Technical Appendix (with quick-reference schematics)
Att 2_Technical Appendix_09292021.docx
Using Real-time Prescription and Insurance Claims Data to Support the HIV Care Continuum
Technical Appendix (with quick-reference schematics)
OMB: 0920-1361
Technical Appendix
Contents
Cluster randomization and assignment to control or treatment group 2
Provider-level intervention (peer-to-peer clinician consultation) 5
Virginia Medicaid data abstraction: 6
Virginia Care Marker data abstraction: 7
Phase I and Phase II patient-level semi-structured interviews: 8
Peer-to-peer clinician consultation: 9
Schematic Summary of AIMS Study Comparison Groups 10
Schematic Summary of Data Sources, Frequency of Collection, and Uses 11
Study overview
The AIMS study is a cluster-randomized controlled Data-to-Care intervention with targeted provider- and patient-level support. The targeted population is persons with HIV who are enrolled in Virginia Medicaid and who have either never filled an ARV prescription or who are > 30 to < 90 days late filling their ARV prescription(s). The Virginia Commonwealth University (VCU, the grantee) and other partners will collaborate to conduct the study.
Eligibility determination
A validated HIV case identification algorithm will be applied to the Virginia Department of Medical Assistance Services (DMAS—Virginia Medicaid) database to identify persons with HIV who have either never filled an ARV prescription or have not filled an ARV prescription within > 30 to < 90 days of the expected fill date. These individuals will be considered potential study participants. Deterministic and probabilistic methods will be used to link the list of potential participants within the Virginia Medicaid database to the Virginia Department of Health (VDH) Care Markers database (an extract of the VDH HIV surveillance database). Data elements listed in Att 3 and Att 4 will be used to match potential participants in the two databases. Individuals that are matched across the two databases (indicating that the persons are both enrolled in Medicaid and confirmed HIV positive) are preliminarily eligible for study participation. The following additional eligibility requirements will apply: continuous enrollment in Virginia Medicaid for the preceding 12 months and age 19 – 63 years. Persons who are eligible for Medicare and persons with other third-party health insurance coverage will be excluded from participation.
Cluster randomization and assignment to control or treatment group
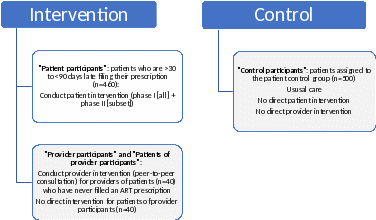
Cluster randomization will occur at the healthcare provider level and will be conducted concurrently with the initial potential participant screening. Providers will be randomized to either the intervention arm or to the usual care (i.e., no intervention or control) arm. Study participants are the patients of the randomized healthcare providers. Participants in the intervention arm will be delegated to either a patient-level or provider-level intervention, depending on need; participants who are > 30 to < 90 days late filling their ARV prescription(s) will receive the patient-level intervention and participants who have never filled an ARV prescription will be delegated to the provider-level intervention. Participants of the provider-level intervention will not receive direct intervention. Instead, the healthcare providers of these patients (henceforth referred to as “provider participants”) will receive the provider-level intervention.
Recruitment and consent
A study Linkage Coordinator will mail all eligible potential participants and provider participants a letter that introduces the study. (Att 5a and Att 5b) A courtesy letter will be sent to the HIV care providers of potential participants, to notify them that some of their patients may be eligible for study participation. (Att 5d) Additionally, a study information sheet will be sent to potential participants and provider participants. (Att 6a and Att 6b)
After randomization, potential participants in the patient-level intervention and potential provider participants in the provider-level intervention will be contacted by a study Linkage Coordinator (Att 7a and Att 7b) who will obtain verbal consent for study participation (Att 8a and Att 8b). The Linkage Coordinator will also get verbal permission from participants in the patient-level intervention to access participants’ identifiable health information (HIPPA authorization). (Att 8d)
Consent procedures will be deferred until after the follow-up period for the intervention arm is complete, for the following groups: 1) eligible potential participants of the provider-level intervention (i.e., patients of the providers who receive the provider-level intervention) and 2) eligible potential participants in the usual care arm (i.e., control participants). Neither of these groups will receive direct intervention. Consenting procedures will be deferred to minimize response bias, which could alter study outcomes.(12) After the follow-up period for the intervention arm is complete, these individuals (both the participants of the provider-level intervention and the control participants) will be mailed an introductory letter (Att 5c) and be contacted by a study Linkage Coordinator (Att 7c)who will obtain verbal consent (Att 8c), for retrospective use of the participants’ health information (Att 3 and Att 4), and HIPPA authorization (Att 8d).
