1-blss_ssa_20210219
1-BLSS_SSA_20210219.docx
Blood Lead Surveillance System (BLSS)
OMB: 0920-0931
Blood Lead Surveillance System (BLSS)
OMB Control No. 0920-0931 (Expiration Date: 05/31/2021)
Request for Extension
National Center for Environmental Health
National Institute for Occupational Safety and Health
Supporting Statement Part A –
Justification
Program Official:
Monica Leonard, CDR
Branch Chief (Acting), Lead Poisoning Prevention and Environmental Health Tracking Branch (LPPEHTB)
Division of Environmental Health Science and Practice (DEHSP)
National Center for Environmental Health (NCEH)
Centers for Disease Control and Prevention (CDC)
4770 Buford Hwy N.E., S106-5
Atlanta, GA 30341
Email: [email protected]
Phone: (404) 498-1826
Fax: (770) 488-3635
Date: February 19, 2021
Table of Contents
A.1. Circumstances Making the Collection of Information Necessary 3
A.2. Purpose and Use of the Information Collection 5
A.3. Use of Improved Information Technology and Burden Reduction 8
A.4. Efforts to Identify Duplication and Use of Similar Information 11
A.5. Impact on Small Businesses or Other Small Entities 13
A.6. Consequences of Collecting the Information Less Frequently 13
A.7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5 14
A.9. Explanation of Any Payment or Gift to Respondents 18
A.10. Protection of the Privacy and Confidentiality of Information Provided by Respondents 18
A.11. Institutional Review Board (IRB) and Justification for Sensitive Questions 21
A.12. Estimates of Annualized Burden Hours and Costs 22
A.13. Estimates of Other Total Annual Cost Burden to Respondents and Record Keepers 24
A.14. Annualized Cost to the Federal Government 25
A.15. Explanation for Program Changes or Adjustments 26
A.16. Plans for Tabulation and Publication and Project Time Schedule 27
A.17. Reason(s) Display of OMB Expiration Date is Inappropriate 28
A.18. Exceptions to Certification for Paperwork Reduction Act Submissions 29
Goal of the information
collection: This
CDC extension information collection request (ICR) includes two data
collection systems that
continue to provide a coordinated, comprehensive, and systematic
public health approach to the surveillance and monitoring of
blood lead levels
(BLLs) for children and occupationally exposed adults in the U.S.
The National Center
for Environmental Health (NCEH) supports
state and local health departments to collect and report
individual-level, laboratory-reported blood lead surveillance data
for children less than 16 years of age
to the Childhood Blood Lead Surveillance (CBLS) system.
The National Institute
for Occupational Safety and Health (NIOSH) works with state labor
and health departments to collect and report laboratory-based blood
lead surveillance data from adults, age 16 years and older, most of
whom are occupationally exposed, to the Adult Blood Lead
Epidemiology and Surveillance (ABLES) program. Intended
use of the resulting data: Data
generated and analyzed from these two programs provide critical
information to monitor trends in BLLs over time. This information is
used for program implementation, policy development, and to target
population-based interventions to children at high risk for lead
exposure and adults who may be exposed to lead in the workplace. Methods
to be used to collect: State
and local health departments submit standardized CBLS data on a
quarterly basis via the
CDC’s Secure Access Management System’s (SAMS) Secure
Data eXchange’s (SDX) integrated Managed File Transfer (MFT)
Platform. CDC staff
import and store the CBLS data on a secure CDC network drive for
processing, analysis, and generation of reports. State health and
labor department’s submit ABLES data on an annual basis
through secure encrypted FTP sites. ABLES data are stored on secure
CDC network drives for processing, analysis, and generation of
reports.
Subpopulation
of interest: Respondents
include state or local agencies, or their bona fide agents, who
submit blood lead surveillance data for children under 16 years of
age to NCEH (n=60) and for adults 16 years of age or older, most of
whom are occupationally exposed, to NIOSH (n=40).
How
data will be analyzed: Data
are analyzed by CDC staff to calculate descriptive statistics and
explore factors that may be related to differences in exposure
levels by location, potential sources of exposure, and over time.
A.1. Circumstances Making the Collection of Information Necessary
The Centers for Disease Control and Prevention (CDC) is requesting a 3-year Paperwork Reduction Act (PRA) clearance for an extension information collection request (ICR) titled the “Blood Lead Surveillance System (BLSS)” [OMB Control Number 0920-0931; expiration date: May 31, 2021]. BLSS is a continuation of a long-term collaboration between the National Center for Environmental Health (NCEH) and the National Institute for Occupational Safety and Health (NIOSH) that has been operating under an approved combined ICR since 2005. Details of the requested extension are provided in Section A.15.
The Childhood Blood Lead Surveillance (CBLS) system is maintained by NCEH. The NCEH Childhood Lead Poisoning Prevention Program (CLPPP)’s mission, to eliminate childhood lead poisoning as a public health problem, is aligned with the Department of Health and Human Services’ (HHS) Healthy People 2030 (HP2030) goal to reduce blood lead levels (BLLs) in children.1 This data collection is authorized under Sections 301(a), 317A and 317B of the 1944 of the Public Health Service Act (42 U.S.C. 241) as amended by the 1988 Lead Contamination Control Act. 2 In addition, this program is also authorized under Section 4002 of the Patient Protection and Affordable Care Act of 2010 (ACA), Public Law (PL) 111-148, (42 U.S.C. Section 300u-11) and under Section 2204 of the Water Infrastructure Improvements for the Nation (WIIN) Act of 2016 (PL 114-322) (Attachment 1a).
The Adult Blood Lead Epidemiology and Surveillance (ABLES) program is conducted by NIOSH. NIOSH’s mission is to promote safety and health at work for all people through research and prevention. NIOSH was established under Section 22 (29 USC 671), and its general authority to conduct the data collection is found in Section 20 (29 USC 669), of the 1970 Occupational Safety and Health Act (Public Law 91-596), as amended (Attachment 1b). Tracking of occupational hazards, exposures, injuries and illnesses is an integral part of the NIOSH mission. This is central to accomplishing ABLES main public health objective of reducing the number of workers with elevated BLLs aligned with the HP2020 objective OSH-7.3
The overarching goal of this information collection is to continue the blood lead surveillance collection for children and occupationally-exposed adults in the U.S. Currently, 53 state and local NCEH-sponsored CLPPPs report information to the CBLS system, and NCEH anticipates funding up to 7 more CLPPPs in FY21 (n=60 total). Additionally, up to 40 states will report information to NIOSH ABLES program.
The 60-day Federal Register Notice for an extension of the BLSS ICR was published on October 13, 2020, Vol. 85, No. 198, pp. 64474 (Attachment 2) and is further discussed in Section A.8.
A.2. Purpose and Use of the Information Collection
This information collection request covers two separate CDC data collection systems with individualized program goals that have been operating under a combined PRA clearance since 2005. The childhood and adult blood lead surveillance data are collected under different statutory mandates, as mentioned in Section A.1.
Childhood Blood Lead Surveillance (CBLS)
CDC’s CLPPP compiles state surveillance data for children less than 16 years of age into a national CBLS system. More information is available at: https://www.cdc.gov/nceh/lead/data/.
