0327 ss 20180625
0327 ss 20180625.docx
Animal Disease Traceability
OMB: 0579-0327
SUPPORTING STATEMENT – OMB NO. 0579-0327
Animal Disease Traceability
June 2018
Explain the circumstances that make the collection of information necessary. Identify any legal or administrative requirements that necessitate the collection. Attach a copy of the appropriate section of each statute and regulation mandating or authorizing the collection of information.
The Animal Health Protection Act of 2002 (7 U.S.C. 8301–8317) is the primary Federal law governing the protection of animal health. The law gives the Secretary of Agriculture broad authority to detect, control, or eradicate pests or diseases of livestock or poultry. Disease prevention is the most effective method for maintaining a healthy animal population and for enhancing the United States’ ability to compete in the world market of animal and animal product trade.
The Veterinary Services unit (VS) of the Animal and Plant Health Inspection Service (APHIS) uses disease control to safeguard U.S. animal health. One important part of disease control is animal disease traceability. Animal disease traceability means being able to document the movement history of an animal throughout its life. Knowing where diseased and at-risk animals have been and are located, as well as when they have been there, is indispensable during an emergency response and important for ongoing disease programs. Epidemiologists use this information to determine the potential spread of a disease. Having the ability to plot locations within a radius of an infected premises helps determine the potential magnitude of a contagious disease and the resources needed to contain it.
Furthermore, as diseases are controlled or eradicated, it is important to document areas, States, or regions of the country that are free from disease. Traceability helps us determine those disease-free zones, thus enhancing the marketability of livestock.
APHIS has established general traceability regulations for livestock moving interstate at title 9, Code of Federal Regulations (9 CFR) part 86. Under the regulations, unless specifically exempted, livestock moved interstate would have to be officially identified and accompanied by an interstate certificate of veterinary inspection or other documentation. The regulations specify approved forms of official identification for each species but allow livestock to be moved between any two States or Tribes with another form of identification as agreed on by animal health officials in the two jurisdictions. This improves APHIS’ ability to trace livestock if disease is found.
The regulations place the greatest information collection burden on the cattle industry, because that sector has had the greatest gaps in traceability and the greatest need for new traceability standards. Previous regulations for the sheep and goat, swine, and poultry sectors provide adequate traceability and the burden associated with those disease programs is contained in information collections related to those programs. However, APHIS adapted investments made in the previously existing National Animal Identification System (NAIS) on information systems, official animal identification devices, and other areas where States and Tribes had achieved progress through cooperative agreements, into the animal disease traceability framework. These burden items, outlined further below, let the traceability program remain essential in helping animal health officials protect U.S. livestock and poultry from disease spread and retaining access to domestic and foreign markets. Timely response to disease outbreaks will:
• Slow disease spread and reduce associated economic impact.
• Lessen disruption to producers and animal owners.
• Speed lifting of quarantine and movement restrictions.
• Lessen the likelihood of animal depopulation.
• Increase consumer confidence.
The ADT framework focuses on interstate movements, with States and Tribes determining requirements for those movements. The basic data APHIS acquires through the ADT system will help achieve timely animal movement tracebacks and trace forwards when responding to an animal disease concern.
Information System
APHIS continues to provide information systems that States and Tribes may use to implement their ADT plans. Producer premises and animal data within the ADT framework necessary to support the framework’s objectives is maintained and controlled at the discretion of States and Tribes.
Cooperative Agreements
Development and implementation of the ADT framework continues to be a partnership involving APHIS, States, Tribes, and industry. States and Tribes enter into cooperative agreements with APHIS to implement their traceability activities. Traceability performance standards measure and document tracing capability resulting from the ADT framework. Agreements will continue to be necessary as APHIS develops and implements this cooperative program.
Also within the ADT framework, the National Uniform Eartagging System (NUES) gives a nationally unique identification number for animals that need official identification. The distribution and use official identification eartags using the NUES requires some information collection activites.
APHIS makes some systems available to tribes. Tribes can designate which premises registration information system they would like to use, if any, by completing VS 1-63, Tribal Location Identification System Implementation Request. The form states what system the Tribes want to use, if any, and lets APHIS know how to help the Tribes use the system.
APHIS is asking OMB to approve, for an additional 3 years, its use of this information collection activity to facilitate animal disease traceability and support disease control, eradication, and surveillance activities.
2. Indicate how, by whom, how frequently, and for what purpose the information is to be used. Except for a new collection, indicate the actual use the agency has made of the information received from the current collection.
APHIS uses the following information collection activities to facilitate animal disease traceability and support disease control, eradication, and surveillance activities.
9 CFR 86.4: Application for Use of More Than One Official Identification (ID) Device (State, Local, and Tribal Governments and Businesses) – New to 0579-0327
A State animal health official, producer, market, accredited veterinarians, or research facilities may make an informal request to a State, Tribe, or VS Assistant District Director for the purposes of using more than one official ID device. The use of multiple official ID devices or methods with multiple official ID numbers for a single animal could impede efforts to track the animal’s movements. A State or Tribal animal health official or VS Assistant District Director could approve an application of a second official ID device by a State animal health official, producer, market, accredited veterinarians, or research facility in specific cases when the need to maintain the identity of an animal is intensified. Such needs could arise where an animal is exported to a country that requires multiple forms of ID, or for research field trials (including field trials conducted by nonprofit entities) where the loss of identification would hurt the outcome of the study. Approval would not be given simply for the convenience of individuals testing livestock or certifying them for interstate movement. The approval process is not formal and would be determined locally. All 50 States and the territories of Puerto Rico and the Virgin Islands may have a few instances each year that would require permission to apply multiple ID devices.
9 CFR 86.1: Application for and Approval of an Approved Tagging Site (State, Local, and Tribal Governments and Businesses) – New to 0579-0327
An approved tagging site is a premises, such as a livestock market or other private sale venue, authorized by APHIS, State, or Tribal animal health officials, where livestock may be officially identified on behalf of their owner or the person in possession, care, or control of the animals when they are brought to the premises. Such sites give producers a safe and convenient alternative, not provided for in the existing regulations, to identifying their animals themselves. The regulation at 9 CFR 86.4(b)(1)(i)(C) allows producers to use this alternative when they cannot tag animals at their farm or ranch. For livestock auction markets, tagging site approval will be incorporated into the process for approving livestock facilities outlined at 9 CFR 71.20. Businesses that do not fit the definition of “approved livestock facility” will request an APHIS evaluation by phone or e-mail. APHIS personnel will then conduct an onsite inspection before approving the site.
9 CFR 86.7: Evaluation of States and Tribes (State, Local, and Tribal Governments) – New to 0579-0327
Because APHIS has not yet finalized the performance standards, it is not presently proposing to add to the regulations a description of the process APHIS will use to evaluate State and Tribal performance or requirements for conducting such evaluations. APHIS is reserving 9 CFR 86.7 for evaluation requirements. APHIS is considering making evaluation part of the administration of the cooperative agreements used to fund traceability in the States and Tribes. The process would involve examination of actual animal traces for disease program work as well as supplemental test exercises. Traceability plans would also be examined during routine disease program and station reviews conducted periodically by the VS Districts. These evaluations would not involve the producers directly, but would focus on the ability of the State or Tribe to conduct specific traceability activities that serve as indicators of tracing capability. Such activities, under the performance standards currently under consideration, would include:
Notification of the State or Tribe where the animal was initially identified by the receiving State or Tribe of the official identification of a reference animal.
Confirmation by the State or Tribe where the animal was initially identified that it was the State or Tribe where the reference animal was officially identified and that it has documentation to that effect. This documentation should contain the contact information for the person who received the identification number.
Notification by the receiving State or Tribe of the State or Tribe from which the reference animal was moved regarding that animal’s official identification number.
Determination by the State or Tribe from which the reference animal was moved of the address from which the reference animal was shipped.
APHIS will collect information on State and Tribal abilities to carry out these four activities for each species covered by the traceability regulation. This information will help APHIS establish firm measurements for evaluating the performance of States and Tribes.
The “reference animals” are the animals used to evaluate State or Tribal ability to meet the performance standards. APHIS could randomly select reference animals for a test exercise or could select animals included in actual disease traceback investigations. States and Tribes would be evaluated on their ability to trace animals moved only in accordance with the new regulation.
9 CFR 86.6: Documentation of Completion of Performance Measures (State, Local, and Tribal Governments) – New to 0579-0327
APHIS does not currently have the data necessary to establish performance standards for States and Tribes and is not proposing to add any to the regulations at this time. APHIS is reserving 9 CFR 86.6 for the performance standards. States and Tribes have been administering defined performance measures as part of the cooperative agreements that provide traceability funding.
9 CFR 86.1 and 86.5: Commuter Herd Agreement (State, Local, and Tribal Governments and Businesses) – New to 0579-0327
A commuter herd agreement is a written agreement between the owner(s) of a herd of cattle or bison and the animal health officials for the States or Tribes of origin and destination specifying the conditions required for the interstate movement between premises during normal livestock management operations. The agreement is usually effective for 1 year and may be renewed annually. The agreement conditions are determined by the animal health officials of the States or Tribes involved in the movement as well as the producer who requests the movement. A copy of the agreement bearing original signatures must accompany each movement. APHIS has no role in preparing or signing the agreement and does not receive a copy; it only requires that the signed document accompany shipments of animals moving interstate.
9 CFR 86.6: Collection of ID Devices at Slaughter (Businesses) – New to 0579-0327
APHIS needs correlation of ID devices with carcasses and/or diagnostic samples collected at harvest facilities to support tracebacks where disease is found in an animal after slaughter. All manmade ID devices affixed to livestock moved interstate must be removed at slaughter and correlated with the carcasses through final inspection. If diagnostic samples are taken, the devices must be packaged with the samples and correlated with the carcasses. Slaughter plant personnel handle this activity under the supervision of the Food Safety and Inspection Service (FSIS) under 9 CFR 310.2. Handling of devices after final inspection is being determined based on the needs of both APHIS and FSIS.