All verbal consent will be documented in a Research Electronic Data Capture (REDCap) database; REDCap is a secure web application for building and managing online surveys and databases.
Patient-level intervention
The patient-level intervention has two phases (Phase I and Phase II) both of which start with a phone consultation with a study Linkage Coordinator. Phase I is intended for patients who are > 30 to < 60 days late filling their ARV prescription(s). The phone consultation will consist of a semi-structured interview. (Att 9) The interview is intended to be a fluid conversation with the participant designed to elicit the participant’s adherence barriers. The Linkage Coordinator will use open-ended questions and then tailor the conversation based on the participant’s responses. Once the participant’s adherence barriers are identified, the patient will be referred (Att 11) to appropriate resources at the participant’s Medicaid Care Organization, or to the participant’s HIV care provider, or to a pharmacist or community resource to assist them in overcoming their adherence barrier(s). If the participant fills their ARV prescription within the subsequent 30 days, the participant will not be contacted further.
Phase II is intended for participants who were enrolled in Phase I who failed to fill their ARV prescriptions in the subsequent 30 days of the Phase I consultation, and for participants who are > 60 to < 90 days late at the time the participant was determined to be study eligible. In Phase II, the Linkage Coordinator will lead a similar semi-structured interview (Att 10) as in Phase I but will probe for more complex adherence barriers (e.g., mental health concerns) and make referrals accordingly. (Att 11)
In Phase II, the participant will also be offered PositiveLinks, an evidence-informed mobile application (“app”) which is designed to support ART adherence and retention in care (https://www.positivelinks4ric.com/).[11] PositiveLinks provides daily queries of stress, mood, and medication adherence; weekly quizzes on general and HIV-specific understanding; appointment and medication reminders, curated resources, a community message board, direct messaging with the study Linkage Coordinator, and contact information for patients’ providers and pharmacies. The Linkage Coordinator will verbally consent and enroll the participants into PositiveLinks. (Att 12a) The enrollment process includes explanation of and participant verbal consent to the previously established app terms of use. Participants who are unsure if they want to enroll into PositiveLinks, may be mailed a copy of the app’s terms of use for review. (Att 12b) The enrollment process also includes: helping participant set up the app for iOS or Android; walking participant through the app login; describing home page features; sending test messages; explaining where contacts are located within the app and having participant practice putting in contact information; setting up the calendar and reminders; and having participant demonstrate understanding of app features. For each participant, a Linkage Coordinator will monitor app usage (i.e., app launches) and follow-up with each participant at one, two, four and twelve weeks to determine if they are having any difficulty using the app and resolve any usability and accessibility problems (e.g., helping participant to reset passwords). Each Virginia Medicaid Care Organization can provide smartphones for their enrollees if necessary.
Provider-level intervention (peer-to-peer clinician consultation)
The provider-level intervention will consist of a peer-to-peer clinician consultation delivered by clinicians from VDH’s Advisory Committee to the Virginia Medication Assistance Program or by another HIV clinical expert. The Advisory Committee provides VDH with clinical expertise on AIDS Drug Assistance Program (ADAP) formulary changes and educational, clinical and social issues concerning HIV treatment. Advisory Committee members represent all five health regions across Virginia and include urban and rural practitioners from a variety of academic and other health systems. Based on the location in which the provider participant practices, an Advisory Committee member or another HIV clinical expert will be assigned as the clinician consultant. The peer-to-peer clinician consultations will occur via phone or in-person, depending on the preference of the provider participant.
The peer-to-peer clinician consultations will include introduction or reinforcement of HIV clinical guidelines for ART initiation, strategies to optimize ART adherence, and resources for supporting adherence for people with HIV. Using a guide of prompts (Att 13a and Att 13b), the clinician consultant will (as appropriate): discuss reasons for lack of ART prescribing; share best practices, based on current HIV treatment guidelines, on ART initiation; share additional guidance and evidence-based recommendations for strategies that address underlying barriers to patient adherence; and give the provider a list of referral or navigation services available for their patients with HIV that may address social or structural barriers to adherence (e.g., locations of nearby substance use treatment facilities). The guide of prompts will be used to elicit information that can be used to inform the consultation. The consultation will be tailored to the needs of the provider participant. After the consultation, the clinician consultant will document the provider participant’s barriers to ART prescribing and recommended resources in a brief post-consultation questionnaire. (Att 14a and Att 14b)
Study outcomes
All outcome analyses will be conducted at the patient level. Participants will be followed for 12 months, either prospectively (for the participants of the patient-level intervention) or retrospectively (for the controls and participants of the provider-level intervention) to compare the primary study outcome of HIV viral suppression (HIV RNA <200 copies/mL). Secondary outcomes are initiation, re-initiation, persistence and adherence to ART, and retention in care. Logistic regression models will be used to test the difference in HIV viral suppression in the intervention arm compared to the control arm after adjusting for potential confounders. The differences in the means of secondary outcomes (e.g., persistence) between intervention and control groups will be tested using a t-test, with a linear regression model used to evaluate the effect, after adjusting for confounders.