The goal of the NCEH CLPPP is to promote primary prevention of exposure to lead in children, and, as a secondary prevention strategy, to promote blood lead testing and surveillance of BLLs in children to ensure that there is a comprehensive system in place for the identification, referral, case management, and follow-up evaluation of lead-exposed children.
In February 1991, the “Strategic Plan for the Elimination of Childhood Lead Poisoning” (HHS, 1991), recommended four immediately essential elements of the effort to eliminate childhood lead poisoning in the U.S., including establishment of national surveillance for children with elevated blood lead levels (BLLs). In 1994, CDC’s CLPPP initially proposed and began collecting surveillance data on BLLs in children less than 16 years of age (Pertowski, 1994) [OMB Control Number: 0920-0337; expiration 04/30/2012]. In 1995, the Council of State and Territorial Epidemiologists (CSTE) designated elevated BLLs as the first noninfectious condition to be notifiable at the national level (CDC, 1996; CDC, 2016). However, mandatory laboratory reporting of all blood lead test results, not just “elevated” BLLs, to state health departments is an essential element of successful childhood blood lead surveillance programs because it allows programs to calculate the denominator of children tested in the state.
According to Federal law, all children enrolled in Medicaid are required to receive blood lead tests at ages 12 months and 24 months. Any child between 24 and 72 months with no record of a previous blood lead test must receive a “catch-up” blood lead test. Additionally, states may have their own laws and regulations regarding more stringent requirements for blood lead testing and reporting of blood lead test results to the state health department.
CBLS collects all laboratory and clinician-reported BLL test results on individual children reported to participating state or local CLPPPs. The de-identified, individual-level case management and follow-up data are forwarded to CDC, imported into CBLS, and a consistent “case” definition is then applied. This allows for comparison across programs (jurisdictions) that would otherwise not be possible. Due to differences in jurisdictional screening practices and laboratory reporting requirements, these data do not provide for valid nationally representative incidence or prevalence estimates. However, when a consistent case definition is applied these data are useful for estimating needs at the Federal, state, and local level which is important for establishing national program goals and objectives.
Currently, CDC funds 53 state and local health departments for lead prevention and surveillance activities (Attachment 3a & 3b). CDC is also planning to fund up to 7 additional awardees over the next fiscal year (total n=60). For FY 2021, CDC has implemented a one-year extension of supplemental funding (Attachment 3c & 3d) of the three-year cooperative agreement (Attachment 3a) and the two-year cooperative agreement, (Attachment 3b). Funding for years 2 and 3 of this ICR is still under consideration by CDC senior leadership.
As part of the funding agreements, cooperative agreement recipients are required to compile and report blood lead surveillance data to CDC on a quarterly basis. The information is used by CDC to monitor short-term trends, progress toward elimination of lead hazards, and to oversee programmatic activities in a timely fashion. Population surveillance of children’s BLLs provides information on how well we are protecting all children from exposure to lead and also provides critical information needed to identify and care for those individual children who are already exposed. Blood lead surveillance data provide the foundation for targeting prevention activities to high risk areas. A summary of CBLS program milestones and accomplishments is found in Attachment 4a.
Adult Blood Lead Epidemiology and Surveillance (ABLES)
The Adult Blood Lead Epidemiology and Surveillance (ABLES) program, a part of CDC’s NIOSH, compiles state surveillance data for adults 16 years of age or greater, most of whom are occupationally exposed. More information is available at: https://www.cdc.gov/niosh/topics/ables/. ABLES is a long-standing state-based surveillance program of laboratory-reported adult BLLs aimed at addressing the national health problem of lead exposure in the workplace. A summary of ABLES program milestones and accomplishments is found in Attachment 4b.
In the U.S., over 95% of adults with BLLs over 25 µg/dL are related to occupational exposure. About 90% of these workers are employed in four main industry sectors4a: manufacturing (NAICS 31-33), construction (NAICS 23), services (NAICS 51-56, 61, 71, 72, 81, 92), and mining (NAICS 21); however, lead is also found in other industries. Moreover, many workers continue to report very high BLLs (above 40 µg/dL) whereas the U.S. national average BLL for adults is 0.92 µg/dL. Pregnant and breastfeeding women employed in lead-related industries may pass lead to their unborn baby or breastfeeding infant. In addition, workers exposed to lead may contaminate their clothing, cars, and homes resulting in lead exposure to their children and others in their household. This is of concern because lead exposure causes acute and chronic adverse effects in multiple organ systems, and even BLLs below 10 µg/dL can negatively affect the neurological, cardiovascular, and reproductive systems. Collecting blood lead data from workers is, therefore, needed to track exposures and to identify interventions that reduce or prevent lead exposures.
Per 29 CFR Part 1910 and 29 CFR Part 1926, the Occupational Safety and Health Administration (OSHA) mandates that medical surveillance, including regular blood lead tests, be conducted for workers who are exposed to an airborne concentration of lead of 30 µg/m3 for 30 or more days. OSHA estimates that approximately 804,000 workers in general industry and an additional 838,000 workers in construction are potentially exposed to lead4b. In addition, states have reporting laws requiring laboratories and providers to submit blood lead results to the health department. States then share these data with NIOSH for monitoring occupational blood lead data and for identifying interventions to reduce or prevent occupational lead exposures. While the current reference BLL is 5 µg/dL, obtaining work-relatedness and industry information for all adults with BLLs >5 µg/dL is resource intensive due to the vast number of cases. Few states are staffed to follow up on these <25 µg/dL cases. Therefore, NIOSH accepts any occupational data that states can provide. Additional details are provided in Section A.3.
Occupational blood lead data are used by states, OSHA, and NIOSH to monitor and develop policies to reduce occupational lead exposure by targeting unsafe conditions or high hazard industries. Case-level data are not shared outside NIOSH. Since over 95% of lead exposure is work-related, ABLES data cannot be generalized to the U.S. population. Aggregated data are available via the NIOSH Workers Health Charts5 and the ABLES website.6 States use the data to provide guidance and information to workers and employers. States also share data with the OSHA for enforcement and compliance assistance activities. In 2008, OSHA updated its “National Emphasis Program – Lead” to reduce occupational lead exposure by targeting unsafe conditions or high hazard industries. OSHA used ABLES data to identify industries where elevated BLLs indicate a need for increased focus. The data are used to track occurrence of elevated BLLs and to provide essential information for setting priorities and goals for research and intervention (e.g., HP2020 OSH-7 objective). In 2010, CDC adopted adult blood lead as a national notifiable condition. ABLES data were also used in support of updates to the case definition for an elevated BLL.
A.3. Use of Improved Information Technology and Burden Reduction
Reporting to the NCEH CBLS System: All CBLS reporting is done by electronic means. CDC software for blood lead surveillance is developed and provided free of charge to state and local programs. Prior to 2007, the software designed and deployed by CDC was called “Systematic Tracking of Elevated Lead Levels and Remediation (STELLAR).” “Healthy Homes and Lead Poisoning Software System (HHLPSS)” is now used by many CLPPPs to collect blood lead surveillance and case management follow-up data. HHLPSS also includes specialized modules for clinical and environmental follow-up for use at the local level only; however, NCEH only requires delivery of certain blood lead case management and follow-up data. NCEH supports ongoing maintenance and development to assure that the software is up-to-date and meets programs’ evolving needs. An additional benefit of using this CDC-deployed software is that surveillance data extracts are built into the system. Some programs, however, use state-based IT systems. All respondent programs have a computerized system for collecting and managing blood lead surveillance data. These programs extract CBLS surveillance data and submit to CDC using the required ASCII text file format (CBLS Variable List).
