Instructions for Dialysis Outpatient Facility COVID-19 Module 20SEP2021
09102021_Dialysis Module TOIs_REVISED.docx
National Healthcare Safety Network (NHSN) Coronavirus (COVID-19) Surveillance in Healthcare Facilities
Instructions for Dialysis Outpatient Facility COVID-19 Module 20SEP2021
OMB: 0920-1317
Instructions for Dialysis Outpatient Facility COVID-19 Module
The following definitions for Confirmed SARS-CoV-2 infection and Suspected SARS-CoV-2 infection apply to the data collection questions below.
Confirmed: A patient with a positive SARS-CoV-2 viral test result. The test result may be from a NAAT/PCR or an antigen test but excludes patients with a negative NAAT/PCR confirmatory test. Note: This does not include serology testing for SARS-CoV-2 antibodies.
Suspected: A patient with signs and symptoms suggestive of COVID-19 but has a pending SARS-CoV-2 test result or a specimen for a viral test has not been collected. Note: Patients with a negative SARS-CoV-2 viral test result, but who continue to be managed or treated with the same precautions as patients with a positive SARS-CoV-2 viral test because of exposure or suggestive signs and symptoms should be included in this count.
Important: The current reporting week is defined as the previous Wednesday to Tuesday. Reporting for this time period must be completed by each Wednesday, no later than 3PM ET. You should report on the same day each week, either by the close of business on Tuesday, or on Wednesday by the 3PM deadline. We advise you not to alternate reporting days.
FOR TRANSIENT PATIENTS, reporting should occur in the clinic where they are currently receiving care.
Note: Examples in this document have attempted to cover the majority of cases. In cases where there are specific examples not covered in this document, please contact the NHSN Help desk at [email protected] for reporting guidance.
*indicate field is required to save and complete
**indicate field is conditionally required to save and complete
Facility Operational Information |
|
Data Field |
|
*Facility ID (OrgID) |
The NHSN-assigned facility ID will be auto-populated by the computer |
*Facility Name |
The Facility Name will be auto-populated by the computer. |
*CMS Certification Number (CCN) |
Enter the assigned CCN of your facility or temporary number. |
*Week of Data Collection |
The week of data collection will be auto-populated by the application by selecting the correct reporting week. |
*Date last modified |
The date is auto-populated by the date of last data entry. |
*In-Center Patient Census |
The in-center patient census numbers will be auto-populated from the outpatient dialysis annual survey, but this field should be updated to reflect the current reporting week. |
*Home Patient Census |
The home patient census numbers will be auto-populated from the home survey but this field should be updated to reflect the current week reporting week. |
*Total Certified Stations
|
The total number of certified stations will be auto-populated from the outpatient survey. If the number needs to be changed and you completed an annual survey, you will need to edit your annual survey. |
*Is your facility a designated COVID unit?
|
Select YES if your facility is a designated COVID unit.
|
*Does your facility have a designated COVID shift(s)?
|
Select YES if your facility has a designated COVID shift(s).
|
*Total number of staff (physician, nurses, techs, environmental services, biomed, etc.) who worked at least 1 day during the current reporting week
|
Enter the total number of all staff who worked or were eligible to work in the clinic/facility at least 1 day during the current reporting week.
Entry must be greater than 0.
|
How many patients on the current in-center census reside in nursing homes? |
Enter the total number of in-center patients from your facility’s census that currently reside in nursing homes during the current reporting week.
|
How many patients on the current home census reside in nursing homes? |
Enter the total number of home patients from your facility’s census that currently reside in nursing homes during the current reporting week.
|
SARS-CoV-2 Positive (+) Patients and Staff |
|
Data Field |
Instructions for Data Collection |
*Number of newly confirmed in-center patients during the current reporting week |
Enter the number of newly confirmed SARS-CoV-2 in-center patients in your facility for the current reporting week.
NOTE: Newly confirmed SARS-CoV-2 in-center patients and newly confirmed SARS-CoV-2 home patients must equal the number of positive tests reported for the current reporting week. (See Test for SARS-CoV-2 Infection section for reporting instructions.)