9 CFR 86.4: Obtaining Official Eartags for Cattle not Currently Required to be Identified with Official Eartags (State, Local, and Tribal Governments and Businesses) – New to 0579-0327
Official eartags are used for official identification of cattle under the existing regulations and will continue to be used under the new traceability regulations. Brands, breed association tattoos, and other forms of identification will no longer be accepted as official ID. Cattle (with some exceptions) will need to be identified with an official eartag. This will initially be limited to breeding animals, dairy breeds, and animals involved in shows, exhibitions, and recreational events. APHIS will make metal eartags available to producers free of charge to facilitate compliance with this requirement. These tags can be ordered through State or Tribal animal health officials in a manner that best fits local needs; State and Tribal animal health officials will process tag orders placed with them and obtain tags for producers. Producers also may obtain official eartags directly from tag manufacturers or distributors; or through accredited veterinarians, livestock market operators, breed associations and livestock production records associations, or other entities. Tag orders may be placed by phone, Internet, fax, or mail.
9 CFR 86.4: Application of State Shield (State, Local, and Tribal Governments) – New to 0579-0327
If States so choose, they can manufacture official eartags bearing the State postal code instead of the U.S. shield that will appear on eartags APHIS supplies. States choosing this option must work directly with the manufacturer to produce the tag. About 10 States have chosen this option.
9 CFR 86.3: Official Identification Device Distribution Records (State, Local, and Tribal Governments and Businesses) – New to 0579-0327
Although applying eartags will be the producers’ responsibility, States and Tribes will be primarily responsible for recording distribution information in a way they can access quickly. Breed and registry associations, accredited veterinarians, and eartag distributors must report to the State or Tribe from which they receive tags the numbers of any tags they issue to livestock operations. States and Tribes must keep the records for a minimum of 5 years, as they are critical in helping APHIS determine the origin of animals that move interstate.
Record of tags issued
Entities that distribute official eartags must keep a record of tags issued to ensure accountability in the distribution system and to ensure that APHIS has the traceability information needed for disease control, eradication, and surveillance efforts. The record may be electronic or paper-based depending on the volume of tags and the needs of the State or Tribe. States, Tribes, and territories must collect sufficient contact information about where official eartags are distributed to meet their traceability needs. At a minimum, the record must include:
The name of the person receiving the tags.
The street address, city, State, and ZIP code where the tags are distributed.
The identification numbers issued.
The date the tags were issued.
The name and contact information of the person issuing the tags.
Record of tags applied
State and Tribal animal health officials and accredited veterinarians using the tags for official disease program work must record sufficient contact information about where official eartags are applied (not just the person to whom they are issued) to meet the traceability needs of the State, Tribe, or territory. Producers will not be required to record or report the application of tags to animals on their livestock operation. At a minimum, the record must include:
The name of the owner of the livestock operation where the tags are applied.
The street address, city, State, and ZIP code where the tags are applied.
The identification numbers applied.
The date the tags were applied.
The name and contact information of the person applying the tags.
If the State, Tribe, or territory uses the Animal Identification Management System (AIMS) to record tag application records, the record must include a premises identification number or State location identifier.
9 CFR 86.3 and 86.5: Certificate of Veterinary Inspection and Recordkeeping (State, Local, and Tribal Governments and Businesses) – New to 0579-0327
Currently, all States require certificates of veterinary inspection (CVIs) for breeding cattle received from other States. The CVI, completed by an accredited veterinarian from information the producer provides, documents that the veterinarian inspected the animals and found them free of reportable disease. The CVI must show the species of animals covered by the certificate; the number of animals covered; the purpose for which the animals are to be moved; the departure address; the destination address; and the names and addresses of the consignor and the consignee (if different from the departure and destination addresses). Additionally, unless APHIS’ species-specific CVI requirements provide an exception, the CVI must list the official identification number of each animal, or group of animals, moved that must be officially identified. If an alternate form of identification has been agreed on by the sending and receiving States or Tribes, the CVI must include a record of that identification.
The traceability regulations establish current State practice as a Federal requirement for interstate movement. A State representative, or an accredited veterinarian, issuing an CVI must enter all the required information, retain a copy for his or her records, provide a copy to accompany the shipment, and forward a copy of the certificate to the State animal health official in the State of origin within 5 business days. The State of origin will forward a copy to the State of destination within 5 business days. APHIS has no role in preparing the CVI and does not receive a copy.
States must retain received CVIs so they can be easily found. The records must be kept for a minimum of 5 years to ensure that information is readily available to facilitate animal disease investigations.
9 CFR 86.4(d): Unauthorized Removal or Loss of Official ID Devices (State, Local, and Tribal Governments and Businesses) – New to 0579-0327
Removal of official identification devices can impair APHIS’ ability to find the source of a disease outbreak or document the absence of disease. Thus, removal needs to be approved except when the animal is presented at slaughter, rendering, diagnostic labs, or other termination points. State animal health officials or the VS Assistant District Director will approve removal on request and after evaluation of the request at the local level by contacting the requestor by phone, fax, or e-mail.
9 CFR 86.4(e): Reporting Retagging Animal Records – Removed, Lost, Stolen, or Misplaced Tags and Recordkeeping (State, Local, and Tribal Governments and Businesses) – New to 0579-0327
If an eartag has to be removed, the State, Tribal, or territorial animal health official will record in a manner that meets State, Tribal, or territorial needs the date the device is removed, contact information (address and phone number) for the location at which the device is removed, the official number of the device being removed, device type, reason for removal, the new identification number, and the type of replacement tag. APHIS requires States and Tribes to keep records of replaced removed tags at the local level, although replacement does not need to be reported to APHIS at this time. The record must be maintained for 5 years to ensure that multiple devices issued to a single animal do not result in multiple traces.
If an animal loses an official identification device and needs a new one, the person (who could be a producer as well as a State, Tribal, or Federal employee) applying the new official identification device must record the following information: The date the new device is added; the official identification number on the device; and the official identification number on the old device if known. The record must be maintained for 5 years to ensure that multiple devices issued to a single animal do not result in multiple traces. Further, VS personnel, State and Tribal animal health officials and their staffs, and accredited veterinarians who use official eartags for program work must report to the Assistant District Director (using the same information) by phone, fax, or e-mail, the loss, theft, or misuse of eartags. This ensures that no one uses tags fraudulently or irresponsibly so as to prevent the accurate traceability of the animal bearing the tag. Private individuals are encouraged but not required to report misused tags.
The ADT information system, initially developed to support the NAIS, is being maintained as an option that States and Tribes may use to support their traceability plans. The systems support the identification of premises, recording information on official animal identification devices and animal events.
9 CFR 86.1 and 86.2: Premises Identification (Business) – New to 0579-0327
The premises identification databases provide a system that can issue premises identification numbers to locations where animals are raised (such as farms and ranches) and held for other purposes (such as markets and clinics). The States and Tribes administer the identification of premises within their geographic areas. APHIS has provided the Standardized Premises Identification System (SPIS) online information system for States and Tribes to use to do this. Forty-one States, seven Tribes, and two Territories currently use the SPIS. APHIS is responsible for its development, enhancement, maintenance, and operation.
States and Tribes may also choose to use a compliant premises identification system. These are either State systems or third-party systems evaluated and determined to be compliant with ADT data standards. These systems are also available online and operate the same way the SPIS does; however, States or third parties administer, develop, enhance, maintain, and operate these systems.
Minimal premises information is forwarded automatically by the information systems to the national repository. Premises owners and operators supply the necessary information to the States or Tribes using the communication method most convenient to them (such as the Internet, hard copy, fax, email, or telephone call). After the initial information is entered, the premises information in the database is updated if premises go out of business, come into existence, change ownership, or experience any other noteworthy changes to their operations that should be recorded.
9 CFR 86.1 and 86.2: Updates to Premises Identification Records (Business) – New to 0579-0327
Updateable premises information maintained on the SPIS includes:
The PIN.
The name of the entity.
The owner or appropriate contact person.
A contact phone number.
The premises address.
The date the operation was activated.
The date the operation changed ownership or ceased operations.
The reason for this event.
The names and phone numbers of previous contact persons.
This data is automatically maintained for 20 years at the State level in the SPIS. This will give animal health officials the proper contact reference when the current contact person was not associated with the premises during the period being researched in a traceback situation.
Contact information maintained in the SPIS opens the lines of communication between producers and animal health officials, which is critical in preventing the spread of disease. State animal health officials can query their respective databases to determine what locations are in a general area during a disease outbreak or incident and can then use the contact information to inform producers and animal owners about potential disease exposure and steps they should take to protect their animals. Although it is impossible to predict how many disease traceback investigations, emergency responses, or disease outbreaks might occur in the next 3 years that would cause State animal health officials to access premises information, these occasions are relatively infrequent and localized.
9 CFR 86.1 and 86.2: Nonproducer Participant Registration (Business) – New to 0579-0327
In addition to the premises identification, the ADT framework provides for the issuance of Nonproducer Participant Numbers (NPN) to entities that do not hold or manage livestock, but are otherwise involved with the food animal industry and could become a vital investigative link during an emergency traceback. These entities include State animal health officials, accredited veterinarians, Animal Identification Number (AIN) managers or resellers (individuals or firms responsible for distributing AIN devices to producers), official identification device manufacturers (companies that manufacture official identification devices), diagnostic laboratories, livestock buyers and dealers, and others who submit data to information systems.