The existing NCEH ICR “CBLS Variables” text file (Attachment 5a) employed by respondents to extract CBLS surveillance data has been updated to include an approved non-substantive change to the race variable options/value sets7 as well as the inclusion of revised general guidance language provided for respondents to improve understanding of CBLS Variables. In addition, based on past experience with a single recipient from a local jurisdiction, NCEH anticipates that one respondent will continue to report annual “CBLS Aggregate Records” in electronic spreadsheet format (Attachment 5b).
Figure A.3.1 provides a graphical overview of the updated respondents’ data delivery into NCEH Childhood Blood Lead Surveillance (CBLS) System.
Procedures used by respondents for data delivery into the NCEH Childhood Blood Lead Surveillance (CBLS) System will be upgraded to employ CDC’s Secure Access Management System’s (SAMS) Secure Data eXchange’s (SDX) integrated Managed File Transfer (MFT) Platform.
Figure
A.3.1.
Overview of the Updated Childhood Blood Lead Surveillance System
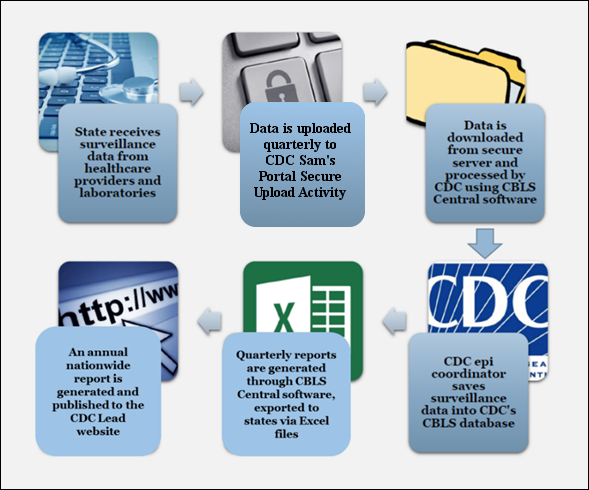
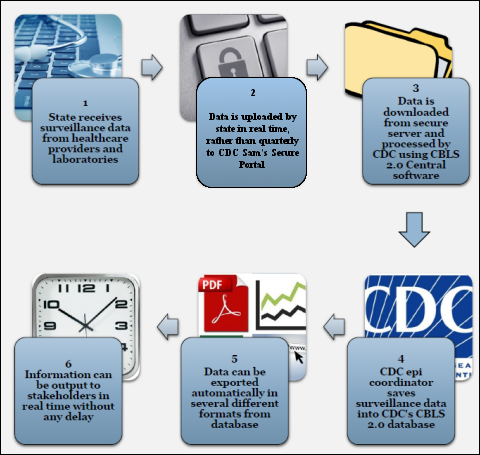
Figure A.3.2 provides an overview of the “Next Generation” CBLS which will be a web-based system that is undergoing development and testing. One of the goals of this developing data system is to provide access to information collection in a “near real-time” manner with less of a delay than in previous systems.
Figure A.3.2. Overview of the “Next Generation” Childhood Blood Lead Surveillance System
Reporting to the NIOSH ABLES System: All participating states transmit adult blood lead data to NIOSH in electronic format (e.g., Excel spreadsheet, CSV format, Access database). States collect adult BLL data from laboratories and physicians. Most of these adults are employed in jobs and industries with potential for lead exposure. OSHA mandates lead surveillance of adults occupationally exposed to lead. States follow-up with workers to confirm industry of employment and occupation; identify work-relatedness of lead exposure; and submit de-identified data annually to NIOSH. Blood lead data transmitted to NIOSH include case-based records or aggregated tables, and a brief narrative report describing any notable lead surveillance activities. An estimated 80% of states will report case records (Attachment 6a) in the required format (Attachment 6a1). An estimated 20% of states may lack adequate resources to submit case records and, to accommodate this situation, they send data to NIOSH in aggregated form (Attachment 6b). States complete either the case-based form or the aggregated form, but not both.
From 1987-2013, NIOSH provided funding that resulted in the expansion of the ABLES program from 4 to 41 states. However, Federal funding for state ABLES programs was discontinued in September 2013. As of August 2020, 37 states collaborate with NIOSH to conduct adult BLL surveillance, and among them, 28 states have submitted blood lead data for 2018. Since 2016, ABLES has been working with NCEH CBLS program to incorporate adult blood lead information into the Healthy Home and Lead Poisoning Software System (HHLPSS). This ongoing initiative was encouraged by state partners to help them reduce personnel and time burden of data collection and data management. At least two states independently modified HHLPSS to collect and manage adult blood lead data.
A.4. Efforts to Identify Duplication and Use of Similar Information
The ICR represents two data collection systems that, when combined, provide a coordinated, comprehensive, and systematic public health approach to the surveillance and monitoring of BLLs for children and occupationally exposed adults in the U.S. NCEH supports state and local health departments to collect and report individual-level, laboratory-reported blood lead surveillance data for children less than 16 years of age to the CBLS system. NIOSH collaborates with state labor and health departments to collect and report laboratory-based blood lead surveillance data from adults, age 16 years and older, most of whom are occupationally exposed, to the ABLES program. No other data collection systems provide the level of detail available under this information collection that is used for program implementation, policy development, and to target population-based interventions at the state and local level.
CDC’s National Health and Nutrition Examination Survey (NHANES) collects BLLs from a nationally representative subset of noninstitutionalized adults and children in the U.S. NHANES data provide the ability to generate a valid estimate of the U.S. population distribution of BLLs (CDC, 2018). Among the statistics generated from NHANES data are the geometric mean BLLs and the national prevalence estimates of BLLs ≥5 µg/dL and ≥10 µg/dL among children and adults. These estimates provide the best available evidence for the U.S. population overall, but the sample size and survey design do not allow for generating estimates at the state or local level. CDC uses BLLs from NHANES to assess national progress towards the HP2020 goals to 1) reduce BLL in children aged 1–5 years and 2) reduce the mean BLLs in children.
In contrast to NHANEs data on children, CBLS respondents report laboratory and clinician-reported BLL test results on individual children. Although these data cannot be used to generate nationally representative estimates, due to differences in jurisdictional screening practices and laboratory reporting requirements, these data are useful for estimating needs at the Federal, state, and local level when a consistent case definition is applied.
Additionally, unlike NHANES adult data, ABLES data are used to describe adult occupational exposure to lead rather than community exposure. Since over 95% of lead exposure in adults is work-related, ABLES data cannot be generalized to the U.S. population. The NHANES sample size and survey design do not allow for generating estimates by industry. The likelihood that a worker exposed to lead on the job may be randomly selected as an NHANES participant is small.
CDC’s Environmental Public Health Tracking Program emphasizes web-based dissemination of various environmental health indicators on environmental hazards, exposures, and health outcomes in aggregate form [OMB Control Number: 0920-1175; expiration date 07/31/2023]. The Tracking Program annually collects aggregate childhood BLL data by age group, BLL category, and geographic location (county) from its 26 cooperative agreement recipients under CDC-RFA-EH17-1702.