Example: Newly confirmed in-center patients plus newly confirmed home patients equal number of positive tests equal sum of vaccination status fields.
F
The patient may have any of the below positive test results:
Enter the number of patients who have been newly identified with a positive SARS-CoV-2 test result during the previous 7 days (current surveillance period). The current reporting week is Wednesday through Tuesday with data entry on Wednesday for the preceding week.
This includes patients who are currently receiving dialysis treatments at the dialysis facility regardless of the patient’s current status with the facility. For example, Ms. L had a positive SARS-CoV-2 test result on Friday and was transferred to the hospital on Sunday. Ms. L should still be included in the Dialysis Facility Confirmed count for that week.
Note: If a hospitalized patient is found to be positive during the current reporting week, please report as newly confirmed regardless of whether they received treatment at the facility during the current reporting week.
Example: A dialysis facility has started reporting SARS-CoV-2 data to the NHSN Module in November. The NHSN user enters the COVID-19 data in the Module once a week on Wednesdays, which includes new counts from the prior Wednesday through Tuesday of each week (current surveillance period). The count includes all confirmed SARS-CoV-2 patients during the surveillance time period who receive dialysis at the facility, even if they weren’t at the facility when the diagnosis was made. For example, the patient was diagnosed after admission to an acute care hospital or diagnosed at nursing home. The home facility reports all information for their patients even if at the time of reporting the patient has been transferred to a COVID isolation facility.
Notes:
Reinfection: Symptomatic patients who newly test positive greater than 90 days after a previous SARS-CoV-2 test result should be included in the Confirmed SARS-CoV-2 count for the reporting period.
|
*Number of newly confirmed in-center patients during the current reporting week that reside in nursing homes |
Enter the number of in-center patients with newly confirmed SARS-CoV-2 patients that resided in a nursing home at the time of dialysis treatment during the current reporting week.
Note: Newly confirmed in-center patients that reside in nursing homes should be a subset of newly confirmed in-center patients
|
*Number of newly confirmed patients during the current reporting week that are home patients |
Enter the number of newly confirmed SARS-CoV-2 patients that are home patients (home hemodialysis or peritoneal dialysis) during the current reporting week.
NOTE: Newly confirmed SARS-CoV-2 in-center patients and newly confirmed SARS-CoV-2 home patients must equal the number of positive tests reported for the current reporting week. (See Test for SARS-CoV-2 Infection section for reporting instructions.)
Example: newly confirmed in-center patients plus newly confirmed home patients equal number of positive tests equal sum of vaccination status fields.
F
|
*Number of newly confirmed staff during the current reporting week |
Enter the number of newly confirmed SARS-CoV-2 staff during the current reporting week.
|
*Number of SARS-CoV-2 patients who are currently admitted to the hospital during the current reporting week |
Enter the number of SARS-CoV-2 patients who are currently admitted to the hospital. Include patients who were recently diagnosed and hospitalized for COVID-19 during the current reporting week.
|
*Number of confirmed patients currently self-monitoring and continuing in-center therapy during the current reporting week |
Enter the total number of previously confirmed patients who have not recovered and are continuing in-center therapy during the current reporting week.
Include patients who were reported as newly confirmed the previous reporting week and continue to be monitored for SARS-CoV-2 illness.
Exclude patients who are newly confirmed during the current reporting week
|
*Number of confirmed patients currently self-monitoring and continuing home therapy during the current reporting week
|
Enter the number of previously confirmed patients who have not recovered and are continuing home therapy during the current reporting week.
Include patients who were reported as newly confirmed the previous reporting week and continue to be monitored for SARS-CoV-2 illness.
Exclude patients who are newly confirmed during the current reporting week.
|
COVID-19 Vaccine Status for Patients with SARS-CoV-2 Infection |
|
Data Field |
Instructions for Data Collection |
* Number of patients who have tested positive this current reporting week and have not received a COVID-19 vaccine or it has been 13 days or less after dose one.
|
Enter the number of patients who tested positive and have not received a COVID-19 vaccine or patients who received a COVID-19 vaccine, but it has been 13 days or less after Dose 1 of Pfizer or Moderna or the Janssen COVID-19 vaccine.