9 CFR 86.1 and 86.2: Official Identification Device Applications and Approved Identification Device Manufacturer Agreements (VS Form 1-64, VS Form 1-65, and VS Form 1-66) (Business) – New to 0579-0327
(This includes applications for all official identification devices provided for through the ADT, primarily AIN devices, visual only eartags, radio frequency identification (RFID) eartags, and RFID injectable transponders)
Approved identification device manufacturers are companies authorized by APHIS to manufacture approved identification devices. They are responsible for the overall production and quality of the devices. Approved identification device manufacturers may only produce devices with the official identification numbers as defined in the agreement.
Approved device manufacturers must:
Complete and submit the Official Identification Device Manufacturer Application.
Abide by the terms and conditions set forth in the agreement.
Imprint the official identification number, the official shield or emblem, and other print criteria as specified in the device application or as otherwise provided by APHIS.
Maintain the uniqueness of the official identification numbers they are permitted to use.
Report the shipment of all AIN devices to the AIN Management System according to established protocols within 24 hours of shipment.
Have an operational computerized system for AIN devices that communicates with the AIN Management System and is compatible with ADT standards to maintain the necessary information, including a database of the manufacturer product codes for all devices that contain an AIN device.
Be able to support the distribution of their official identification devices.
Agree to discontinue the printing of any identification numbering system as prescribed by USDA if USDA phases out the numbering system.
Maintain a current inventory of official identification devices and make it available to USDA on request. (Note: This does not require additional recordkeeping; the manufacturer must simply agree that USDA has the right to review the current inventory of AIN devices at any time.)
Designate nonproducer participants they wish to use as AIN device managers into the AIN Management System.
APHIS provides device manufacturer applications which can be requested by contacting ADT staff directly via email or phone. Device manufacturers provide information about their devices, which APHIS uses to determine whether to approve the company’s or entity’s device as an official device for use in the ADT system.
9 CFR 86.1 and 86.2: AIN Device Manufacturer Updates and Recordkeeping (Business) – New to 0579-0327
After obtaining an NPN, entities update information as the need arises (such as when a contact person or phone number changes). Nonproducer participants who submit information to the AIN Management System routinely maintain records associated with their animal identification activities for 5 years. This recordkeeping is standard business procedure, and is often required by Federal regulations if the entity or individual is involved in other USDA programs. This information is maintained because it could provide critical information during a disease traceback.
9 CFR 86.1 and 86.2: AIN Device Managers Registration and Agreement (Business) – New to 0579-0327
AIN device managers are individuals, organizations, or companies that provide AIN devices to livestock owners or another AIN device manager or reseller. The AIN device manager must have an established relationship with an AIN device manufacturer.
To be an authorized AIN Device Manager, the individual or firm must agree to:
Abide by the terms and conditions set forth in the AIN Device Manager Agreement (Note: The agreement simply asks the individual or firm to abide by the terms or conditions listed below. All of the information about the manager or reseller will already be in the AIN Management System because only entities with an NPN will be eligible to become AIN device managers.)
Maintain an ongoing list of inventoried AIN devices received from an authorized AIN manufacturer device manufacturer or AIN manager and make that list available to USDA on request. (Note: This list does not require additional recordkeeping; the manager must simply agree that USDA has the right to review the current inventory of AIN devices at any time.)
Validate the PIN or NPN of the premises or entity that is to receive AIN devices and provide such PIN or NPN to the entity shipping the tags. The shipper reports the distribution record to the AIN Management System or to the AIN device manufacturer for orders shipped direct from the manufacturer.
Submit a record to the AIN Management System of all AINs they possess and ship or deliver to a premises, in accordance with prescribed protocols.
Educate customers on the proper use of official identification devices.
The AIN device managers will apply online using the AIN Management System, confirming the AIN device manufacturers or other device managers with whom they have an established relationship. The entity will be recognized as an AIN manager on confirmation of an established relationship with an AIN device manufacturer.
9 CFR 86.1 and 86.2: AIN Device Manager Updates (Business) – New to 0579-0327
AIN device managers provide updated information regarding changes in status (contact person’s name, phone number, etc.) as the need arises. This information is maintained because it could provide critical information during a disease traceback.
9 CFR 86.1 and 86.2: Cooperative Agreements (State, Local, and Tribal Governments and Businesses) – New to 0579-0327
USDA has been working with States, Tribes, and industry to advance animal disease traceability since June 2004. USDA has provided funding through cooperative agreements to participants to further develop and implement the system components. USDA and APHIS have used Government-wide fund allocation (SF-424, SF-424A, and SF-424B) and lobbying disclosure (SF-LLL) forms in processing cooperative agreement request packages.
9 CFR 86.1 and 86.2: Cooperative Agreement Application (State, Local, and Tribal Governments) – New to 0579-0327
APHIS provides Federal support for ADT implementation activities and infrastructure within participating States, Tribes, and territories through cooperative agreements. Cooperative agreement awards require quarterly reporting and Federal oversight of the successful completion of the goals and objectives outlined in the cooperative agreement workplan (a part of the application package).
ADT cooperative agreement funding is provided to advance animal disease traceability. Each participant will be required to evaluate, describe, and identify animal disease traceability risks within State or Tribal boundaries. Workplans will describe how each applicant will reduce those risks and advance animal disease traceability. Because States, Tribes, and territories have made varying progress to date, this approach will allow each applicant the flexibility needed to advance animal disease traceability appropriately for its individual situation.
9 CFR 86.1 and 86.2: State/Tribe Quarterly Reports (State, Local, and Tribal Governments) – New to 0579-0327
States and Tribes give APHIS quarterly accomplishment reports. The reports include accomplishments achieved as defined in their cooperative agreement workplans. States and Tribes also use the reports to acknowledge current tracing capabilities based on APHIS’ current traceability performance measures documents. Statistics relative to interstate movements of livestock are similarly reported.
9 CFR 86.1 and 86.2: ANIMAL DISEASE TRACEABILITY (ADT) ROAD MAP
Develop ADT Roadmap using templates
Submit ADT Roadmap for approval
(State, Local, and Tribal Governments)
The ADT Road Map serves as a strategic 3-year plan for advancing animal disease traceability within the jurisdiction of each State, Tribe, or territory. Each applicant must prioritize its program objectives and needs according to its traceability capability. By forecasting objectives and needs by 3 years, applicants can plan more consistently year to year and substantially reduce the time involved in planning and developing annual cooperative work plans. Concurrently, APHIS will have collective information to better project and enhance justification and submission of annual Federal budget requests and associated accountability.
APHIS has an ADT Road Map template. Initial Road Maps, updates, and renewals must be approved by VS District personnel and filed in the appropriate District Offices. The Districts will approve the Road Maps using a checklist addressing the items in the Table of Contents.
Each applicant may address each item or add to the Road Map in a way that will benefit its own traceability needs. The template includes six required items that directly support the implementation framework and measure national traceability capability:
1. The applicant must list at least four animal disease traceability performance measures.
2. The applicant must indicate intended use of official metal ear tags to be applied by persons other than State or Federal animal health officials or accredited veterinarians.
3. The applicant must identify its tag distribution recordkeeping system(s).
4. The applicant must identify how and when animal disease traceability data will be shared with other States, Tribes, territories, and APHIS.
5. The applicant must describe an outreach plan that explains its traceability plan and the ADT Road Map to accredited veterinarians, livestock markets, and industry, including small producers and those in underserved areas.
6. The applicant must describe a plan to monitor the activities described in the Road Map and to report interstate movement activity. This plan must include:
a. The number of ICVIs and other interstate movement documents created within the State, Tribe, or territory.
b. The number of ICVIs and other interstate movement documents received for animals moved into the State, Tribe, or territory.
c. The number of animals by species and class moving into the State, Tribe, or territory, indicating the number of animals officially identified and the number not officially identified.
d. The number of animals by species and class moving out of the State, Tribe, or territory.
e. Volume of distribution for each official numbering system used or device issued, including backtags.
The ADT Road Map prioritizes implementation objectives and is updated with every cooperative agreement workplan. This provides consistency and transparency in implementing animal disease traceability.
USE OF NUES EARTAGS: GENERAL PROVISIONS
9 CFR 86.4: Eartag Orders (State, Local, and Tribal Governments)
To obtain NUES eartags from USDA through the VS supply warehouse, the State, Tribe, or accredited veterinarian, will need to provide the designated VS order taker their mailing address and the number of tags they are ordering. Tag orders may be placed by telephone, fax, or mail. Orders may also be placed online; the next item covers orders placed over the Internet. Because VS uses tag information to trace animals during disease events, NUES tags are accountable property. Those who distribute, receive, and apply them must maintain appropriate records as described below and keep the tags in a secure place before applying them.
9 CFR 86.1 and 86.2: Program Site Tag Information Sheet (APHIS Form 453-R) (State, Local, and Tribal Governments)
Users will also be able to order NUES eartags through the National Logistics Support Center Web site operated by the National Oceanic and Atmospheric Administration using APHIS form 453-R. Users will be able to obtain this form online. The form asks requestors to provide their shipping address, the date of the request, a telephone number, the type and number of tags requested, and yearly usage. Once the supply warehouse receives the form, the requestor receives account access with a user ID and password, and can then use the site to order tags.