CBLS data are fundamentally different from the Tracking Program’s annual aggregate counts because CBLS collects de-identified, individual-level data used for program management and public health intervention at the state and local level. There is some duplication of effort between CBLS and the Tracking Program, since some states participate in both CDC programs; however, the duplication is minimal as the data required by the Tracking Program is readily available among CBLS recipients. Currently, there are four states that are part of Tracking Program, and not part of CBLS, but within these four states CDC supports two local jurisdictions for CBLS. The Notices of Funding Opportunity (NOFOs) that guide the Tracking and CBLS program goals and requirements are not on the same schedule, so the overlap between these two programs changes over time. Additionally, some states have data sharing agreements with CDC that must be modified before sharing between CDC programs can occur.
The Centers for Medicare & Medicaid Services (CMS) requires that all children enrolled in Medicaid receive blood lead screening tests at ages 12 months and 24 months.8,9 In addition, any child between ages 24 and 72 months with no record of a previous blood lead screening test must receive one. The Medicaid requirement is met only when one of the two blood lead screening tests identified above (or a catch-up blood lead screening test) are conducted. These test results are captured by state-based CLPPPs, where these programs exist and as state blood lead reporting laws require. State Medicaid agencies are required to submit Early Periodic Screening, Diagnosis, and Treatment (EPSDT) data annually using Form CMS-416, line 1410 [Annual EPSDT Participation Report; OMB Control Number: 0938-0354; expiration date 05/31/2023], including the total aggregate number of blood lead screening tests for children enrolled in Medicaid, from birth to age 6 years.
CBLS data fundamentally differ from the CMS data in that CBLS is more complete because it does not restrict test results to children of a certain age or socioeconomic status. In addition, as stated previously, CBLS data represent individual-child data that is de-identified prior to submission as opposed to aggregate counts of tests or children tested.
A.5. Impact on Small Businesses or Other Small Entities
The collection of this information does not directly impact small businesses or small entities. CDC collects only the minimum data necessary to carry out the goals for CBLS and ABLES directly from state and local agencies or their bona fide agents.
A.6. Consequences of Collecting the Information Less Frequently
There are no technical or legal obstacles to reducing burden.
As part of program requirements, respondents will submit their data quarterly to CBLS. Prior to 2005, childhood blood lead surveillance data (on individual children’s blood lead tests) was collected annually at the end of the 1st quarter of the subsequent calendar year and programmatic data (aggregate counts of the number of children tested) were collected quarterly to monitor program progress towards strategic goals (such as prevention activities including blood lead screening, follow-up testing, and identification and mitigation of lead sources). In 2005, based on feedback received from state partners, the decision was made to increase the frequency of surveillance data collection from once a year (on an annual basis) to four times a year (on a quarterly basis) to supplant the need for separate quarterly programmatic reporting. CBLS currently uses quarterly surveillance data for technical assistance and program management purposes and updates the childhood blood lead reports once a year when the updated information is posted to the general public website.
The ABLES program updates the adult blood lead database once a year and reports are shared with federal agencies, states, and the general public annually.
A.7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
There are no special circumstances with this information collection package. This request fully complies with the regulation 5 CFR 1320.5.
A.8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
A. A 60-day Federal Register Notice was published in the Federal Register on October 13, 2020, Vol. 85, No. 198, pp. 64474 (Attachment 2). CDC/ATSDR did not receive public comments related to this notice.
B. CBLS has regularly engaged with outside consultations with experts and stakeholders during the:
Biannual meetings of the Lead Exposure and Prevention Advisory Committee (LEPAC) (initiated in 2018)
biannual meetings of NCEH/ATSDR Board of Scientific Counselors (BSC) (disbanded in June 2019) (https://www.atsdr.cdc.gov/science/members.html);
ad hoc meetings of the BSC Lead Poisoning Prevention Subcommittee (disbanded in June 2019) (https://www.atsdr.cdc.gov/science/lpp/lppmembership.html); and
annual meeting of state and local cooperative agreement recipients
(https://www.cdc.gov/nceh/lead/programs/)
ad hoc meetings of the Lead Subcommittee of the President’s Task Force for Environmental Health and Safety Risks to Children
(https://ptfceh.niehs.nih.gov/activities/lead-exposures/)
During the past three years, CBLS has specifically engaged in consultations with:
The Lead Exposure and Prevention Advisory Committee (LEPAC) which was approved on Jan 19, 2018. LEPAC is charged with:
reviewing federal programs and services available to lead-exposed individuals and communities;
reviewing current research on lead poisoning to identify additional research needs;
reviewing and identifying best practices, or the need for best practices, regarding lead screening and prevention of lead poisoning; and
identifying effective services for individuals and communities affected by lead exposure.
A list of LEPAC members is included below.
NCEH/ATSDR Lead Exposure and Prevention Advisory Committee (LEPAC) |
|
CHAIR Matthew
Ammon, M.S. |
DESIGNATED FEDERAL OFFICER Perri
Ruckart, M.P.H. |
MEMBERS |
|
Tammy
Barnhill-Proctor, M.S. |
Jeanne
Briskin, M.S. |
Wallace
Chambers Jr., M.P.H. |
Tiffany
DeFoe, M.S. |
Michael
Focazio, Ph.D., M.S. |
Monique
Fountain Hanna, M.D., M.P.H., M.B.A. |
Nathan
Graber, M.D., M.P.H. |
Karla
Johnson, M.P.H. |
Donna
Johnson-Bailey, MPH, RD |
Erika
Marquez, PhD, M.P.H. |
Howard
Mielke, Ph.D., M.S. |
Anshu
Mohllajee, Sc.D., M.P.H. |
Jill
Ryer-Powder, Ph.D., M.N.S.P. |
|
The NCEH/ATSDR Board of Scientific Counselors and the Lead Poisoning Prevention Subcommittee advised the program until their term ended on June 14, 2019 as part of the Executive Order on Evaluating and Improving the Utility of Federal Advisory Committees (EO 13875).
NCEH/ATSDR Board of Scientific Counselors (up to 06/14/2019) |
|
CHAIR Melissa J. Perry ScD,
MHS |
DESIGNATED FEDERAL OFFICER William Cibulas, PhD,
MS |
MEMBERS |
|
Babafemi
A. Adesanya, Ph.D. |
Kenneth Aldous,
PhD |
Paloma
Beamer, PhD |
Aaron Bernstein, MD
MPH |
Darryl Brown, PhD
MPA |
Suzanne Condon,
MSM |
Roberta Grant,
PhD |
Daniel
Hryhorczuk, MD, MPH |
Joyce M. Martin, MA
JD |
Ralph McCullers,
MPA |
John Meeker, ScD,
CIH |
Devon Payne-Sturges,
DrPH MPH |
Joan
B. Rose, PhD |
Marilyn
C. Underwood, Ph.D. |
Nsedu Obot Witherspoon,
MPH |
|
FEDERAL EXPERTS |
|
Wayne E. Cascio, MD,
FACC, FAHA |
Ruth M. Lunn, DrPH Director, Office of the Report on Carcinogens, Division of the National Toxicology Program, NIEHS/NIH |
Paul J. Middendorf, PhD, CIH Deputy Associate Director for Science, CDC/NIOSH/OD/ADSO |
Joey Y. Zhou, PhD Senior Epidemiologist, U.S. Department of Energy |
NCEH/ATSDR BSC Lead Poisoning Prevention Subcommittee (up to 06/14/2019) |
|
CHAIR Matthew Strickland,
BA, MA, MPH, PhD. |
DESIGNATED FEDERAL OFFICER William Cibulas, PhD,
MS, CAPT |
MEMBERS |
|
John G. Belt, MS |
Elizabeth A.
