
Quantity Dispensed
Days Supply
Prescriber ID Qualifier
Prescriber ID
Prescription Origin Code
Sixteen non-financial data elements are unique to Part D:
Record ID
Sequence No.
Claim Control Number
Paid Date
Adjustment Deletion Code
Non-Standard Format Code
Pricing Exception Code
Date Original Claim Received
Claim Adjudication Began Timestamp
OAP Indicator
Pharmacy Service Type
Patient Residence
Submission Clarification Code
Adjustment Reason Code Qualifier
Adjustment Reason Code
Type of Fill Code
Dollar Fields: The PDE Record layout includes 16 fields that must be populated with dollar amounts. These 16 fields can be categorized as detail cost fields, summary cost fields, patient liability payment fields, and plan payment fields. Each of these fields impacts Part D payment. In cost fields, plans must report the dollar amount paid to the pharmacy.
Ingredient Cost Paid
Dispensing Fee Paid
Total Amount Attributed to Sales Tax
Gross Drug Cost Below Out-of-Pocket Threshold (GDCB)
Gross Drug Cost Above Out-of-Pocket Threshold (GDCA)
Patient Pay Amount
Other TrOOP Amount
Low Income Cost Sharing Subsidy Amount (LICS)
Patient Liability Reduction Due to Other Payer Amount (PLRO)
Covered D Plan Paid Amount (CPP)
Non-Covered Plan Paid Amount (NPP)
Estimated Rebate at POS (Point-of-Sale)
Vaccine Administration Fee
Total Gross Covered Drug Cost Accumulator
True Out-of-Pocket Accumulator
Reported Gap Discount
Benefit Design Identifiers: Seven data elements, which are unique to Part D, provide information regarding the benefit design under which the PDE was adjudicated.
Drug Coverage Status Code
Catastrophic Coverage Code (optional for PDEs with DOS January 1, 2011 and forward)
Beginning Benefit Phase
Ending Benefit Phase
Brand/Generic Code
Tier
Formulary Code
The 57 data elements on the PDE record are:
Field # |
Field Name |
Description |
1 |
Record ID |
Identifies record as a detail record. |
2 |
Sequence No. |
Identifies the detail record submitted. |
3 |
Claim Control Number |
This field is an optional, free-form field. It may be used by plans to identify unique events for any other plan purpose. The data in this field will be reported back to a plan in the event a batch or individual record is rejected at some point in processing. |
4 |
Medicare beneficiary identifier |
This field contains the unique number assigned to identify every Medicare beneficiary. Starting in April 2018, a new identifier, the Medicare Beneficiary Identifier (MBI) will be issued to beneficiaries. All drug events submitted to DDPS should use the MBI when available. If the MBI is not available, the HICN or RRB number should be used. The Medicare beneficiary identifier will also permit linkage of Part D drug event data to Parts A and B claims data, eligibility and enrollment data, and risk adjustment data. |
5 |
Cardholder ID |
CMS collects the plan-assigned number used to identify the beneficiary. This number verifies beneficiary identity and will be used to help plans map transactions to their databases and for program oversight functions. |
6 |
Patient Date of Birth (DOB) |
Patient date of birth (DOB) is optional and is used in conjunction with Mbi and gender to verify beneficiary identity. It is used as a cross-reference to ensure the event has identified the correct beneficiary. |
7 |
Patient Gender Code |
Together with Mbi and DOB (when reported), gender confirms the identity of the beneficiary. |
8 |
Date of Service (DOS) |
Date of Service (DOS) is the date on which the prescription was filled. |
9 |
Paid Date |
This field shall be populated with the date the plan originally paid the pharmacy for the prescription drug. (If the plan subsequently adjusts payment, the plan will report the original paid date in the adjustment PDE). Paid Date is a mandatory field for fallback plans, and is optional for all other plan types. CMS uses Paid Date to reconcile drug costs reported on PDE records to withdrawals for drug costs from the fallback plan's drawdown account. |
10 |
Prescription Service Reference Number |
This field contains the prescription service reference number assigned by the pharmacy at the time the prescription is filled. It enables DDPS to identify a unique prescription drug event. |
12 |
Product Service ID |
This field identifies the dispensed drug using a National Drug Code (NDC). The NDC is reported in an 11 digit format. In instances where a pharmacy formulates a compound containing multiple NDC drugs, the NDC of the most expensive Part D covered drug ingredient shall be used. |
13 |
Service Provider ID Qualifier |
This field indicates the type of pharmacy provider identifier used. |
14 |
Service Provider ID |