9 CFR 86.4: Record of Tags Issued and Applied and Recordkeeping(State, Local, and Tribal Governments and Businesses)
Federal employees of the VS supply warehouse keep a record of tags issued to States and Tribes, and States and Tribes also must keep records of tags issued to accredited veterinarians, field employees, and producers. Accredited veterinarians will also apply tags and keep records in the course of disease program work. The recordkeeping ensures accountability in the distribution system so that tags can be quickly matched to animals during a disease event. The records may be electronic or paper-based depending on the volume of tags and the needs of the State or Tribe. States and Tribes (and accredited veterinarians as appropriate) must collect sufficient contact information about the NUES tag recipients to meet the traceability needs of the State or Tribe, which in turn ensures that not only State but Federal animal health officials have the traceability information needed for disease control, eradication, and surveillance efforts. At a minimum, the record must include:
1. The name of the person receiving the tags.
2. The street address, city, State, and ZIP code where the tags are distributed.
3. The identification numbers issued.
4. The date the tags were issued.
5. The name and contact information of the person issuing the tags.
States and Tribes will need to maintain a record of NUES eartags issued for a minimum of
5 years to support Federal animal disease tracing ability.
9 CFR 86.3: Coordination of Tag Orders with Manufacturers and Recordkeeping (State, Local, and Tribal Governments and Businesses)
States and Tribes (as well as Dairy Herd Information Association members) may choose to purchase their own tags and not use the free ones available from VS. If so, they will need to make sure that the manufacturer and tags are APHIS-approved and that the number sequence is carefully coordinated with the manufacturer to avoid duplicate tag numbers. States and Tribes will also need to maintain a record of these NUES eartags, including all contact and issuance information, for a minimum of 5 years to support Federal traceability efforts.
9 CFR 86.4(b)(1): Reporting Loss, Theft, or Misuse of NUES Tags (State, Local, and Tribal Governments and Businesses)
VS personnel, State and Tribal animal health officials and their staffs, and accredited veterinarians who use NUES tags for program work must report loss, theft, or misuse of NUES tags to the District Director. This ensures that no one uses tags fraudulently or in a manner that would prevent VS’s ability to accurately trace the animal to which the tag was applied. Producers are encouraged to report, but are not required to do so. VS does not have an established reporting method, but the following information, at a minimum, should be reported:
1. Name and address of the reporting individual.
2. The tag numbers of lost, stolen, or misused tags.
3. The date of the occurrence.
4. A description of what happened.
9 CFR 86.4: Removal or Replacement of Eartags (State, Local, and Tribal Governments and Businesses)
If an eartag has to be removed, the State or Tribal animal health official or accredited veterinarian will record in a manner that meets State or Tribal needs the date the device is removed, contact information (address and phone number) for the location at which the device is removed, the official number of the device being removed, device type, reason for removal, the new identification number, and the type of replacement tag. The information will support VS’ ability to trace animals during a disease event. For that reason, VS requires that the records be retained for 5 years.
USE OF NUES EARTAGS IN OFFICIAL DISEASE PROGRAM ACTIVITIES
9 CFR 86.4: Record of Tags Applied (Recordkeeping) (State, Local, and Tribal Governments and Businesses)
State and Tribal animal health officials and accredited veterinarians using NUES tags for official disease program work must record and make available to VS sufficient contact information about where the eartags are applied (not just the person to whom they are issued) to meet the traceability needs of the State or Tribe. This ensures that not only State but Federal animal health officials have the traceability information needed for disease control, eradication, and surveillance efforts. At a minimum, the record must include:
The name of the owner of the livestock operation where the tags are applied.
The street address, city, State, and ZIP code where the tags are applied.
The identification numbers applied.
The date the tags were applied.
The name and contact information of the person applying the tags.
If the State or Tribe uses the AIMS to record tag application records, the record must include a premises identification number or State location identifier.
The operation owner names and addresses are drawn from existing information collected by the States and Tribes for disease program purposes and do not need to be collected separately when tags are applied. The person who applies the tags for disease control programs will be a State, Tribal, or Federal animal health official, who would only have to provide the date the tags were applied.
NUES EARTAGS DISTRIBUTED OUTSIDE OF DISEASE PROGRAMS
State and Tribal animal health officials may provide NUES eartags to producers who wish to use them for official identification and other purposes without administering the eartags through a specific disease program. This lets producers use the eartags to qualify their animals for interstate movement under the ADT framework. Distribution of tags is conducted in a way that meets local needs and resources. State or Tribal animal health officials will always oversee the integrity of the information collected when NUES tags are distributed outside of specific disease programs.
To comply with the ADT framework, and ensure that APHIS has adequate data to trace animals, State and Tribal animal health officials must conduct the following information collection or recording activities:
9 CFR 86.3: Informing VS of Tag Orders( State, Local, and Tribal Governments and Businesses)
VS needs to know which States and Tribes wish to distribute tags to producers so that it can make enough tags available and can budget accordingly. States and Tribes need to let VS know which numbering format they wish to use because the number-prefixed tags are purchased directly by VS, and the alpha-prefixed tags are purchased by the States and Tribes. The State or Tribal designated official lets the District Director know that the State or Tribe wishes to distribute tags and the District Director reports the information to a member of the VS animal traceability staff.
9 CFR 86.3: Tribe Tag Distribution (State, Local, and Tribal Governments and Businesses)
Tribes will need to inform VS which of the following options they will use to issue tags so that their sovereign decision is clear:
1. Obtaining NUES eartags through the State in which the Tribe is located, and working with the State to maintain records as set forth under the general provisions section of this collection.
2. Obtaining NUES eartags through the appropriate VS District Office, and working with the Area Office to maintain records as set forth under the general provisions section of this collection.
3. Issuing Tribe-specific NUES eartags to Tribal producers, and maintaining records according to the needs of the Tribe.
USE OF NUES EARTAGS IN THE DAIRY HERD INFORMATION ASSOCIATION (DHIA)
9 CFR 86.4(b)(1): DHIA Eartag Distribution Plan (State, Local, and Tribal Governments and Businesses)
Each State or Tribe where DHIA has tag distribution must have an eartag distribution plan prepared, agreed to, in writing, and signed by the District Director, the State or Tribal animal health official, and the State DHIA representative. The information in the plan coordinates DHIA tag use with Federal traceability information needs. The plan will set aside a block of numbers for cattle to be identified, a description of the current recordkeeping system, and a description of the DHIA responsibilities for maintaining a database of all DHIA eartag numbers. The plan will also set forth methods for recording information about the herd to which the eartags are applied or distributed to meet the traceability needs of the State or Tribe where the animals are identified. The District Director, the State or Tribal animal health official, and the State DHIA representative, as well as the tag manufacturer, each receive a copy of the plan.
9 CFR 86.1 and 86.2: VS 1-63, Tribal Location Identification System Implementation Request (State, Local, and Tribal Governments)
Tribes can designate which premises information system they would like to use, if any, by completing VS 1-63. The form states what system the Tribes want to use, if any, and lets APHIS know how to help Tribes in using the system. APHIS will use the information provided on VS 1-63 to contact the respondents and help them use the premises information system they selected. APHIS will access this information only once to initiate the process. The information also helps ensure that when multiple parties claim to represent Tribes in managing location identification information, USDA only deals with those entities the Tribe has officially recognized.
Recordkeeping for VS 1-63
The Tribal organization will retain the VS 1-63 for 3 years to answer any inquiries concerning which system the Tribe is using.
9 CFR 86.4(b)(1): DHIA Tag Application Record (Businesses)
DHIA will record, in a database, the identification tag numbers when the animals are identified (or when tags are issued to producers to apply) and information about the herd in which the eartags are issued or applied. The record should include sufficient information about the location where NUES eartags are distributed or applied to meet the traceability needs of the State or Tribe and ensure that not only State but Federal animal health officials have the traceability information needed for disease control, eradication, and surveillance efforts. At a minimum, the record must include:
1. The name of the owner of the herd where tags are issued or applied.
2. The street address, city, State, and ZIP code of the livestock operation where the animal identification tags are issued or applied.
3. The official identification numbers issued to the producer or applied to the animals.
4. The date the tags are issued or applied.
5. Name and contact information of the person who issued or applied the tags.
6. If the State or Tribe uses the AIMS to enter tag distribution records, a premises identification number or State location identifier is required.
The herd owner names and addresses are drawn from DHIA’s member database and do not need to be collected separately when tags are issued. If a DHIA field representative applies the tags, he or she would only have to provide the date the tags were applied and the official identification numbers used. If the tags are provided to the producer to apply, the DHIA field representative would only need to provide the date the tags were issued and the official identification numbers. The producer has no recordkeeping or reporting responsibility.
The DHIA must keep records of the numbers for a minimum of 5 years after the eartags are applied, as the records are critical in helping VS determine the origin of animals during a disease investigation. DHIA representatives must also send a report to VS at least monthly. The report must include the information that DHIA records when tags are issued or applied.
3. Describe whether, and to what extent, the collection of information involves the use of automated, electronic, mechanical, or other technological collection techniques or other forms of information technology, e.g., permitting electronic submission of responses, and the basis for the decision for adopting this means of collection. Also describe any consideration of using information technology to reduce burden.
Application for Use of More Than One Official ID Device
This will be managed at the local level in a way that best suits the needs of the State or Tribe; therefore, whether it is, or can be, automated, is not determinable at the Federal level.
Application for and Approval of an Approved Tagging Site
States handle approving tagging sites. At least some States have formal applications for approval; such applications ask for the name of the entity seeking approval; the name, address, email address, and phone and fax number of the person responsible for the site; the site’s PIN, if applicable; and the type of tags to be applied at the site. Both the responsible person and the State animal health official sign the document. The document also sets forth the regulatory responsibilities of the approved tagging site and the State animal health official relative to issuing and applying tags. APHIS has a system for evaluating livestock markets to be federally approved livestock facilities, and the approval of a tagging site could also become part of that process to consolidate functions and eliminate redundancies. The current market approval system is not automated because it requires onsite inspections.