Colón |
Kim N. Dietrich,
Ph.D. |
Nathan M. Graber, MD,
MPH, FAAP |
Michael J. Kosnett, MD,
MPH |
Jennifer A. Lowry,
MD |
Patrick J. Parsons, PhD,
Chem., FRSC |
|
FEDERAL EXPERTS |
|
Mark A. Maddaloni, MS,
DrPH |
|
Federal and state partners of the ABLES program meet at the Council of State and Territorial Epidemiologists (CSTE) (http://www.cste.org/) annual conference to exchange experiences and provide feedback on the ABLES program. In addition, state partners participate with NIOSH and NCEH in the ongoing process of integrating ABLES into HHLPSS.
ABLES Contact References
Barbara Materna – California Department of Public Health, (510) 620-5730
Kathy Leinenkugel – Iowa Department of Public Health, (515) 281-4930
Barbara Grajewski – Wisconsin Division of Public Health, (608) 266-0923
A.9. Explanation of Any Payment or Gift to Respondents
CBLS respondents are funded through a cooperative agreement (CDC-RFA-EH17-1701-PPHF17 (Attachment 3a); CDC-RFA-EH17-1701SUPP20 (Attachment 3c); CDC–RFA–EH18–1806 (Attachment 3b); CDC-RFA-EH18-1806 SUPP20, (Attachment 3d) to develop and sustain programs aimed at childhood lead poisoning prevention and surveillance. CBLS data submission on a quarterly basis is a requirement of these agreements. Cooperative agreement recipients will not receive additional payments or gifts for providing information.
Data submission to the ABLES Program is voluntary and completed through data sharing agreements with state agencies or their bona fide agents.
A.10. Protection of the Privacy and Confidentiality of Information Provided by Respondents
CBLS: The NCEH/ATSDR PRA Contact has reviewed this extension ICR and has determined that the Privacy Act does not apply. CBLS does collect potentially personally identifiable information (PII) in the form of date of birth; however, records will not be retrieved by PII (Attachment 7a – question 22). The NCEH/ATSDR Information Systems Security Officer (ISSO) conducted an annual review of the CBLS and has determined that a new CDC Security Assessment and Authorizations (SA&A) is required (Attachment 7a).
State or local health departments receive data from health care providers, laboratories, hospitals, or other facilities that analyze blood samples for lead and then store these data on secured servers housed on their respective premises. Reporting is conducted through a variety of modes following the specific jurisdiction’s system design including: 1) Health Level 7 (HL7) data format via secure, encrypted transfer; 2) Excel sheets via secure, encrypted FTP; or 3) secure delivery of paper records, such as via secure fax. Health departments are responsible for following all local or state personal privacy protection laws and state IT security protocols and processes, such as security options to enter data into password-protected Microsoft SQL databases.
This reporting from providers and laboratories to state or local CLPPPs is not included in the burden table in section A.12., consistent with 5 CFR §1320.3(b)(2) and (b)(3),11 because the initial collections and reporting to the state is required by state or local public health law, statutes, or regulations that require blood lead testing and reporting within their jurisdictions independent from CDC’s collection of this information (NCSL, 2010). The reporting, recordkeeping, or disclosure activities needed to comply within jurisdictions are usual and customary and would be required by law even in the absence of the federal requirement.
As part of funding agreements, CBLS recipients are required to submit quarterly data to CDC with a one-quarter lag (e.g., data collected during the first quarter is due by the end of the second quarter). All required data are extracted from the respondent’s secure server and transmitted to CDC via the CDC’s Secure Access Management System’s (SAMS) Secure Data eXchange’s (SDX) integrated Managed File Transfer (MFT) Platform. Data submitted in text files to CDC are processed and maintained in the CBLS database. Data are delivered in separate text files following formatting requirements (Attachment 5a) then processed using CBLS Central software into linkable tables. For the regular quarterly CBLS data submissions, all children are assigned a unique child identifier by the state or local program, a unique address identifier (ID), and certain medical information (blood lead test results only). CBLS does not collect any personally identifiable information (PII) including but not limited to child name, address, phone numbers, medical record or other identification numbers, or financial account information.
Each record contains a file identifier (FILEID), a program identifier (PGMID), and record-specific information to create a unique record identifier. Each table has one or more key variables that can be used to merge the data between multiple tables. Three important key variables for linking tables are: 1) Program ID – identifies the recipient jurisdiction; 2) Child ID; and 3) Address ID, which in combination are used to create a unique ID per individual or per address. A single local recipient reports annual summary “CBLS Aggregate Records” in spreadsheet format (Attachment 5b). We anticipate this recipient will continue to report in aggregate.
In special circumstances when state or local programs request technical assistance from CDC, or CDC makes a data request for its own sponsored projects, CDC will receive data that may include additional IIF that may be linked to the CBLS records delivered in the quarterly reports. In those situations, data will be transferred to CDC via the CDC’s Secure Access Management System’s (SAMS) Secure Data eXchange’s (SDX) integrated Managed File Transfer (MFT) Platform in the same manner as the quarterly data submissions. Data will be maintained on secure CDC servers. Each request outside of the CBLS quarterly collection will undergo a separate research and PRA determination and is not covered by this ICR. The ability to receive IIF will be approved when the CDC Security Assessment and Authorization (SA&A) for CBLS is completed. The extension CBLS data collection will not begin until the Authority to Operate (ATO) is granted. Social Security numbers are not provided to nor requested by CDC.
If there is a security breach for the CBLS data stored at CDC, some effect on the respondent’s privacy could occur; however, to minimize this risk, there are a variety of safeguards in place as described in the applicable Privacy Act System of Records Notice (SORN 09-20-0136 “Epidemiologic Studies and Surveillance of Disease Problems”).12
No consent form for the CBLS collection is required as the data are part of state or local surveillance efforts, under the Health Insurance Portability and Accountability Act of 1996 (HIPAA) section on disclosures for public health activities.13 Consent to share data with other federal agencies is not required when it involves enforcement of the Federal Lead Disclosure Rule Section 1018 of Title X and Lead-Safe Housing Rule (24 CFR Part 35).14
All CBLS data is secured in a password-protected surveillance system. Only CDC staff will have access to the raw data in CBLS. Data from state or local programs are sent electronically to CDC via the CDC’s Secure Access Management System’s (SAMS) Secure Data eXchange’s (SDX) integrated Managed File Transfer (MFT) Platform. Physical controls will also be implemented. Data will be stored on highly secured CDC servers in Atlanta, GA. Access to all CDC campuses is restricted by armed guards. The servers are housed in a secure computer room complete with climate control, emergency power, and an uninterruptible power supply (UPS). Daily back-ups and integrated security are implemented through the CDC computer services infrastructure. All data access is password-protected, and all network communications use encryption. All servers and PCs that are part of the CDC infrastructure are protected by both host-based firewalls and software in order to prevent the undetected installation of "spyware."