Date vaccine received is counted as Day 1. |
* Number of patients who have been vaccinated with dose one of the Pfizer-BioNTech COVID-19 vaccine and have tested positive for COVID-19 14 days or more after receiving the vaccine : Dose 1_______ |
Required: Enter the number of patients that test positive 14 days or more after receiving Pfizer-BioNTech COVID-19 vaccine.
Enter number of patients in Dose 1:
AND
If there were no patients that tested positive after receiving Pfizer-BioNTech COVID-19 vaccine, enter zero for Dose 1.
Date vaccine received is counted as Day 1. |
* Number of patients who have been vaccinated with dose one and dose two of the Pfizer-BioNTech COVID-19 vaccine and have tested positive for COVID-19 14 days or more after receiving dose two. Dose 2_______ |
Required: Enter the number of patients that test positive 14 days or more after receiving dose 2 of Pfizer-BioNTech COVID-19 vaccine.
If the COVID-19 test was positive 13 days or less after the second dose, enter number of patients in Dose 1.
If there were no patients that tested positive after receiving Dose 2 of the Pfizer-BioNTech COVID-19 vaccine, enter zero for Dose 2.
Date vaccine received is counted as Day 1. |
* Number of patients who have been vaccinated with dose 1 of the Moderna COVID-19 vaccine and have tested positive for COVID-19 14 days or more after receiving the COVID-19 vaccine: Dose 1 ______ |
Required: Enter the number of patients that test positive 14 days or more after receiving the Moderna COVID-19 vaccine.
Enter number of patients in Dose 1:
AND
If there were no patients that tested positive after receiving Dose 1 of the Moderna COVID-19 vaccine, enter zero for Dose 1. Date vaccine received is counted as Day 1. |
* Number of patients who have been vaccinated with dose 1 and dose 2 of the Moderna COVID-19 vaccine and have tested positive for COVID-19 14 days or more after receiving the COVID-19 vaccine: Dose 2 _______ |
Required: Enter the number of patients that test positive 14 days or more after receiving dose 2 of Moderna COVID-19 vaccine.
As explained above, if the COVID-19 test was positive 13 days or less after the second dose, enter number of patients in Dose 1.
If there were no patients that tested positive after receiving Dose 2 of the Moderna COVID-19 vaccine, enter zero for Dose 2.
Date vaccine received is counted as Day 1. |
*Number of patients who have been vaccinated with Janssen COVID-19 vaccine and have tested positive 14 days or more after receiving the COVID-19 vaccine Dose 1 _______ |
Required: Enter the number of patients that test positive 14 days or more after receiving the Janssen COVID-19 vaccine.
If there were no patients that tested positive after receiving the Janssen COVID-19 vaccine, enter zero.
Date vaccine received is counted as Day 1. |
* Number of patients who have been vaccinated with dose 1 of an Unspecified COVID-19 vaccine and have tested positive for COVID-19 14 days or more after receiving the COVID-19 vaccine:
____________ |
Required: Enter the number of patients that test positive 14 days or more after receiving an initial dose of an Unspecified COVID-19 vaccine.
This category also includes patients who have an unknown number of doses as well as an unspecified manufacturer.
Enter number of patients in Dose 1:
AND
If there were no patients that tested positive after receiving an Unspecified COVID-19 vaccine, enter zero for Dose 1.
Date vaccine received is counted as Day 1. |
* Number of patients who have been vaccinated with dose 1 and dose 2 an Unspecified COVID-19 vaccine and have tested positive for COVID-19 14 days or more after receiving the COVID-19 vaccine.
____________ |
Required: Enter the number of patients that test positive 14 days or more after receiving a complete series.
Unspecified should be used in the event of receiving a primary series comprised of an unknown manufacturer, mixed COVID-19 vaccines (for example: one Pfizer vaccine and one Modern vaccine), or if patient’s vaccination status is unknown.
The category also includes Individuals who received all recommended doses of a COVID-19 vaccine that is neither approved nor authorized by FDA but listed for emergency use by the World Health Organization (WHO) if they provide documentation of vaccination. Please refer to Interim Clinical Considerations for Use of COVID-19 Vaccines and CDC for the complete list of COVID-19 vaccines that have received an emergency use listing from WHO.