This field identifies the pharmacy where the prescription was filled. This data helps CMS identify a unique prescription drug event. Prior to National Provider Identifier (NPI) implementation in May 2008, the National Council of Prescription Drug Plans (NCPDP) Provider ID number was primarily reported. Since May 2008 the NPI is primarily reported in this field. CMS prefers the use of the NPI in this field. For standard format PDEs, if the NPI is unavailable, the field must contain the NCPDP number, which all NCPDP billers are assigned. If neither the NPI number nor the NCPDP number is available for a provider who submitted in Non-Standard Format (e.g., home infusion, physicians when providing vaccines), then the UPIN, State License Number, Federal Tax Identification Number (TIN) or |
|
|
Employer Identification Number (EIN) or” other” will be the required identifier. |
15 |
Fill Number |
This field indicates the number fill of the current dispensed supply. |
16 |
Dispensing Status |
On PDEs with DOS prior to January 1, 2011, this field indicates how the pharmacy dispensed the prescribed quantity of the prescription. When the pharmacy partially fills a prescription, this field indicates a partial fill. When the full quantity is dispensed at one time, this field is blank. When the pharmacy dispenses a partial fill, the plan has the option to submit two PDE records, one for the partial fill and a second for completion of the partial fill. If the plan prefers, the plan can defer PDE submission for a reasonable amount of time until the plan receives transactions for both the partial and complete fill. At that point, the plan may summarize the multiple transactions in a single PDE, reporting a blank in Dispensing Status. On PDEs with DOS on and after January 1, 2011, this field must be blank. |
17 |
Compound Code |
This field indicates whether or not the dispensed drug was compounded or mixed. This distinction will ensure that correct payments are made to the plan for mixed or compounded drugs. Plans may adjust the dispensing fee to include additional labor costs in the delivery of the compounded pharmaceutical item. |
18 |
Dispense As Written (DAW)/Product Selection Code |
This field indicates the prescriber’s instruction regarding substitution of generic equivalents or order to dispense the specific product as written. |
19 |
Quantity Dispensed |
This field indicates how many dosage units of the medication were dispensed in the current drug event (e.g., number of tablets, grams, milliliters, or other unit). |
21 |
Days Supply |
This field indicates the number of days supply of medication dispensed by the pharmacy and consists of the amount the pharmacy enters for the prescription. |
22 |
Prescriber ID Qualifier |
This field indicates the type of identifier that is used in the Prescriber ID field. |
23 |
Prescriber ID |
This field contains the prescriber’s unique identification number. Effective January 1, 2013, CMS requires sponsors to submit an active and valid NPI. Beginning May 6, 2013, only Type 1 NPIs will be accepted in this field. |
24 |
Drug Coverage Status Code |
This field indicates whether or not the drug is covered under the Medicare Part D benefit and/or a specific PBP. |
25 |
Adjustment/ Deletion Code |
This field distinguishes original from adjusted or deleted PDE records so that the DDPS can adjust claims and make accurate payment for revised PDE records. When DDPS receives a PDE record with Adjustment/Deletion Code = A (adjustment) or D (deletion), DDPS will search the database for a current active PDE record with matching values in key fields (key fields are Mbi, Service Provider ID, Service Provider ID Qualifier, Prescription/Service Reference Number, Date of Service, Fill Number and Dispensing Status), plus the contract number and PBP ID. |
26 |
Non-Standard Format Code |
This data element is used by DDPS to identify PDE records that are compiled from non-standard sources. NCPDP is the standard electronic format in which plans receive data from pharmacies. |
27 |
Pricing Exception Code |
This field indicates that the PDE reports an out-of-network or Medicare as Secondary Payer (MSP) service that is subject to unique pricing rules. |
28 |
Catastrophic Coverage Code |
For DOS prior to January 1, 2011, this field indicates that a beneficiary has reached the out-of-pocket (OOP) threshold. At this point, catastrophic coverage provisions begin, namely reinsurance and reduced beneficiary cost sharing. For DOS on and after January 1, 2011, this field is optional. |
29 |
Ingredient Cost Paid |
This field contains the amount paid for the drug itself. Dispensing fees or other costs shall not be included in this amount except as allowed on nonstandard format claims. |
30 |
Dispensing Fee Paid |
This field contains amounts paid to the pharmacy for dispensing the medication. Include only those activities related to the transfer of possession of the drug from the pharmacy to the beneficiary, including charges associated with mixing drugs, delivery, and overhead as delineated in the final rule §423.100, the preamble to the rule and in chapter 5 of the Prescription Drug Benefit Manual. No other costs shall be included in this field. The fee may be negotiated with pharmacies at the plan or PBM level. |
31 |
Total Amount Attributed to Sales Tax |
This field contains the sum of all amounts paid to cover sales tax. |
32 |
Gross Drug Cost Below Out-Of-Pocket Threshold (GDCB) |
This field represents the gross drug cost (Ingredient Cost Paid + Dispensing Fee Paid + Total Amount Attributed to Sales Tax + Vaccine Administration Fee) paid below the OOP threshold for a given PDE for a covered Part D drug. For claims before a beneficiary has reached the out-of-pocket threshold, this field will list a positive dollar amount. For claims above the out-of-pocket threshold, this field will have a zero-dollar value. For a claim on which the out-of-pocket threshold is reached, there will be a positive dollar amount in this field and there is likely to be a positive dollar amount in the GDCA field. |
33 |
Gross Drug Cost Above Out-Of-Pocket Threshold (GDCA) |
This field represents the gross drug cost (Ingredient Cost Paid + Dispensing Fee Paid + Total Amount Attributed to Sales Tax+ Vaccine Administration Fee) paid above the OOP threshold for a given PDE for a covered Part D drug. For claims before a beneficiary has reached the out-of-pocket threshold, this field will list a zero-dollar amount. For claims above the out-of-pocket threshold, this field will have a positive dollar value. For a claim on which the out-of-pocket threshold is reached, there is likely to be a positive dollar amount in this field and there will be a positive dollar amount in the GDCB field. |
34 |
Patient Pay Amount |
This field lists the dollar amount the beneficiary paid that is not reimbursed by a third party (e.g., co-payments, coinsurance, deductible or other patient pay amounts). This amount contributes to a beneficiary’s TrOOP only when it is payment for a covered Part D drug. Plans are responsible for ensuring that beneficiaries are charged amounts that are consistent with their benefit packages as approved in the bidding process. Note: Payments actually made by a beneficiary shall be recorded in this field, and CMS expects amounts paid by friends or family to also be reported under Patient Pay Amount. However, other third-party payments made on behalf of a beneficiary that contribute to TrOOP shall be reported in the Other TrOOP Amount field, payments that do not contribute to TrOOP shall be reported in the PLRO field, and payment by the government under the low-income cost-sharing subsidy shall be reported in LICS Amount. |
35 |
Other TrOOP Amount |
This field records all qualified third-party payments that contribute to a beneficiary's TrOOP, except for LICS and Patient Pay Amount. Examples include payments made on behalf of a beneficiary by qualified SPAPs, charities, or other TrOOP-eligible parties. Note: LICS amounts and payments by beneficiaries or friends or family, which count towards TrOOP, shall not be reported in this field; they are reported in the LICS and Patient Pay Amount fields. Also, the Other TrOOP field does not include payments by other parties that do not contribute to TrOOP; those amounts are reported in the PLRO field. |
36 |
Low-Income Cost- Sharing Subsidy Amount (LICS) |
The Act provides for Medicare payments to plans to subsidize the cost sharing liability of qualifying low-income beneficiaries at the point of sale. In accordance with statutory language, CMS refers to these amounts as Low-Income Cost-Sharing Subsidies or LICS amounts. The LICS field will contain plan-reported LICS amounts per drug event, so that CMS systems can reconcile prospective LICS payments made to plans with actual LICS amounts incurred by the plan at POS. |
37 |
Patient Liability Reduction Due to Other Payer Amount (PLRO) |