Virginia Commonwealth University will be given DMAS and VDH affiliate status which allows them to access the Virginia Medicaid and Virginia Care Marker databases on the DMAS and VDH servers, respectively. Data necessary for the study will be placed in study specific files on the secure DMAS and VDH servers by DMAS and VDH personnel. VCU will only have access to the study files. VCU will have access to these data through study-specific amendments to existing cross-agency and cross-institutional data use agreements.
The AIMS study will collect data for the patient- and provider-level interventions. The information collection has five primary components: 1) Virginia Medicaid claims abstraction 2) Virginia Care Marker data abstraction 3) Phase I and Phase II patient-level interviews 4) Peer-to-peer clinician consultation and 5) PositiveLinks mobile app abstraction.
Virginia Medicaid data abstraction:
The Virginia Medicaid data are existing data that are not collected for the purpose of this study but are routinely collected by DMAS for payment of administrative insurance claims. The Virginia Medicaid database contains demographics and medical diagnosis, procedure and pharmacy claims for Virginia Medicaid enrollees. The database also includes identifying information such as name, contact information, and prescriber information. Secondary data from the Virginia Medicaid database will be abstracted. The purpose of the data abstraction is three-fold: 1) to determine study eligibility 2) to conduct the patient- and provider-level interventions and 3) to determine study outcomes.
A VCU Data Analyst will abstract data elements listed in Att 3 to determine study eligibility. These data will be abstracted monthly until 500 participants of the patient-level intervention and provider participants are enrolled and 500 controls are identified; enrollment is estimated to take six months to complete. These data will be securely transferred to the VDH server where they will be matched to the Virginia Care Marker data; the match will determine study eligibility. Although these database queries will abstract personally identifiable information (PII) no PII will be accessed outside of the DMAS servers (which routinely contain these data) and none will be sent to CDC. The PII collected will also be used to conduct the patient- and provider-level interventions (e.g., PII will be used to contact potential participants and enrollees).
After enrollment, data will be abstracted for participants of the patient-level intervention, quarterly for 12 months. Additionally, a one-time abstraction will occur at the end of the intervention follow-up period for the controls and participants of the provider-level intervention; this abstraction will contain 12 months of data retrospective to the date of consent. A VCU Data Analyst will abstract these data. De-identified data elements listed in Att 3 will be sent to CDC, quarterly, for participants of the patient-level intervention, and one time at the end of the intervention follow-up period for the controls and participants of the provider-level intervention. These data will be analyzed to determine study outcomes (e.g., viral suppression).
Virginia Care Marker data abstraction:
The Virginia Care Markers data are existing data that are not collected for the purpose of this study but are routinely collected by VDH for HIV surveillance. The Virginia Care Markers database contains information on all people with HIV in Virginia and includes HIV surveillance data (e.g., HIV viral load, CD4 counts), care reports for persons receiving ADAP benefits, vital status, demographics and some care utilization data for people receiving care within the Ryan White HIV care program (e.g., dates of medical visit, antiretroviral therapy prescriptions). Secondary data from the Virginia Care Markers database will be abstracted. The purpose of the data abstraction is three-fold: 1) to determine study eligibility and 2) to conduct the patient- and provider-level interventions and 3) to determine study outcomes.
A VCU Data Analyst will abstract data elements listed in Att 4 to determine study eligibility. These data will be abstracted monthly until 500 participants of the patient-level intervention and provider participants are enrolled and 500 controls identified; enrollment is estimated to take six months to complete. These data will be matched to the Medicaid data; the match will determine study eligibility. Although these database queries will abstract personally identifiable information (PII) no PII will be accessed outside of the VDH servers (which routinely contain these data) and none will be sent to CDC. The PII collected will also be used to conduct the patient- and provider-level interventions (i.e., PII will be used to contact potential participants and enrollees).