If there were no patients that tested positive after receiving an Unspecified COVID-19 vaccine, enter zero.
Date vaccine received is counted as Day 1. |
|
|
COVID-19 Vaccine Status for Patients with SARS-CoV-2 Infection Additional Dose |
|
Data Field |
|
* Number of patients who have received an additional dose or booster dose of the Pfizer-BioNTech COVID-19 vaccine and have tested positive for COVID-19 14 days or more after receiving the additional dose or booster dose: Additional dose or booster_______ |
*Required: Enter the number of patients that test positive 14 days or more after receiving an additional dose of Pfizer-BioNTech COVID-19 vaccine.
NOTE: Any patient who has received an additional or booster dose of the Pfizer-BioNTech COVID-19 vaccine and has tested positive should be reported as also having received a completed primary series in the above COVID-19 Vaccine Status section. . |
* Number of patients who have received an additional dose or booster dose of the Moderna COVID-19 vaccine and have tested positive for COVID-19 14 days or more after receiving the additional dose or booster dose. Additional dose _______ |
*Required: Enter the number of patients that test positive 14 days or more after receiving an additional dose of Moderna COVID-19 vaccine.
NOTE: Any patient who has received an additional or booster dose of the Moderna COVID-19 vaccine and has tested positive should be reported as also having received a completed primary series in the above COVID-19 Vaccine Status section. |
* Number of patients who have received an additional dose or booster dose of the Janssen COVID-19 vaccine and have tested positive for COVID-19 14 days or more after receiving the additional dose or booster dose. Additional dose _______ |
*Required: Enter the number of patients that test positive 14 days or more after receiving an additional dose or booster dose of a Janssen COVID-19 vaccine.
NOTE: Any patient who has received an additional or booster dose of the Janssen COVID-19 vaccine and has tested positive should be reported as also having received a completed primary series in the above COVID-19 Vaccine Status section. |
* Number of patients who have received an additional dose or booster dose of an Unspecified COVID-19 vaccine and have tested positive for COVID-19 14 days or more after receiving the additional dose or booster dose. Additional dose _______ |
*Required: Enter the number of patients that test positive 14 days or more after receiving an additional dose or booster dose of an Unspecified COVID-19 vaccine.
NOTE: Any patient who has received an additional or booster dose of an Unspecified COVID-19 vaccine and has tested positive should be reported as also having received a completed primary series in the above COVID-19 Vaccine Status section. |
SARS-CoV-2 Positives (+) that have recovered |
|
Data Field |
Instructions for Data Collection |
*Number of Patients recovered during the current reporting week |
Enter the number of patients that are known to have recovered from Wednesday to Tuesday of the current reporting week.
Note: Recovered means no longer treated as having COVID-19 (e.g., resolved signs/symptoms and no longer being cared for using transmission-based precautions).
|
*Number of new Staff recovered during the current reporting week |
Enter the number of staff that are known to have recovered from Wednesday to Tuesday of the current reporting week.
Note: Recovered means no longer treated as having COVID-19 (e.g., resolved signs/symptoms and no longer being cared for using transmission-based precautions).
|
Suspected or Confirmed SARS-CoV-2 deaths |
|
Data Field |
Instructions for Data Collection |
*Number of patients with suspected or confirmed SARS-CoV-2 infection that have died during the current reporting week |
Enter the number of patients with suspected or confirmed SARS-CoV-2 infection that have died during the current reporting week.
Suspected or confirmed SARS-CoV-2 Deaths is defined as a staff member with suspected or confirmed SARS-CoV-2 viral test result who died before fully recovering from SARS-CoV-2 infection.
|
*Number of staff with suspected or confirmed SARS-CoV-2 infection that have died since last reporting |
Enter the number of staff with suspected or confirmed SARS-CoV-2 infection that have died during the current reporting week.