Evaluation of States and Tribes
We do not currently have an automated system to evaluate State or Tribal traceability plans or adherence to traceability performance standards. As we continue to collect baseline traceability implementation data over the next several years, we expect to have the necessary information to design and implement an electronic evaluation system. At the moment we expect the work to involve onsite review of documents and procedures which would not qualify this task for electronic submission.
Documentation of Completion of Performance Measures
At the moment we expect the work to involve onsite review of documents and procedures which would not qualify this task for electronic submission.
Commuter Herd Agreement
An original copy of the Commuter Herd Agreement must accompany the herds during movement, and is therefore not a candidate for electronic submission.
Collection of ID Devices at Slaughter
The original devices must physically accompany samples to the laboratory for identification purposes. The devices must also be available to collect information in or on the device that could be useful for tracebacks. For instance, a backtag with a barcode might also have attached some of the hair of the animal from which it was removed. The hair sample could help identify the animal and would be lost if only the number of the backtag was recorded. Thus, this activity is not a candidate for complete electronic submission.
Applying and Obtaining Official Eartags for Cattle Not Currently Identified
USDA has provided a Web-based system, the Animal Identification Management System (AIMS), to order official eartags and track the order to the livestock location to which they are issued. We will make this system available to any State or Tribe that wishes to use it, but will not require its use. The AIMS also serves as a record of eartag distribution.
Official Identification Device Distribution Records
This will be managed at the local level in a way that best suits the needs of the State or Tribe; therefore, whether it is, or can be, automated, is not determinable at the Federal level.
Certificate of Veterinary Inspection
The CVI bearing original signatures must accompany the shipment of animals and thus is not a candidate for electronic submission.
Unauthorized Removal of Official ID Devices
Approval of removal of official ID devices will be managed at the local level in a way that best suits the needs of the State or Tribe; therefore, whether it is, or can be, automated, is not determinable at the Federal level.
Reporting Retagging Records – Removed and Lost Tags
Records regarding replacement of lost or removed tags are kept at the local level. APHIS expects States to keep track of tags distributed to them, which would include keeping information on the AIMS system regarding the disposition of such tags.
Information on premises, nonproducer participant and animal identification devices, and animal event information are all submitted to APHIS electronically. State, Tribe, territory and industry cooperator participants in cooperative agreements submit quarterly reports electronically to the District offices.
Premises Identification
SPIS
APHIS provides the SPIS to States and Tribes that elect to use the system. Each State using the SPIS has its own URL to provide access. Data for States and Tribes is partitioned so that users who log into their State or Tribal system can only access information pertaining to that State or Tribe.
States and Tribes may enable their SPIS for public access so premises owners and operators with Internet access can enter their premises information online. For producers who do not have Internet access, States and Tribes also allow them to submit premises information for entering into the SPIS by mail, fax, email, or phone.
When premises information is entered in the SPIS, whether by a premises owner or operator or by a State or Tribal administrator, the information is entered directly into the State premises identification system database. At a minimum, the address, city, State, and ZIP code is also forwarded to the Premises Allocator/Repository. On address validation, the Premises Allocator assigns a premises number. These electronic transactions occur automatically on submission of premises information. Having only one data entry operation eliminates problems and errors that arise from reentering data at another stage of the registration process.
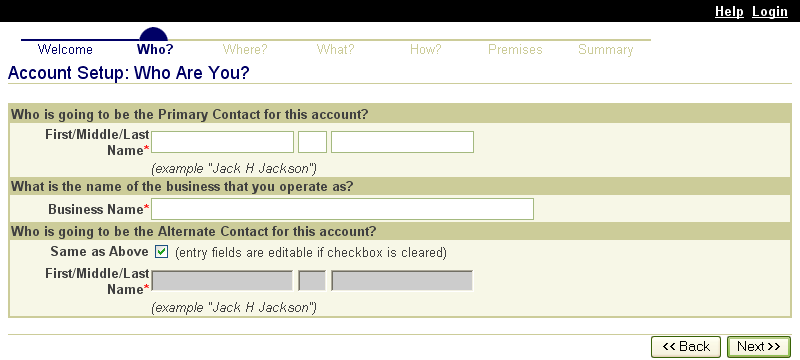
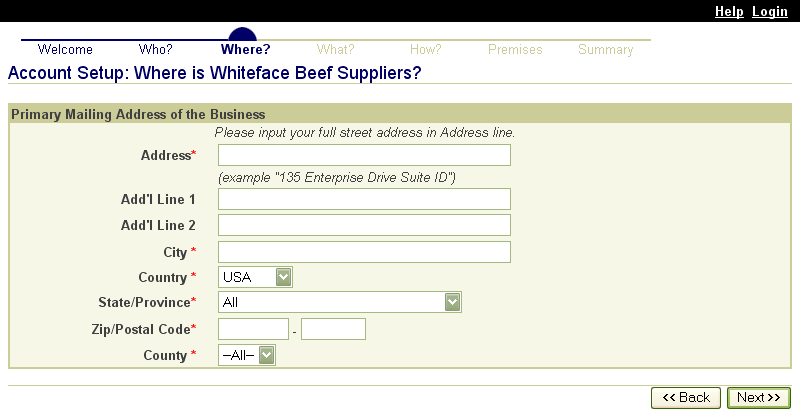
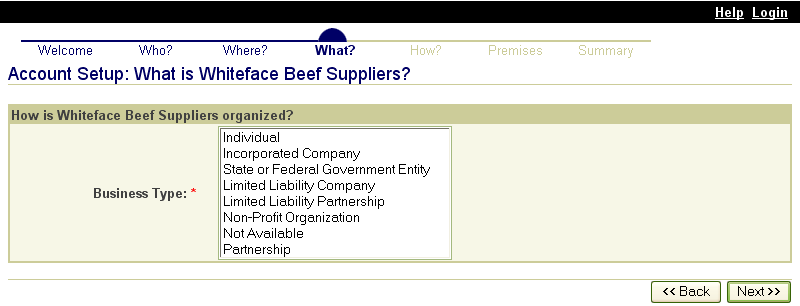