ABLES: States collect data on adult BLLs and provide NIOSH with the minimum number of fields that are needed for NIOSH to process and analyze the data. The CDC Chief Privacy Officer has conducted a review and has determined that the Privacy Act does apply (Attachment 7b). The applicable Privacy Act System of Records Notice is SORN No. 09-20-0149 titled “Morbidity Studies in Coal Mining, Metal and Non-metal Mining and General Industry,” for which records are retrievable by name and/or assigned numerical identifier, plant name and study name are some of the indices used to retrieve records from this system. Social security numbers, supplied on a voluntary basis may occasionally be used for data retrieval [Federal Register: February 14, 2018; Volume 83, Number 31; Pages 6591-6600 (Attachment 7b – question 22)].
NIOSH collects the date of birth to calculate age since state and local health departments vary in their rounding methods. NIOSH uses the ID field for analysis and for communicating with the states regarding data quality issues.
Data are stored on CDC facilities, using CDC data network within CDC’s firewalls. The CDC network is in a limited access server room in a building secured with guards, id badges, key cards and closed-circuit TV. Access to project data is granted to badged staff assigned to the project and on a need to know basis. User access is removed upon transfer or termination.
State agencies (departments of health or labor departments) share adult BLLs with the NIOSH ABLES program for occupational surveillance under data sharing agreements. States submit adult BLLs through encrypted FTP sites.
A.11. Institutional Review Board (IRB) and Justification for Sensitive Questions
The NCEH/ATSDR Human Subjects coordinator has reviewed the existing FY17 three-year cooperative agreement (CDC-RFA-EH17-1701-PPHF17; CDC-RFA-EH17-1701SUPP20) and the existing FY18 two-year cooperative agreement (CDC–RFA–EH18–1806; CDC-RFA-EH18-1806 SUPP20) and has determined that this program is non-research; therefore, review and approval by the CDC Institutional Review Board (IRB) is not required (Attachment 8a). The NIOSH Human Subjects coordinator has determined that the ABLES collection is non-research and that review and approval by the NIOSH IRB is not required (Attachment 8b).
The purpose of these activities is to identify and control a public health problem, specifically lead exposures that may lead to adverse health outcomes for children and adults.
Intended benefits of the projects are primarily or exclusively for the children and adult workers at risk for lead exposure.
The data collected are needed for state or local health departments to identify children and adults in need of referral for medical monitoring or management.
The knowledge that is generated does not extend beyond the scope of the activities and project activities are not experimental.
Justification for Sensitive Questions:
Questions that could be considered sensitive by at least a segment of the population, such as information on pregnancy and race/ethnicity, are integral to accomplishing the purpose of CBLS and ABLES. Table A.11.1 describes the specific use of the potentially sensitive questions.
Table A.11.1. Specific Uses of Questions of a Sensitive Nature
Questions |
Specific uses of information |
Pregnant at time of test? (at time of blood lead test)
|
To assess prevalence of pregnant women with elevated blood lead which provides important data for clinical follow-up of women and their unborn babies. |
Race/ethnicity? |
For targeting resources to subpopulations with high risk for elevated blood lead or housing risk factors |
A.12. Estimates of Annualized Burden Hours and Costs
The total annualized time burden requested by the CDC for this ICR is 1,226 hours. This is the same as the previously approved 1,226 hours for BLSS ICR [OMB Control Number: 0920-0931; expiration date 05/31/2021]. We are requesting to again include up to 60 CBLS respondents and up to 40 ABLES respondents. Burden hours and costs are provided separately for CBLS and ABLES by data collection method (individual records and aggregate records).
CBLS Respondents: NCEH respondents include up to 60 cooperative agreement recipients from state or local health departments, or their bona fide agents, who receive support to develop and implement a CLPPP. There are two types of NCEH respondents. First, are the 48 cooperative agreement partners funded solely under the FY17 Program and its corresponding Supplemental funding. Second, are the remaining 5 cooperative agreement partners funded solely under the FY18 Program and its corresponding Supplemental funding. We anticipate funding up to another 7 respondents in years 2 & 3 of this ICR.
In this PRA clearance, 59 respondents will submit quarterly CBLS text files (944 annualized hours) (Attachment 5a) and a single respondent will submit quarterly CBLS Aggregate Records Forms (2 annualized hours) (Attachment 5b). The total annual time burden requested by NCEH is 946 hours for each year of PRA clearance.
ABLES Respondents: Over the next three years, up to 40 participating states will submit adult BLL data to NIOSH. Over the past three years, 32 states reported BLL data to NIOSH. Over the next three-years, NIOSH aims to collaborate with up to 8 additional states. The total burden hours are calculated for 40 respondents. On an annual basis, states submit either case records or aggregate counts of adult blood lead test results (Attachments 6a, 6a1, 6b). NIOSH estimates that 80 percent of states (respondents) (n=32) will spend 256 burden hours submitting case records and 20 percent (n=8) will spend 24 burden hours submitting aggregate records. The total annual time burden requested by NIOSH is 280 hours (Table A.12.1).
Table A.12.1. Estimated Annualized Burden Hours
Type of Respondents |
Form Name |
Number of Respondents |
Number of Responses per Respondent |
Average Burden per
Response |
Total |
||
State or Local Health Departments, or their Bona Fide Agents |
CBLS Variables (ASCII Text Files) |
59 |
4 |
4 |
944 |
||
CBLS Aggregate Records Form (Excel) |
1 |
1 |
2 |
2 |
|||
ABLES Case Records Form |
32 |
1 |
8 |
256 |
|||
ABLES Aggregate Records Form |
8 |
1 |
3 |
24 |
|||
Total |
|
1,226 |
|||||
The total annualized burden cost is $51,013.86 which includes $39,363.06 for CBLS and $11,650.80 for ABLES (Table A.12.2). The hourly wage for respondents is estimated to be $41.61 per hour. This is based on the Bureau of Labor Statistics May 2019 median hourly rate of pay for a computer programmer (see http://www.bls.gov/oes/current/oes151251.htm).
15-1251 Computer
Programmers -
Create,
modify, and test the code, forms, and script that allow computer
applications to run. Work from specifications drawn up by software
developers or other individuals. May assist software developers by
analyzing user needs and designing software solutions. May develop
and write computer programs to store, locate, and retrieve specific
documents, data, and information.
Table A.12.2. Estimated Annualized Burden Costs
Type of Respondents |
Form Name |
Number of Respondents |
Total |
Hourly Wage Rate |
Total |
State or Local Health Departments, or their Bona Fide Agents |
CBLS Variables (ASCII Text Files) |
59 |
944 |
$41.61 |
$39,279.84 |
CBLS Aggregate Records Form (Excel) |
1 |
2 |
$41.61 |
$83.22 |
|
ABLES Case Records Form |
32 |
256 |
$41.61 |
$10,652.16 |
|
ABLES Aggregate Records Form |
8 |
24 |
$41.61 |
$998.64 |
|
Total |
|
$51,013.86 |
|||
A.13. Estimates of Other Total Annual Cost Burden to Respondents and Record Keepers
The estimate of the total annual cost burden to respondents or record keepers is based on the optional implementation of the HHLPSS software in state or local programs. The base software is provided at no cost to programs and NCEH provides technical support for implementation at no cost. The total capital and start-up cost component include approximately $40,000 for computer hardware and software; however, state or local programs (e.g., health departments) may already have existing equipment that can be used. Total operation and maintenance and purchase of services for the maintenance of HHLPSS is approximately $5,000 per year; however, state or local programs (e.g., health departments) may already have existing computer software and servicing contracts in place that can be used for HHLPSS.