Suspected or confirmed SARS-CoV-2 Deaths is defined as a staff member with suspected or confirmed SARS-CoV-2 viral test result who died before fully recovering from SARS-CoV-2 infection.
|
Staff and/or Personnel Impact |
|
Data Field |
Instructions for Data Collection |
Will your facility have a critical shortage of staff and/or personnel within the next week? (Select: Yes or No). |
Select “Yes” if your facility is currently experiencing a critical staff and/or personnel shortage in the following groups: Nursing Staff: registered nurse, licensed practical nurse, vocational nurse Clinical Staff: physician, physician assistant, advanced practice nurse Tech: dialysis technician Other staff or facility personnel, regardless of clinical responsibility or patient contact not included in the categories above (for example, environmental services, biomed)
Select “No” if your facility is not currently experiencing a critical staff and/or personnel shortage.
Note: Each organization should define critical staffing shortage based on facility-specific needs.
|
Supplies & Personal Protective Equipment (PPE) |
|
Data Field |
Instructions for Data Collection |
Do you currently have any supply? (Select: Yes or No)
|
Select “Yes” if your facility currently has a supply of the following supplies & PPE:
Select “no” if your facility does not have a supply for the supply items. If no, next question will automatically populate as “no”.
|
Do you have enough for one week if using conventional strategies? (Select: Yes or No). |
Select “Yes” if your facility currently has a supply of the following supplies & PPE for at least one week
Select “no” if your facility does not have a week’s supply for the supply items.
Note: Answer question based on use of conventional strategies for PPE.
|
Laboratory Testing |
|
Data Field |
Instructions for Data Collection |
Does your facility have the ability to collect specimens onsite for SARS-CoV-2 testing?
|
Select “yes” if your facility can perform onsite sample collection for SARS-CoV-2 tests. These samples can be sent offsite for testing.
Select “no” if your facility does not have onsite sample collection for SARS-CoV-2 tests
Additional fields requiring responses will open depending on this response.
|
**If yes, what types of specimens are being collected? |
Conditionally Required. If answered “yes” to: Does your facility have onsite sample collection for SARS-CoV-2, select which tests are being performed. Select all that apply.
|
**If yes, to viral (PCR) tests, what types of specimens are being collected? |
Conditionally Required. If answered “yes” to Viral (PCR) test are performed at your facility, indicate which type of sample is being collected. Select all that apply.
|
**If no, indicate reasons why specimens are not being collected onsite for SARS-CoV-2 testing? |
Conditionally Required. If answered “no” to: Does your facility have the ability to collect specimens onsite for SARS-CoV-2 testing, indicate why your facility does not have the ability to collect specimens onsite for SARS-CoV-2 testing. Select all that apply.
Lack of recommended personal protective equipment (PPE) for personnel to wear during specimen collection Lack of supplies for specimen collection Lack of access to a laboratory for submitting specimens Lack of access to trained personnel to perform testing Uncertainty about testing reimbursement
Notes:
|
**If yes, does your facility have an in-house point-of-care test machine (capability to perform SARS-CoV-2 testing within your facility)? (Select: Yes or No)
|
Select YES or NO if your facility has an in-house point-of-care test machine that is capable of performing SARS-Co-V-2 testing.
In-house is defined as available for use within your dialysis facility.
|

| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | Instructions for Dialysis Outpatient Facility COVID-19 Module |
| Subject | NHSN Dialysis COVID-19 Module |
| Author | CDC/DDID/NCEZID/DHQP |
| File Modified | 0000-00-00 |
| File Created | 2021-10-11 |
© 2026 OMB.report | Privacy Policy
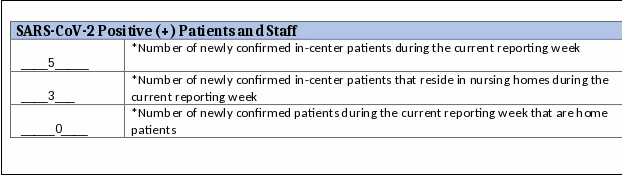 or
Example:
Facility A had 5
newly confirmed in-center patients, 3 nursing home patients, and 0
home patients. The user should enter the following values for the
relevant fields:
or
Example:
Facility A had 5
newly confirmed in-center patients, 3 nursing home patients, and 0
home patients. The user should enter the following values for the
relevant fields: