Summary of Changes 2021
OMBMemo_NFLIS-MEC_ChangesSummaryMemo_08022021.docx
The National Forensic Laboratory Information System Collection of Analysis Data
Summary of Changes 2021
OMB: 1117-0034
August 2, 2021
Change Request
National Forensic Laboratory Information System (NFLIS)
2022 Medical Examiner and Coroner Office Survey and Continuous Data Collection
OMB Control No: 1117-0034
The Drug Enforcement Administration (DEA), Diversion Control Division, is requesting approval of changes made to the previously approved National Forensic Laboratory Information System (NFLIS) Medical Examiner/Coroner Office (NFLIS-MEC) Survey and continuous data collection. DEA had previously shared the survey instrument and description of the continuous data collection with OMB in June 2019 with its formal package request.
This document summarizes the instrument modifications and provides reasons for their importance. The changes were made on the basis of:
An assessment of the data from MECs based on data reviews we have done with the current and ongoing NFLIS-Tox data collection;
Ongoing project needs to ensure medical examiner/coroner frame integrity;
An internal review of the data from the NFLIS-MEC 2017 Survey, which included an assessment of the data quality and how those data were subsequently used;
A seven-member expert panel that reviewed the survey during June 2021; and
Cognitive interviews with seven participants conducted during July 2021.
Following is a summary of the changes to the NFLIS-MEC Survey:
Nine questions from the NFLIS-Tox 2017 Survey were eliminated from the 2022 survey because the data received were not useful, had low item response, and/or did not ultimately serve the project goals. The eliminated questions are documented in Table 1 in the appendix.
Seventeen questions were modified slightly based on the 2017 data assessment and feedback from DEA, the expert panel, cognitive interviews, or internal/editorial review. The changes to these questions are summarized in Table 2 in the appendix and reflect mostly modest edits to the question stem and/or response options and were made to enhance clarity and data quality and/or to reduce burden.
Non-substantive changes to the survey that are not included in Table 2 included renumbering the questions to be plainer for the field, cutting down on many question subparts in favor of merely adding more plain numbers. For some of the numeric fields (e.g., Question 10 regarding number of cases referred to the MEC in calendar year 2021), we included boxes for each number with a comma for the thousandths place (versus having a blank line which was done in the 2017 administration). This slight change in formatting will help with data quality. We also included auto-fill responses for confirmation in the web survey, where possible, to reduce survey burden.
Ten new questions were added. The following narrative provides details about these new items.
DEA has reviewed Statements A and B and has determined that some changes will need to be made to the original Statement B that were submitted to OMB on XXX and approved by OMB on XXX. Specifically:
The sampling strategy for the NFLIS-MEC continuous data collection has changed based on our experience with the NFLIS-Tox recruitment effort and new knowledge of the MEC community.
We have added eight core data elements for the continuous NFLIS-MEC data collection based on expert inputs and data needs that we have determined through our work with the NFLIS-Tox data collection.
Notably, the following items remain the same and thus, have not changed in Statements A or B.
The survey goals are the same.
The uses and audience for the anticipated data remain the same.
The content is the same since each of the new questions reflect the same concepts that were surveyed previously only now we are proposing stronger measures that will reduce burden based on field input.
The eligibility criteria are the same.
The analytic plan remains the same.
The estimated burden to complete the survey remains the same.
The cost to the government remains the same.
Summarized below are more details about the ten new survey items for the NFLIS-MEC Survey.
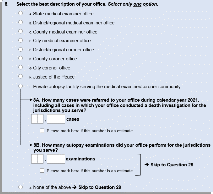
When we examined responses for Question 5 from the 2017 administration, we realized there were more private autopsy facilities that operate in the United States than previously known. Thus, Questions 8A and 8B were added to ask about the caseload and number of examinations of these private autopsy facilities to gain an understanding of their workload (Figure 1).
Following responses to questions 8A and 8B, the private autopsy facilities will follow a skip pattern to the end of the survey.
Figure 1. 2022 NFLIS-MEC Instrument, Question 8
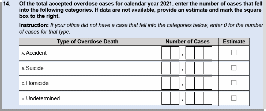
Questions 13 and 14 were added to the 2022 instrument to gain an understanding of the total number of overdose death cases accepted by the MEC in 2021 (Question 13; Figure 2) and how these cases were classified (Question 14; Figure 2). This information will help DEA get a timely sense of the 2021 caseload for overdoses since official death statistics are released at least 12 months after the close of a calendar year. An estimate box is provided to facilitate answering the question and reduce survey burden.
Figure 2. 2022 NFLIS-MEC Instrument, Questions 13-14

The free text responses provided in Question 13 from the 2017 NFLIS-MEC administration provided the inputs needed to create a new Question 16 (Figure 3) which asks respondents about the instances that warrant specific testing for drugs. Because this new question is a “check all that apply” option and covers the free text responses we received in 2017, as well as the feedback we received from the field via the expert panel and cognitive interviews, this two-question series will be less burdensome than the one question with an open text field.
Figure 3. 2022 NFLIS-MEC Instrument, Questions 15-16

Question 17 was added to the 2022 NFLIS-MEC Survey because it is important that we gain an understanding of whether an MEC performs immunoassay testing prior to sending the samples to toxicology laboratories (Figure 4). This question is straightforward for the NFLIS-MEC respondent to answer.
Figure 4. 2022 NFLIS-MEC Instrument, Question 17

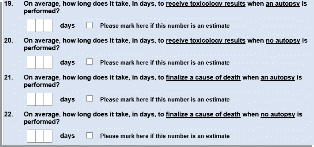
Questions 19-22 were added to better gain an understanding of how long cases involving toxicology testing take to complete, which will help DEA understand how long data lags will take for reporting results out. Both of these questions tested well during cognitive interviews (Figure 5).
Figure 5. 2022 NFLIS-MEC Instrument Questions 19-22
Question 27 was added into the 2022 NFLIS-MEC instrument on the basis of DEA’s need to understand how testing results from off-site laboratories are incorporated into the MEC’s records management system. The new question tested well during our cognitive interviews.
Figure 6. 2022 NFLIS-MEC Instrument, Question 27

We welcome any comments or questions from OMB regarding these changes.
Appendix
Table 1. 2017 NFLIS-MEC Survey Questions That Were Eliminated for the 2022 NFLIS-MEC Survey Administration
Question No. |
2017 Questions That Were Eliminated |
8 |
Enter the total population of the jurisdiction(s) your office serves. |
9 |
Is your office accredited by any organizations? Select all that apply. A. National Association of Medical Examiners (NAME) B. International Association of Coroners & Medical Examiners (IAC&ME) C. Other – please specify |
15 |
In general, what is the average turnaround time, in days, for completing a case (defined here as completion of a death certificate)? |
22 |
Does your information management system have the ability to export customized files? Select all that apply. A. Comma-separated values (CSV) file B. Tab-delimited text C. XML D. Database (DBF, SQL) E. Text (TXT) F. Excel (XLS, XLSX) G. Access (MDB, ACCDB) H. Crystal Reports I. Other (please specify) J. None of the above K. Don’t know |
23 |
Does your office have the ability to electronically transfer exported files? Select all that apply. A. E-mail B. SFTP upload C. HTTP upload D. Other (please specify) E. None of the above F. Don’t know |
25 |
What types of assistance would ease your participation in NFLIS? Select all that apply. A. Computer hardware B. Computer software C. Assistance with programming D. Direct financial assistance to support data acquisition and reporting E. Other (please specify) F. None of the above |
26 |
Of the types of assistance that you specified in Question 25 that would ease your participation in NFLIS, which one is the most important? Select only one option. A. Computer hardware B. Computer software C. Assistance with programming D. Direct financial assistance to support data acquisition and reporting E. Other (please specify) |
27 |
Does your office participate in any other drug-related data collection efforts? Select all that apply. A. National Violent Death Reporting System (NVDRS) B. Fatality Analysis Reporting System (FARS) C. State-based drug-related data collection (please specify) D. Other (please specify) E. None of the above |
28 |
Generally, what are the main potential barriers for your office to participate in data collection efforts? Select all that apply. A. Lack of electronic records B. Lack of resources for data conversion to other systems C. Concerns about privacy D. Unavailable personnel to work on project E. Unavailable personnel for software, IT, and so forth needed for this project F. Unwillingness to share data with Federal agencies G. Political climate or restrictions H. Resource limitations I. Concerns that the effort will not benefit my jurisdiction, office, or laboratory J. Other barriers (please specify) K. None of the above |
Table 2. Summary of Minor Changes to NFLIS-MEC Survey Questions
Question No. Survey Year |
Description of Change |
||
2017 |
2022 |
||
2 |
2 |
|
|
3 |
3 |
|
|
4 |
4 |
|
|
4a |
5 |
|
|
4a1 |
6 |
|
|
6 |
9 |
|
|
10 |
10 |
|
|
11 |
11 |
|
|
12 |
12 |
|
|
13 |
15 |
|
|
14 |
18 |
|
|
17 |
23 |
|
|
18 |
25 |
|
|
19 |
25 |
|
|
20 |
|||
21 |
26 |
|
|
24a & 24b |
28 |
|
|
29 |
29 |
|
|
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Smiley-McDonald, Hope |
| File Modified | 0000-00-00 |
| File Created | 2021-11-16 |
© 2026 OMB.report | Privacy Policy