This field takes into account coordination of benefits that result in reduced patient liability, excluding any TrOOP-eligible payers. This field shall contain amounts by which patient liability is reduced due to payments by other payers that do not participate in Part D and are not TrOOP-eligible. PLRO amounts are excluded from Part D payment, and the PLRO field documents these benefits so that CMS can exclude them from risk corridor calculations and from TrOOP accumulation. Note: This field should not include payments or other patient liability reductions due to coverage under qualified SPAPs or any other Troop eligible third-party payer. All TrOOP-eligible amounts should be reported in the Patient Pay Amount field (if paid by the beneficiary, family, or friends) or in Other TrOOP Amount (if paid by another qualified third party). |
38 |
Covered D Plan Paid Amount (CPP) |
This field contains the net amount the plan paid for basic benefits (covered Part D drugs). In other words, the field reports the plan-paid amount for drugs with Drug Coverage Code = C. If Drug Coverage Code = E or O, the CPP field is zero. DDPS will use this field to facilitate reconciliation calculations, especially determining allowable risk corridor costs. |
39 |
Non-Covered Plan Paid Amount (NPP) |
This field shall contain the net amount paid by the plan for non-Part D covered benefits. Thus, this value includes all plan payment for supplemental benefits (supplemental drugs and supplemental cost sharing) as well as over-the-counter drugs paid under plan administrative costs for basic prescription drug coverage in accordance with CMS policy. The amount recorded in NPP is excluded from risk corridor payment and from TrOOP accumulation. DDPS may also use this data to assure that coverage provisions are in accordance with the approved plan benefit structure from its bid. |
40 |
Estimated Rebate at Point-of-Sale (POS) |
This field contains the estimated amount of rebate that the plan sponsor has elected to apply to the negotiated price as a reduction in the drug price made available to the beneficiary at the point of sale. This estimate should reflect the rebate amount that the plan sponsor reasonably expects to receive from a pharmaceutical manufacturer or other entity. |
41 |
Vaccine Administration Fee |
This field contains the fee reported by a pharmacy, physician, or provider to cover the cost of administering a vaccine, excluding the ingredient cost and dispensing fee. |
42 |
Prescription Origin Code |
This field contains the code indicating the origin of the prescription (i.e., not specified, written, telephone, electronic, facsimile). |
43 |
Date Original Claim Received |
This field contains the date the sponsor receives the original claim. The field is required for PDEs with DOS on and after January 1, 2011. |
44 |
Claim Adjudication Began Timestamp |
This field contains the date and time the sponsor begins adjudicating the claim in Greenwich Mean Time. The field is required on PDEs with DOS on and after January 1, 2011. |
45 |
Total Gross Covered Drug Cost Accumulator |
This field is the sum of the beneficiary’s covered drug costs for the benefit year known immediately prior to adjudicating the claim. The field is required on PDEs with DOS on and after January 1, 2011. |
46 |
True Out-of-Pocket Accumulator |
This field is the sum of the beneficiary’s incurred costs (Patient Pay Amount, LICS, Other TrOOP Amount, Reported Gap Discount Amount) |
|
|
for the benefit year known immediately prior to adjudicating the claim. The field is required on PDEs with DOS on and after January 1, 2011. |
47 |
Brand/Generic Code |
The plan reported value indicating whether the plan adjudicated the claim as a brand or generic drug. The field is required on PDEs with DOS on and after January 1, 2011. |
48 |
Beginning Benefit Phase |
This field is the plan-defined benefit phase in effect immediately prior to the time the sponsor begins adjudicating the individual claim being reported. The field is required on PDEs with DOS on and after January 1, 2011. |
49 |
Ending Benefit Phase |
This field is the plan-defined benefit phase in effect upon the sponsor completing adjudication of the individual claim being reported. The field is required on PDEs with DOS on and after January 1, 2011 |
50 |
Reported Gap Discount |
This field contains the reported amount that the sponsor advanced at point of sale for the Gap Discount for applicable drugs. This field is required on PDEs with DOS on and after January 1, 2011. |
51 |
Tier |
This field contains the formulary tier in which the sponsor adjudicated the claim. This field is required on PDEs with DOS on and after January 1, 2011. |
52 |
Formulary Code |
This field indicates if the drug is on the plan’s formulary. This field is required on PDEs with DOS on and after January 1, 2011. Va lid values are F – formulary, N –Non-formulary. |
53 |
OAP Indicator |
This is a placeholder field related to Prescriber ID editing. Field should be blank until further notice.
Note: This field replaced Gap Discount Override Code on 5/5/16. |
54 |
Pharmacy Service Type |
This field will contain a code that represents the type of service being performed by a pharmacy. The field is required on PDEs with DOS on and after February 28, 2013. |
55 |
Patient Residence |
This field will contain a code that identifies the patient’s place of residence. The field is required on PDEs with DOS on and after February 28, 2013. |
56 |
Submission Clarification Code |
This field will contain a code that indicates the pharmacist is clarifying the submission for short-cycle dispensing in long-term care. The field is required on PDEs with DOS on and after February 28, 2013. |
57 |
Adjustment Reason Code Qualifier |
This field, in combination with the Adjustment Reason Code field, will assist CMS in tracking the reason for an adjustment or deletion of a PDE. |
58 |
Adjustment Reason Code |
This field, in combination with the Adjustment Reason Code Qualifier, will assist CMS in tracking the reason for an adjustment or deletion of a PDE. |
59 |
Type of Fill Code |
This is a placeholder field related to Prescriber ID editing. This field should be blank until further notice. |
13. Capital Costs
There are no significant maintenance costs that are directly associated with this effort. Any administrative and/or capital costs incurred will be recouped through the bidding process and secured through the reinsurance and/or risk corridors processes. The average number of Part D contracts per year is 739 (based on 2017, 2018, and 2019 data). These entities have sufficient capital assets in place to address reporting drug data. MA-PD plans also have sufficient capital assets in place to address drug data reporting.
14. Cost to Federal Government
CMS has estimated that the total cost of the PDE data submission activities utilizing the PDFS and DDPS will be approximately $13 million.
|
PDFS |
DDPS |
Labor |
$1.8 Million |
$8.8 Million |
Infrastructure (or other Direct Costs) |
$1.4 Million |
$1.0 Million |
Total |
$3.2 Million |
$9.8 Million |
15. Changes to Burden
The burden estimate has been increased approximately 6.3% (the responses increased from 1,409,828,464 to 1,499,238,090 while the hours increased from 2,820 to 2,998)
from the burden estimate in the last approved PRA package. The increase is a result of the growth of Medicare beneficiaries enrolled in Part D, which also resulted in an increase in the average number of PDEs per year. This change reflects actual numbers from the most recent 3 reconciled years of the Part D program. The average number of Part D contracts has decreased by 40, from 779 in our currently approved ICR to 739 in this iteration.
16. Publication/Tabulation Dates
The purpose of this data submission request is to support the payment of outpatient prescription drugs for beneficiaries who are members of Part D plans and who receive services under the Medicare Part D benefits program. There are no publication and tabulation dates.
17. Expiration Date
The expiration date is displayed within the PRA Disclosure Statement and can be found on Reginfo.gov under OMB Control No. 0938-0982 (available at https://www.reginfo.gov/public/Forward?SearchTarget=PRA&textfield=09380982&Image61.x=12&Image61.y=20).
18. Certification Statement
CMS has no exceptions to Item 19, “Certification for Paperwork Reduction Act Submissions” of OMB Form 83-I.
B. Collection of Information Employing Statistical Methods
Requirements for this data collection do not employ statistical methods.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | PDE Supporting Statement - PRA |
| Author | CMS |
| File Modified | 0000-00-00 |
| File Created | 2021-09-08 |
© 2026 OMB.report | Privacy Policy