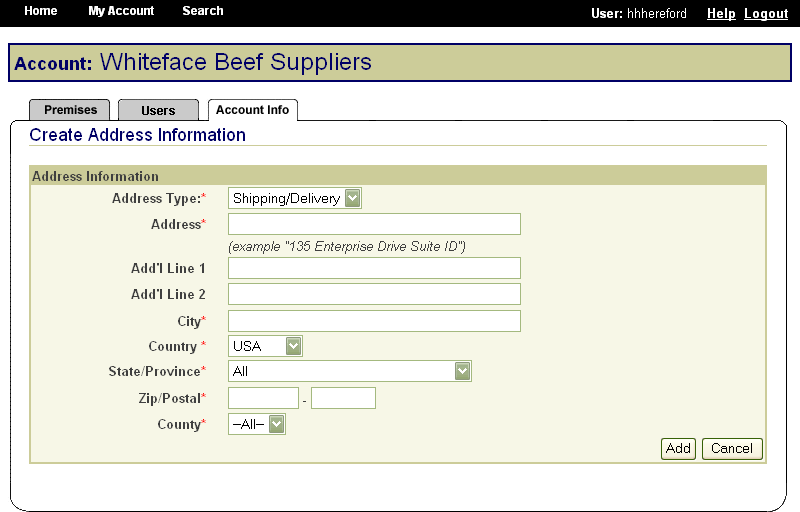
These screen shots from the SPIS are typical of the whole application. The successive screens allow either a premises owner or operator or a State administrator working on the owner or operator’s behalf to submit premises information directly to the State premises identification system and to the Premises Repository/Allocator. Data is entered only once, and some data is stored in both the State and Federal databases. Thus, there is no redundancy in data input, no errors from repeated input of the same data, and no paperwork. This ensures an effective, efficient method for premises registration. |
|
These screen shots from the SPIS are typical of the whole application. The successive screens allow either a premises owner or operator or a State administrator working on the owner or operator’s behalf to submit premises information directly to the State premises identification system and to the Premises Repository/Allocator. Data is entered only once, and some data is stored in both the State and Federal databases. Thus, there is no redundancy in data input, no errors from repeated input of the same data, and no paperwork. This ensures an effective, efficient method for premises registration. |
|
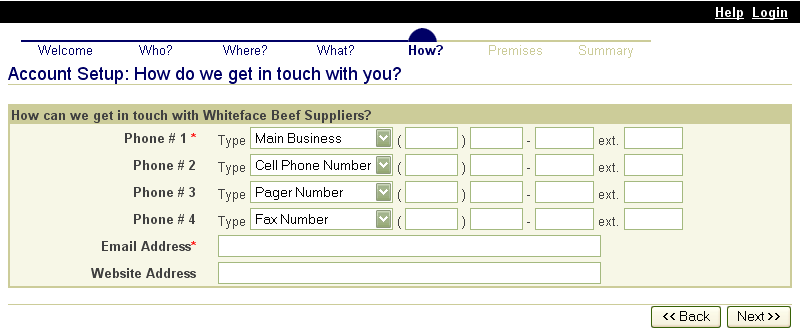
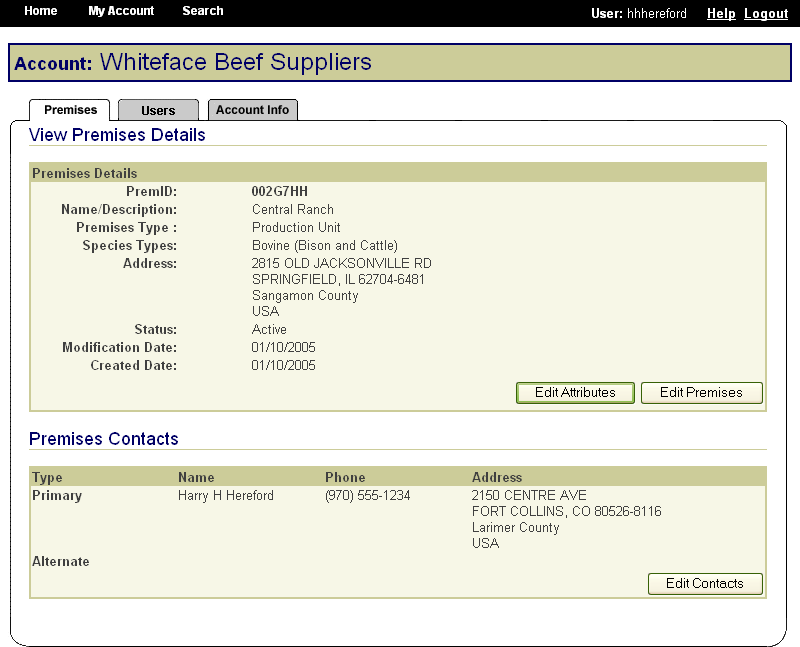
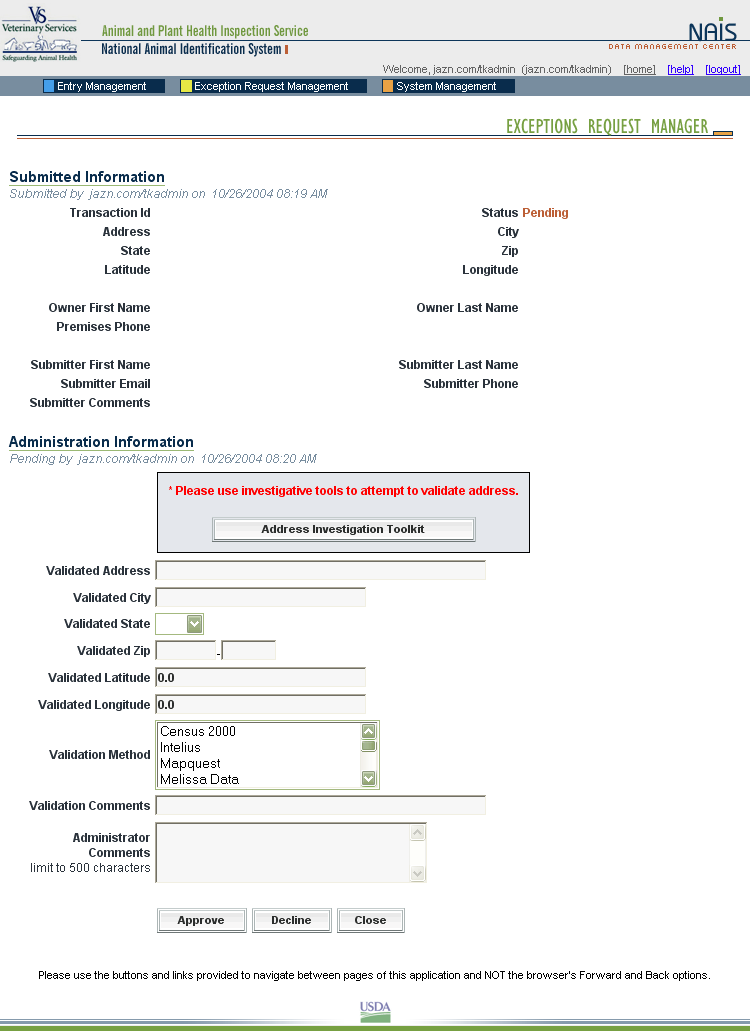
In the same way, State animal health officials who need to view premises information to carry out their duties during a traceback can instantly obtain the required information through the SPIS. State officials can access information for premises locations within their State. This screen is typical of the kind of report that an authorized user can access. |
|
Compliant Premises Identification System (CPIS)
States may also use a CPIS approved by APHIS. Such systems are similar to the premises registration system provided by APHIS.
Exceptions Processing – DMC
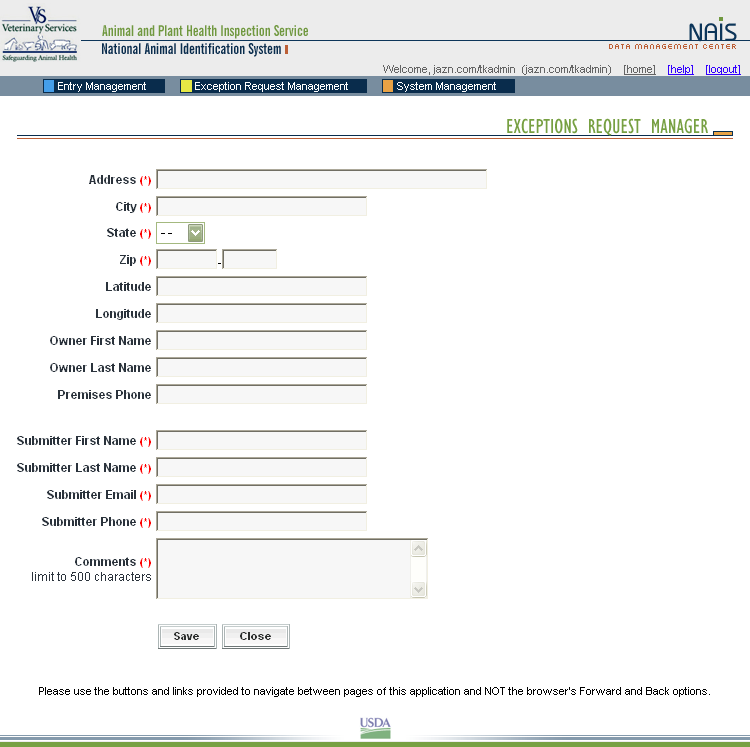
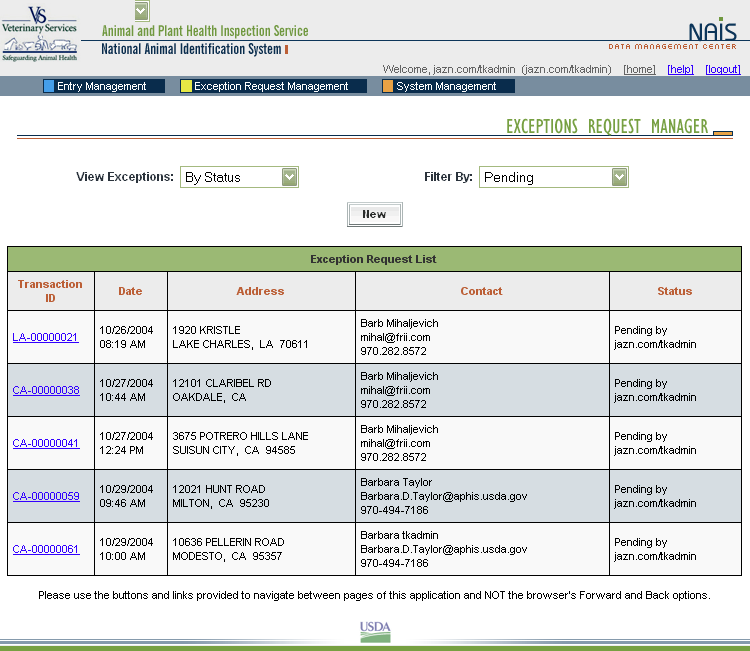
When a premises address is submitted to the Premises Allocator through the SPIS, and the submitted address does not validate, it becomes necessary to process the premises address as an exception. The user sends relevant premises information, including a property description, address information, and GPS coordinates (latitude and longitude). This information is submitted via email to the ADT Help Desk, which processes the exception request. Any additional information that may be required from the premises owner or operator to process the request is obtained either via email or over the phone.
When premises are submitted for exceptions processing, an exceptions manager enters the data received from the administrator for the State where the premises are located into the Data Management Center (DMC). After researching the address, using a resource toolkit of address databases, the exceptions manager will either grant or deny the exception, depending on whether the premises’ location can be accurately identified.
Once an address exception is entered into the DMC, the steps of address validation, research, and exceptions processing are all conducted through the Web-based DMC, which is linked to the Premises Allocator/Repository. Links have been created to other databases to permit research without reentering data, eliminating input errors.
|
|
Animal Identification
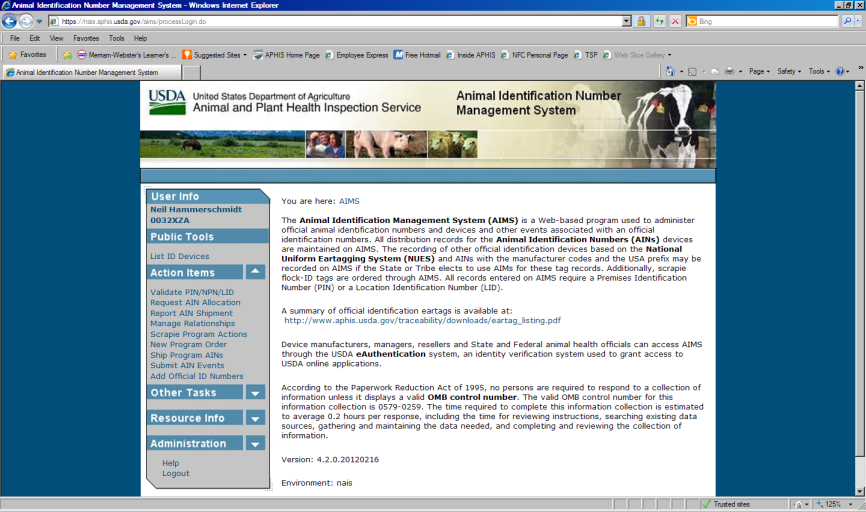
Animal Identification Management System
The Animal Identification Management System (AIMS) allocates Animal Identification Numbers (AINs) at the request of AIN device manufacturers. A record of AINS allocated to each manufacturer is automatically generated on AIMS. Whenever an AIN is shipped to another entity such as an AIN manager, an AIN reseller, or a producer’s premises, the shipment is recorded in AIMS. Those records include the PIN or NPN of the entity that distributed and received the device and the date of distribution.
|
|
Nonproducer Participants
Device manufacturers, managers, and resellers (distributors) are referred to as nonproducer participants. Each nonproducer participant obtains a Nonproducer Participant Number (NPN) through the premises registration system in the State where the headquarters is located (see screenshots under SPIS above, for example of screens that nonproducer participants would fill out online). For example, if the company’s corporate office is in Kansas, it will obtain an NPN through the Kansas premises registration system. All NPNs are unique seven-character numbers similar to PINs.