After enrollment, data will be abstracted for participants of the patient-level intervention, quarterly for 12 months. Additionally, a one-time abstraction will occur at the end of the intervention follow-up period for the controls and for participants of the provider-level intervention; this abstraction will contain 12 months of data retrospective to the date of consent. A VCU Data Analyst will abstract these data. De-identified data elements listed in Att 4 will be sent to CDC, quarterly, for participants of the patient-level intervention, and one time at the end of the intervention follow-up period for the controls and participants of the provider-level intervention. These data will be analyzed to determine study outcomes (e.g., viral suppression).
Phase I and Phase II patient-level semi-structured interviews:
A one-time Phase I semi-structured interview (Att 9) will be administered by a study Linkage Coordinator for participants identified as > 30 to < 60 days late filling ARV prescriptions and a one-time Phase II semi-structured interview (Att 10) will be administered by a study Linkage Coordinator for participants identified as > 60 to < 90 days late filling ARV prescriptions. The purpose of the Phase I and II interviews is to identify participants’ adherence barriers; this information will be used to refer participants to appropriate resources to assist their adherence to ART. The Linkage Coordinator will enter all data from the Phase I and II interviews directly into a REDCap database during the interviews. De-identified data elements will be sent quarterly to CDC for analysis to determine study outcomes.
Peer-to-peer clinician consultation:
A one-time peer-to-peer clinician consultation will be administered by a member of VDH’s Advisory Committee to the Virginia Medication Assistance Program or by another HIV clinical expert. The clinician consultant will use a guided prompt to elicit information which can be used to inform the consultation. (Att 13a and 13b) After the consultation, the clinician consultant will document the provider’s barriers to ART prescribing and recommended resources in a brief post-consultation questionnaire. (Att 14a) The questionnaire will be directly entered into a REDCap database, via a secure link provided by the Linkage Coordinator. (Att 14b) De-identified data elements will be sent quarterly to CDC for analysis to determine study outcomes.
PositiveLinks mobile app:
A Linkage Coordinator will download app data through the app’s administrative web portal. These data will be collected quarterly and include response rates for daily queries about medication, mood and stress, response rates for weekly quizzes, posts to the community message board and messages to the study Linkage Coordinator. (Att 15) The Linkage Coordinator will use Google Analytics to measure app launches. For all participants, the Linkage Coordinator will monitor the community message board daily for misinformation and inflammatory comments (which will be removed). The Linkage Coordinator will monitor direct messages daily to respond to participants’ inquiries. De-identified data elements will be sent quarterly to CDC for analysis to determine study outcomes (Att 15).
Schematic Summary of AIMS Study Comparison Groups
Retrospective data collection
(12 months)
Prospective data collection
(12 months)

Schematic Summary of Data Sources, Frequency of Collection, and Uses
Data Source |
1 |
2 |
3 |
4 |
5 |
Virginia Medicaid Data Abstraction |
Virginia Care Marker Database |
Semi-structured Interviews with Patients |
Peer-to-peer Clinician consultation |
PositiveLinks app |
|
Source Type |
Existing Medicaid database for claims administration |
Existing VDH HIV surveillance system |
New information collection for the AIMS study |
New information collection for the AIMS study |
Existing mobile app |
Data Type(s) |
Demographics Medical diagnoses Procedure(s) Prescriptions (Att 3) |
Demographics Lab data (e.g., HIV viral load) Care services (Att 4)
|
Barriers to ART use |
Barriers to ART prescribing |
Queries and reports |
Use(s) |
|
|
Referral to resources to promote/support ART use |
Address barriers to ART prescribing |
Communication with Linkage Coordinator and patient access to peer network and resources |
Frequency of Collection |
Monthly abstraction to identify eligible patients; quarterly abstraction post-enrollment for; patients in control group; retrospective 12-month abstraction for control patients |
Monthly abstraction to identify eligible patients; quarterly abstraction post-enrollment for; patients in control group; retrospective 12-month abstraction for control patients |
One-time Phase I interview for patients in the direct patient intervention group; one-time Phase II interview for Phase I interviewees who fail to fill an ARV prescription within subsequent 30 days |
One-time consultation |
Quarterly |
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Macaluso, Renita (CDC/DDPHSS/OS/OSI) |
| File Modified | 0000-00-00 |
| File Created | 2021-10-20 |
© 2026 OMB.report | Privacy Policy