For the ABLES program, there are no capital/start-up or ongoing operation/maintenance costs associated with this information collection.
A.14. Annualized Cost to the Federal Government
The combined annualized cost to the federal government for CBLS and ABLES is $12,717,882. The cost for each program is outlined below.
CBLS annualized estimated cost to the federal government is $12,662,882 and is based on the following:
Table A14.1. CBLS Annualized Costs
FY21 Cooperative Agreements for surveillance activities |
$11,000,000 |
FTE Salaries for surveillance activities |
$512,882 |
FTE Travel (to meet with CBLS stakeholders; site visits) |
$50,000 |
HHLPSS IT Support Services (firm fixed price contract) |
$1,060,000 |
Contractor Travel (HHLPSS support provide to state & local agencies) |
$40,000 |
Total Costs |
$12,662,882 |
The annual FY21 cooperative agreement program budget for surveillance activities is estimated to be $11,000,000 per year. The annual federal personnel salary cost for surveillance activities is $512,882 based on 40 percent FTE of the total salary estimate of $1,282,206 for the following positions: Program Chief, Deputy Program Chief, 6 Project Officers, 1 IT Specialist, 2 Epidemiologists, 1 Communications Specialist.15 Annual FTE travel cost is $50,000 based on the need for site visits, training, and meeting attendance. In FY21, the total overall operational and maintenance costs for HHLPSS is $1,100,000 for a firm, fixed-price contract (which includes $40,000 estimated travel to support state and local health departments). The contract provides for the services of five (5) IT specialists ranging from database administrator to direct user support with a focus primarily on management and operations of the system and a minimal amount of development and upgrades related to state/local health agency user requests. Other tasks include data extraction, formatting, and validation to process data submitted to CDC.
ABLES annualized estimated cost to the federal government is $50,000 and is based on the following:
Table A.14.2. ABLES Annualized Costs
FTE Salaries |
$52,000 |
FTE Travel (to meet with ABLES stakeholders) |
$3,000 |
Total Cost |
$55,000 |
NIOSH staff working in the ABLES Program includes four part-time subject matter experts that total 0.5 FTE. Their duties are management and oversight of the multi-state ABLES surveillance system including data management, data analysis, dissemination of findings, responding to public requests, and providing technical assistance to states. These employees spend approximately 1,044 hours per year working on the surveillance program. Using an estimated salary of $50 per hour, personnel costs will total $52,000 annually. One ABLES staff person also attends the annual national CSTE conference and makes occasional trips to attend other meetings with ABLES stakeholders. States are providing data to the ABLES Program on a voluntary basis and no direct funding to states is provided by NIOSH in support of the ABLES Program.
A.15. Explanation for Program Changes or Adjustments
This information collection request for two separate CDC data collection systems has operated under a combined PRA clearance since 2005 (Attachment 9).
On May 15, 2018, the Terms of Clearance16 specified that
Within two months of the approval of this ICR, CDC will submit a non-substantive change request confirming updates to the public-facing website reflecting the new language as indicated in the supplementary document associated with this package.
Within two weeks, NIOSH submitted and OMB approved the request updating the NIOSH website to include ABLES standardized variables, format, and instructions for data submission.17
On July 10, 2019, OMB approved a second non-substantive change request,18 in which CDC’s CLPPP updated the existing NCEH CBLS Variable and NIOSH updated the ABLES variable list to include the modified race variable options/value sets.
As an extension ICR, no changes are requested to the currently approved information collection forms, the requested burden hours, or the number of respondents on an annual basis.
A.16. Plans for Tabulation and Publication and Project Time Schedule
CBLS Data Processing and Report Dissemination: Respondents are required to submit quarterly data to NCEH by the end of the subsequent quarter. Data submitted in text files to NCEH are processed and maintained in the CBLS database. NCEH uses its processing software, CBLS Central, to perform data checks on recipient text files for required formatting. Text files are parsed into separate linkable data tables (e.g., Child, Address, Lab tests, Investigation) (Attachments 5a). Processing reports are generated and sent back to recipients via the CDC’s Secure Access Management System’s (SAMS) Secure Data eXchange’s (SDX) integrated Managed File Transfer (MFT) Platform, to indicate how many records were properly parsed and entered into the CBLS database and how many records were not successfully loaded, with an explanation of the rejection. Data corrections are returned in the next quarterly report. Therefore, NCEH has a one to two quarter lag time for reporting results. CBLS Annual Reports are based on the calendar year.
Table A.16.1. CBLS Project Time Schedule
Activity |
Time Schedule |
Programs deliver FY1 Q1 data (Oct-Dec) |
By end of FY1 Q2 (March 31) |
CBLS Q1 Data Cleaning and Quality Control Processing Reports sent back to Programs |
FY1 Q3 (April-June) |
Programs deliver FY1 Q2 (Jan-Mar) data |
By end of FY1 Q3 (June 30) |
CBLS Q2 Data Cleaning and Quality Control Processing Reports sent to Programs |
FY1 Q4 (July-September) |
Programs deliver FY1 Q3 (Apr-Jun) data |
By end of FY1 Q4 (September 30) |
CBLS Q3 Data Cleaning and Quality Control Processing Reports sent to Programs |
FY2 Q1 (October-December) |
Programs deliver FY1 Q4 (Jul-Sep) data |
By end of FY2 Q1 (December 31) |
CBLS Q4 Data Cleaning and Quality Control Processing Reports sent to Programs |
FY2 Q2 (January-March) |
Annual Calendar Year Reports sent to Programs when Programs deliver FY2 Q1 (Oct-Dec) data |
By end of FY2 Q2 (March 31) |
Post Annual Data on Web and/or Publish Annual Calendar Year Report |
By end of FY2 Q3 (June 30) |
CBLS Publications and Results Dissemination: NCEH shares the de-identified aggregate/summary data without restriction through its public website19 and through publications such as MMWR Surveillance Summary reports. CBLS datasets include some required variables with potentially personally identifiable information (PII) (e.g., age, race, sex, county of address) which may present privacy concerns due to small numbers for some of the variables. Therefore, small cell sizes (counts <5) will be redacted from all datasets or summary tables that are disseminated in any way. According to the 2018 BLSS Terms of Clearance,20 dissemination of the aggregate data set and statistics generated from the aggregate data set will always be accompanied by the following caveats:
“Approved consistent with CDC’s commitment to always communicate that these data do not provide for nationally representative prevalence estimates, due to the fact that not all states participate in CBLS and ABLS, as well as differences in jurisdictional screening practices and laboratory reporting requirements among state and local jurisdictions. However, use of the consistent case definition allows for estimating needs at the Federal, state, and local level which is important for establishing national program goals and objectives. In addition, CDC commits to working with CMS to better capture Medicaid-required test results and decrease duplicative requirements on States.”
Table A.16.2. ABLES Project Time Schedule (Example recurring timeline)
ABLES Project Time Schedule |
|
Activity |
Time Schedule |
Request 2019 Data from States |
February – March 2020 |
Receive 2019 Data from States |
By June 30, 2020 |
Data Cleaning and Data Quality Control |
March – July 2020 |
Work on Report Based on 2018 ABLES data |
July – August 2020 |
Post and/or Publish Report |
August – December 2020 |
ABLES publication and dissemination: Summary data from the ABLES program will be posted to the ABLES website.21 In addition, aggregate ABLES data will also be made available on the NIOSH Worker Health Charts,22 a data query and visualization tool. Data will be updated annually.
A.17. Reason(s) Display of OMB Expiration Date is Inappropriate
The display of the OMB expiration date is appropriate.
A.18. Exceptions to Certification for Paperwork Reduction Act Submissions
There are no exceptions to the certification.
References
American College of Obstetricians and Gynecologists (ACOG). Lead screening during pregnancy and lactation. Committee Opinion No. 533. Obstet Gynecol 2012; 120:416–20. Available at: http://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Obstetric-Practice/Lead-Screening-During-Pregnancy-and-Lactation/
Centers for Disease Control and Prevention (CDC). Fourth National Report on Human Exposure to Environmental Chemicals. Updated Tables, March 2018. Available at: https://www.cdc.gov/exposurereport/
Centers for Disease Control and Prevention (CDC). Changes in national notifiable diseases data presentation. MMWR 1996; 45:41–2.
Centers for Disease Control and Prevention (CDC). National Notifiable Diseases Surveillance System (NNDSS). Diseases and Conditions – Lead, Elevated Blood Levels, Adult (≥16 Years) and Children (<16 Years). Atlanta: 2016. Available at: https://wwwn.cdc.gov/nndss/conditions/lead-elevated-blood-levels/case-definition/2016/
Council for State and Territorial Epidemiologists (CSTE). Public Health Reporting and National Notification for Elevated Blood Lead Levels (Position Statement 15-EH-01). (Revised 2015, December). Available at: http://c.ymcdn.com/sites/www.cste.org/resource/resmgr/PS1/15-EH-01_revised_12.4.15.pdf
U.S. Department of Health and Human Services (HHS). Strategic Plan for the Elimination of Childhood lead Poisoning. Developed for the Risk Management Subcommittee, Committee to Coordinate Environmental Health and Related Programs. Atlanta: 1991, February. Available at: http://files.eric.ed.gov/fulltext/ED344700.pdf
National Conference of State Legislatures (NCSL). State Lead Poisoning Prevention Statutes. Denver: 2010 March. Compiled by Farquhar D. Available at: http://www.ncsl.org/documents/environ/stlaws10.pdf
Newman N, Jones C, Page E, Ceballos D, Oza A. Investigation of Childhood Lead Poisoning from Parental Take-Home Exposure from an Electronic Scrap Recycling Facility — Ohio, 2012. MMWR 2015 Jul 17;64(27):743-5. Available at: https://www.cdc.gov/mmwr/pdf/wk/mm6427.pdf
Occupational Safety and Health Administration (OSHA). Safety and Health Topics: Lead. Available at: https://www.osha.gov/SLTC/lead/
Pertowski C. Lead Poisoning. From Data to Action: CDC’s Public Health Surveillance for Women, Infants, and Children. Atlanta, GA: U.S. Department of Health and Human Services. 1994.
1 HP2030 objective EH-04 Reduce blood lead levels in children aged 1 to 5 years. Available at: Reduce blood lead levels in children aged 1 to 5 years — EH‑04 - Healthy People 2030 | health.gov
2 The 1988 Lead Contamination Control Act amended the Public Health Service Act to authorize the Secretary of Health and Human Services to make grants to state and local governments for the initiation and expansion of community programs designed to: (1) screen infants and children for elevated blood lead levels; (2) assure referral for treatment of, and environmental intervention for, infants and children with such blood lead levels; and (3) provide education about childhood lead poisoning. It requires that grant priority be given to programs which will serve areas with a high incidence of elevated blood levels in infants and children. Available at: https://www.congress.gov/bill/100th-congress/house-bill/4939.
3 HP2020 objective OSH-7 Reduce the proportion of persons who have elevated blood lead concentrations from work exposures. Available at: https://www.healthypeople.gov/2020/topics-objectives/topic/occupational-safety-and-health/objectives
4a The North American Industry Classification System (NAICS) is the standard used by Federal statistical agencies in classifying business establishments for the purpose of collecting, analyzing, and publishing statistical data related to the U.S. business economy. https://www.bls.gov/bls/naics.htm
b Occupational Safety and Health Administration: https://www.osha.gov/SLTC/lead/index.html Accessed: 2019
5 Worker Health Charts : https://wwwn.cdc.gov/Niosh-whc/chart/ables-ab/exposure
6 ABLES website: https://www.cdc.gov/niosh/topics/ables/data.html
7 https://www.reginfo.gov/public/do/PRAOMBHistory?ombControlNumber=0920-0931#
8 CMS: Accessed 01/27/2017 at https://www.medicaid.gov/federal-policy-guidance/downloads/cib113016.pdf
9 CMS: Accessed 01/27/2017 at https://www.medicaid.gov/federal-policy-guidance/downloads/cib-06-22-12.pdf
10 CMS: Accessed 01/27/2017 at https://www.cms.gov/Medicare/CMS-Forms/CMS-Forms/Downloads/CMS416.pdf
11 5 CFR § 1320.3 – Definitions: For purposes of implementing the PRA, the following terms are defined as follows in Paragraph (b):
(2) The time, effort, and financial resources necessary to comply with a collection of information that would be incurred by persons in the normal course of their activities (e.g., in compiling and maintaining business records) will be excluded from the “burden” if the agency demonstrates that the reporting, recordkeeping, or disclosure activities needed to comply are usual and customary.
(3) A collection of information conducted or sponsored by a Federal agency that is also conducted or sponsored by a unit of State, local, or tribal government is presumed to impose a Federal burden except to the extent that the agency shows that such State, local, or tribal requirement would be imposed even in the absence of a Federal requirement.
13 See https://www.gpo.gov/fdsys/pkg/CFR-2011-title45-vol1/pdf/CFR-2011-title45-vol1-sec164-512.pdf and the discussion of 45 CFR 164.512(b) at https://privacyruleandresearch.nih.gov/pdf/ocr_publichealth.pdf.
14 See Final Rule - 24 CFR Part 35 - Lead Safe Housing Rule; Docket No. FR–5816–F–02]. Available at: https://www.gpo.gov/fdsys/pkg/FR-2017-01-13/pdf/2017-00261.pdf
15 Based on OPM Atlanta Locality Pay for Grade and Step 5 Salary Table at https://www.opm.gov/policy-data-oversight/pay-leave/salaries-wages/salary-tables/20Tables/html/ATL.aspx.
19 National and state aggregate data are provided at: https://www.cdc.gov/nceh/lead/data/
21 ABLES website: https://www.cdc.gov/niosh/topics/ables/data.html
22 Worker Health Charts: https://wwwn.cdc.gov/Niosh-whc/chart/ables-ab/exposure
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Richardson, Tony (CDC/OD/OADS) |
| File Modified | 0000-00-00 |
| File Created | 2021-10-04 |
© 2026 OMB.report | Privacy Policy