AIN Device Manufacturers and Managers
USDA has provided information technology to allow manufacturer and managers to select the most convenient application method for them. Once entities have an NPN (see above), identification device manufacturers, AIN device managers, and resellers use AIMS to indicate their interrelationships; this allows them all to be part of the AIN device distribution process. AIN device managers, once they have obtained their NPN, indicate in the system which device manufacturers they have a marketing agreement with. The system then automatically brings up the following online agreement:
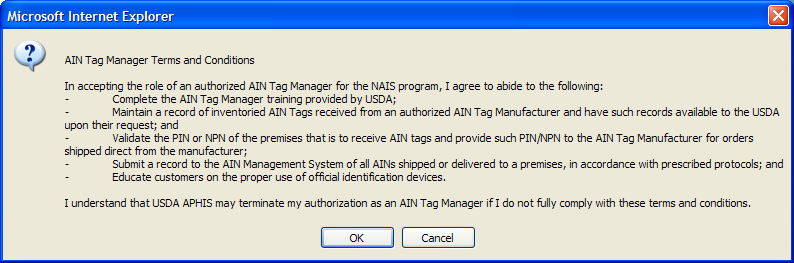
The online reseller agreement is identical except that all references to “manager” are changed to “reseller.”
Animal Trace Processing System
Working with State and industry partners, APHIS developed the Web-based portal that State and Federal animal health officials will use to request information from the animal tracking database (ATD) administrators to address animal disease events. Known as the Animal Trace Processing System, this information system provides secure access and auditing functions and is now operational at the Federal level. However, full integration of participating ATDs, and full development of the query form that State and Federal animal health officials will use, are not yet complete. As with the AIN Management System, participation in the ATPS will be completely electronic.
The Road Map template will be used as a guide for the States, Tribes, and territories to complete their Road Map. The Road Maps will be developed using standard word processing software (e.g., MS Word) and submitted on paper to the VS regional offices or by attaching an electronic document to an email directed to the VS regional offices.
APHIS continues to collect baseline traceability implementation data. We hope to have gained the necessary information to design and implement an electronic evaluation system.
USE OF NUES EARTAGS: GENERAL PROVISIONS
Eartag Orders
Respondents can call, fax, mail, or email orders.
APHIS uses the AIMS for tracking official eartag orders. Although the system was initially designed to track the distribution of 840 (USA country code) Animal Identification Number (AIN) eartags, it is now available to record the distribution of NUES tags. The AIMS is available at no charge to any State or Tribe (or accredited veterinarian) wishing to use it. It is also integrated with approved tag manufacturers. Another option for recording tag distribution is the Surveillance Collaboration Services (SCS) which APHIS is now using to replace the Generic Database. SCS is a commercial off-the-shelf product used to meet most of VS’ data entry needs for animal disease program management.
Program Site Tag Information Sheet (APHIS Form 453-R)
Tags may also be ordered through the National Logistics Support Center Web site operated by the National Oceanic and Atmospheric Administration using APHIS Form 453-R as described below. This form will be available on our APHIS Web site.
Record of Tags Issued and Recordkeeping
The AIMS must be used to maintain a record of all 840 AIN tags issued from the manufacturer to States, Tribes, tag dealers and resellers, and producers. Whoever has possession of a tag is responsible for recording to whom the tag is delivered up to the point it is issued to a livestock operation. The producer does not need to record anything in the system. The AIMS can also be used to track the issuance of NUES tags, but it is not required to be used for this.
Coordination of Tag Orders with Manufacturer and Recordkeeping
When States, Tribes, or DHIA members choose to order their own tags, they can use their own electronic system or the AIMS.
Reporting Loss, Theft, or Misuse of NUES Tags
Reporting is usually done by telephone or e-mail, depending on the method determined by each State or Tribe to best suit its needs for accepting accurate reports while preserving the reporter’s anonymity. Privacy concerns preclude using an electronic system.
Removal or Replacement of Eartags and Recordkeeping
Requests to remove or replace tags are usually handled by telephone or email. These methods are easier to administer and cost less than an electronic database set up solely for this purpose. An electronic database is not justified because VS receives few requests to remove or replace tags.
USE OF NUES EARTAGS IN OFFICIAL DISEASE PROGRAM ACTIVITIES
Record of Tags Applied (Recordkeeping)
The APHIS Mobile Information Management System (MIMS) (fully electronic device) is used at the field level to enter data when animals are processed for disease control programs.
NUES EARTAGS DISTRIBUTED OUTSIDE OF DISEASE PROGRAMS
Informing VS of Tag Orders
Reporting to VS is usually done by telephone or email, or as part of the cooperative agreement workplan for traceability.
Tribe Tag Distribution
Reporting to VS is usually done by telephone or email, or as part of the cooperative agreement workplan for traceability.
USE OF NUES EARTAGS IN THE DHIA
DHIA Eartag Distribution Plan
The distribution plan is a written document agreed on and signed by State and Federal animal health authorities and the DHIA. Since few of these documents are generated and they are variable it would not be cost effective to set up an electronic system to monitor and support them.
VS 1-63
VS 1-63 is available on the APHIS Web site. In addition, respondents can request, receive, and submit the VS 1-63 via email or fax.
https://www.aphis.usda.gov/library/forms/pdf/VS_1_63.pdf
DHIA Tag Application Record
The DHIA will have access to MIMS if they want to use it, although DHIA offices have highly automated electronic systems of data recording. Reports to States or Tribes are expected to be in the form of an electronic file transmitted in a way most appropriate for local needs, such as an e-mail attachment or Internet data transfer. The DHIA data processing centers are capable of long-term electronic data storage; no additional systems are needed.
4. Describe efforts to identify duplication. Show specifically why any similar information already available cannot be used or modified for use for the purpose described in item 2 above.
The information collected in connection with this activity is not available from any other source. APHIS is the only Federal agency responsible for tracing animal disease outbreaks. However, APHIS coordinates with three other USDA agencies (the Food Safety Inspection Service, the Agricultural Marketing Service, and the Grain Inspection, Packers, and Stockyards Administration) and with Health and Human Services’ Food and Drug Administration in certain aspects of this traceability work.
5. If the collection of information impacts small businesses or other small entities, describe any methods used to minimize burden.
APHIS developed these regulations to minimize burden on small businesses, because most producers who move livestock interstate (the principal respondents) are small entities. Such entities would not be required to maintain official identification records (other than for retagging of animals, which is expected to occur infrequently) or copies of CVIs. About 80 percent of the entities that would be affected by these requirements are considered small entities.
Moreover, Federal traceability requirements do not apply in the following instances:
Movement entirely within Tribal land that straddles a State line, if the Tribe has a separate traceability status from the States in which its lands are located;
Movement to a custom slaughter facility in accordance with Federal and State regulations for preparation of meat for personal consumption;
Movement as part of a commuter herd with a copy of the commuter herd agreement;
Movement directly from one State through another State and back to the original State; or
Movement to an approved tagging site, if the animals are officially identified there before they are commingled with cattle and bison from other premises.
6. Describe the consequence to Federal program or policy activities if the collection is not conducted or is conducted less frequently, as well as any technical or legal obstacles to reducing burden.
If the information was collected less frequently or not collected, APHIS’ ability to trace and appropriately address the outbreak of disease would be significantly hampered. This could have a tremendous impact on the health of U.S. livestock, and on the viability of industries dependent on U.S. livestock.
7. Explain any special circumstances that require the collection to be conducted in a manner inconsistent with the general information collection guidelines in 5 CFR 1320.5.
requiring respondents to report information to the agency more often than quarterly;
APHIS requires monthly reporting of DHIA tag application records because animal diseases can affect all ages and classes of livestock, and the ability to quickly find animals of interest is essential to effective disease control. APHIS provides an electronic database to record tag distribution information when tags are distributed, but use of this system is not required. DHIA centers that wish to use their own systems may do so if they forward application and distribution records to VS at least monthly to provide the information needed for rapid disease response.
requiring respondents to prepare a written response to a collection of information in fewer than 30 days after receipt of it;
An animal health official or accredited veterinarian issuing a CVI must forward a copy to the State or Tribe of origin within 5 working days. The State of origin is also required to forward copies of CVIs it receives to the destination State within 7 calendar days. These requirements are based on the speed, frequency, and volume of interstate livestock movements in today’s marketing environment and the threat of rapid disease spread that movement poses. APHIS needs information that supports rapid and effective traceability to stop disease outbreaks and prevent recurrences; the 7-day requirement provides it in a timeframe that is workable at the State level.
requiring respondents to submit more than an original and two copies of any document;
requiring respondents to retain records, other than health, medical, government contract, grant-in-aid, or tax records for more than 3 years;
The retention of official identification device distribution records and reports of removed, lost, stolen, or misused tags used for cattle, bison, sheep, goats, cervids, and equines is required for 5 years, as is retention of NUES identification device issuance records, DHIA tag application records, and reports of removed or replaced tags. This requirement is based on the fact that livestock animals, especially cattle, typically live to be more than 3 years old and animal diseases can affect all ages and classes of livestock. Therefore, traceability information that fully supports disease control, eradication, and surveillance needs to be maintained for longer than 3 years. APHIS also requires 5-year retention of records associated with animal movement kept by producers and operators of feedlots, markets, buying stations, and slaughter plants. The 5-year requirement brings consistency throughout APHIS regulations.
in connection with a statistical survey, that is not designed to produce valid and reliable results that can be generalized to the universe of study;
requiring the use of a statistical data classification that has not been reviewed and approved by OMB;
that includes a pledge of confidentiality that is not supported by authority established in statute or regulation, that is not supported by disclosure and data security policies that are consistent with the pledge, or which unnecessarily impedes sharing of data with other agencies for compatible confidential use; or
requiring respondents to submit proprietary trade secret, or other confidential information unless the agency can demonstrate that it has instituted procedures to protect the information's confidentiality to the extent permitted by law.
There are no other special circumstances and this information collection is conducted in a manner consistent with the guidelines established in 5 CFR 1320.5.
8. Describe efforts to consult with persons outside the agency to obtain their views on the availability of data, frequency of collection, the clarity of instructions and recordkeeping, disclosure, or reporting form, and on the data elements to be recorded, disclosed, or reported. If applicable, provide a copy and identify the date and page number of publication in the Federal Register of the agency's notice, soliciting comments on the information collection prior to submission to OMB.
APHIS has engaged in productive consultations with the following individuals concerning the information collection activities associated with this program:
Dr. Keith Roehr
State Veterinarian
Division of Animal Industry
Colorado Department of Agriculture
700 Kipling Street, Suite 4000
Lakewood, CO 80215
(303) 239-4161
Vicky LeBeaux
Intertribal Agriculture Council
100 North 27th Street
Suite 500
Billings, MT 59101
Phone: 406-259-3525
Fax: 406-259-9980
Email: [email protected]
David Hecimovich
Animal ID Program Manager
Washington State Department of Agriculture
2nd Floor, Natural Resources Building
1111 Washington St. SE
Olympia, WA 98504-2560
(360) 725-5493
Cell: (360) 507-6383
On Tuesday, March 28, 2017, APHIS published in the Federal Register, pages 15320-15321, a 60-day notice seeking public comments on its plans to request a 3-year renewal of this collection of information. No comments from the public were received.
9. Explain any decision to provide any payment or gift to respondents, other than remuneration of contractors or grantees.
This information collection activity involves no payments or gifts to respondents.
10. Describe any assurance of confidentiality provided to respondents and the basis for the assurance in statute, regulation, or agency policy.
No additional assurance of confidentiality is provided with this information collection. However, the confidentiality of information is protected under 5 U.S.C. 552a.
11. Provide additional justification for any questions of a sensitive nature, such as sexual behavior or attitudes, religious beliefs, and other matters that are commonly considered private. This justification should include the reasons why the agency considers the questions necessary, the specific uses to be made of the information, the explanation to be given to persons from whom the information is requested, and any steps to be taken to obtain their consent.
This information collection activity will ask no questions of a personal or sensitive nature.
12. Provide estimates of the hour burden of the collection of information. Indicate the number of respondents, frequency of response, annual hour burden, and an explanation of how the burden was estimated.
• Indicate the number of respondents, frequency of response, annual hour burden, and an explanation of how the burden was estimated. If this request for approval covers more than one form, provide separate hour burden estimates for each form and aggregate the hour burdens in Item 13 of OMB Form 83-I.
See APHIS form 71.
• Provide estimates of annualized cost to respondents for the hour burdens for collections of information, identifying and using appropriate wage rate categories.
Respondents are producers; State, Tribal, and territorial animal health officials; accredited veterinarians; livestock production and breed associations; livestock market operators; eartag manufacturers and distributors; designated laboratories and harvest facility operators. APHIS estimates the total annualized cost to these respondents to be $34,511,820. APHIS arrived at this figure by multiplying the hours of estimated response time (1,314,736 hours) by the estimated average hourly wage of the above respondents ($26.25). Estimated hourly wages for the respondents were determined from the U.S. Department of Labor, Bureau of Labor Statistics May 2017 Report – National Compensation Survey: Occupational Employment and Wages. Seehttps://www.bls.gov/news.release/pdf/ocwage.pdf
Farm, ranch, and other agricultural managers - $38.62
Farmers and ranchers - $13.38
Veterinarians - $48.81
Laboratory staff: $33.10 (animal scientists)
Slaughterers and meat packers - $13.38
(Staff of State and Tribal animal health officials; DHIA employees) Agricultural and food science technicians - $20.63
(Staff of State and Tribal animal health officials) Agricultural inspectors - $21.60
(Eartag manufacturers and distributors) First-line supervisors/managers of production and operating workers - $30.13
(Eartag manufacturers and distributors) Assemblers and fabricators, all others - $16.62
13. Provide estimates of the total annual cost burden to respondents or recordkeepers resulting from the collection of information, (do not include the cost of any hour burden shown in items 12 and 14). The cost estimates should be split into two components: (a) a total capital and start-up cost component annualized over its expected useful life; and (b) a total operation and maintenance and purchase of services component.
No annual cost burden is associated with capital and startup costs, operation and maintenance expenditures, and purchase of services.
14. Provide estimates of annualized cost to the Federal government. Provide a description of the method used to estimate cost and any other expense that would not have been incurred without this collection of information.
See APHIS Form 79. The annualized cost to the Federal government is estimated at
$24,770,309.
15. Explain the reasons for any program changes or adjustments reported in Items 13 or 14 of the OMB Form 83-1.
ICR Summary of Burden: |
|
Requested |
Program Change Due to New Statute |
Program Change Due to Agency Discretion |
Change Due to Adjustment in Agency Estimate |
Change Due to Potential Violation of the PRA |
Previously Approved |
Annual Number of Responses |
10,272,160 |
0 |
7,514,682 |
373 |
0 |
2,757,105 |
Annual Time Burden (Hr) |
1,314,736 |
0 |
476,949 |
-1,813 |
0 |
839,600 |
Annual Cost Burden ($) |
0 |
0 |
0 |
0 |
0 |
0 |
There is an overall increase of 7,515,055 responses and 475,136 burden hours due to adjustments and potential violations of the Paperwork Reduction Act (entered in ROCIS as program changes).
Adjustments
The “Develop ADT Roadmap using templates – States and Tribal Governments” hours per response increased from 4 to 8 hours resulting in an increase of 216 burden hours.
The “Removal or Replacement of Eartag” for both State and Tribal Governments and Businesses’ burden increased 163 burden hours to more accurately reflect activities.
The “DHIA Tag Application Record – Businesses” burden decreased 2,192 hours due to more accounting of hours per response.
Potential Violations of the Paperwork Reduction Act (entered in ROCIS as Program Changes)
The following burden items have been added to this information collection for first time resulting in 7,514,682 additional responses and 476,949 additional burden hours. They were previously covered under 0579-0259 and 0579-0388 which have expired.
Application for Use of More Than One Official Identification (ID) Device (State, Local, and Tribal Governments and Businesses)
Application for and Approval of an Approved Tagging Site (State, Local, and Tribal Governments and Businesses)
Evaluation of States and Tribes (State, Local, and Tribal Governments)
Documentation of Completion of Performance Measures (State, Local, and Tribal Governments)
Commuter Herd Agreement (State, Local, and Tribal Governments and Businesses)
Collection of ID Devices at Slaughter (Businesses)
Obtaining Official Eartags for Cattle not Currently Required to be Identified with Official Eartags (State, Local, and Tribal Governments and Businesses)
Application of State Shield (State, Local, and Tribal Governments)
Official Identification Device Distribution Records (State, Local, and Tribal Governments and Businesses)
Certificate of Veterinary Inspection and Recordkeeping (State, Local, and Tribal Governments and Businesses)
Unauthorized Removal or Loss of Official ID Devices (State, Local, and Tribal Governments and Businesses)
Reporting Retagging Animal Records – Removed, Lost, Stolen, or Misused Tags and Recordkeeping (State, Local, and Tribal Governments and Businesses)
Premises Identification (Business)
Updates to Premises Identification Records (Business)
Nonproducer Participant Registration (Business)
Official Identification Device Applications and Approved Identification Device Manufacturer Agreements (Business)
AIN Device Manufacturer Updates and Recordkeeping (Business)
AIN Device Managers Registration and Agreement (Business)
AIN Device Manager Updates (Business)
Cooperative Agreements (State, Local, and Tribal Governments and Businesses)
Cooperative Agreement Application (State, Local, and Tribal Governments)
State/Tribe Quarterly Reports (State, Local, and Tribal Governments)
16. For collections of information whose results are planned to be published, outline plans for tabulation and publication.
APHIS has no plans to publish information it collects in connection with this activity.
17. If seeking approval to not display the expiration date for OMB approval of the information collection, explain the reasons that display would be inappropriate.
Not applicable. APHIS will display the expiration date.
18. Explain each exception to the certification statement identified in the "Certification for Paperwork Reduction Act."
APHIS can certify compliance with all provisions under the Act.
B. Collections of Information Employing Statistical Methods
No statistical methods are associated with the information collection activities used in this program.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | Department of Agriculture |
| Author | eamarkese |
| File Modified | 0000-00-00 |
| File Created | 2021-11-30 |
© 2026 OMB.report | Privacy Policy