Supporting Statement A
Supporting Statement A.docx
Identification of Behavioral and Clinical Predictors of Early HIV Infection (Project DETECT)
OMB: 0920-1100
Identification of Behavioral and Clinical Predictors of Early HIV Infection
(Project DETECT)
OMB No. 0920-1100
October 8, 2021
Supporting Statement
Part A
CONTACT:
Kevin Delaney
Epidemiologist, Special Studies and Diagnostics Team
Division of HIV/AIDS Prevention
Centers for Disease Control & Prevention
1600 Clifton Rd, NE, MS E-46
Phone (404) 639-8630
Fax (404) 639-8640
Table of Contents
Section
A. Justification
Circumstances Making the Collection of Information Necessary
Purpose and Use of the Information Collection
Use of Improved Information Technology and Burden Reduction
Efforts to Identify Duplication and Use of Similar Information
Impact on Small Businesses or Other Small Entities
Consequences of Collecting the Information Less Frequently
Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
Explanation of Any Payment or Gift to Respondents
Protection of the Privacy and Confidentiality of Information Provided by Respondents
Institutional Review Board (IRB) and Justification for Sensitive Questions
Estimates of Annualized Burden Hours and Costs
Estimates of Other Total Annual Cost Burden to Respondents and Record Keepers
Annualized Cost to the Government
Explanation for Program Changes or Adjustments
Plans for Tabulation and Publication and Project Time Schedule
Reason(s) Display of OMB Expiration Date is Inappropriate
18. Exceptions to Certification for Paperwork Reduction Act Submissions
Exhibits
Table A12A. Estimate of Annualized Burden Hours
Table A12B Annualized Cost to Respondents
Table A14A. Annualized Cost to Government
Table A16. Project Time Schedule
List of Attachments
Attachment number |
Document description |
1 |
Section 301 of the Public Health Service Act |
2 |
60-Day Federal Register Notice |
3 |
References |
4 |
IRB approval |
5 |
Project Determination |
6 |
No Material or Non-substantive Change Approval |
7a |
Phase 1 Enrollment Survey (English) |
7b |
Phase 1 Enrollment Survey (Spanish) |
8a |
Phase 2 Behavioral Survey (English) |
8b |
Phase 2 Behavioral Survey (Spanish) |
9a |
Phase 2 HIV Symptom and Care Survey (English) |
9b |
Phase 2 HIV Symptom and Care Survey (Spanish) |
10a |
Phase 1 Consent Form (English) |
10b |
Phase 1 Consent Form (Spanish) |
11a |
Phase 2 Consent Form (English) |
11b |
Phase 2 Consent Form (Spanish) |
12a |
Survey Screen Shots (English) |
12b |
Survey Screen Shots (Spanish) |

Goals
of the study:
The goals of the project are to: 1) characterize the performance of
new HIV tests for detecting established and early HIV infection at
the point of care (POC), relative to each other and to currently
used gold standard, non-POC tests, and 2) identify behavioral and
clinical predictors of early HIV infection.
Intended
Use:
CDC provides guidelines for HIV testing and diagnosis for the United
States, as well as technical guidance for its grantees. CDC will use
the HIV testing data collected in this project to update these
guidance documents to reflect the latest available testing
technologies and their performance characteristics. CDC will use the
information on behavioral and clinical characteristics of persons
with early infection to help HIV test providers (including CDC
grantees) more effectively target the tests designed to detect early
HIV infection, which are the most expensive HIV tests, and are most
appropriately used to test those at highest risk of infection.
Methods
to be used to collect data:
Persons at high risk of HIV infection will be identified via a
standard intake form when they present to the main study site
clinics or Emergency Department for HIV testing, and persons with
established and early HIV infection will be identified from
participating clinics through routine HIV testing. In Phase 1,
biological specimens from all persons who consent to participate
will be tested with up to seven HIV tests under investigation. Test
performance and socio-demographic, behavioral and medical data
collected via the Phase I enrollment questionnaire will be compared
for persons at high risk, and persons with established and early
infection. In Phase 2, participants with discordant test results in
Phase 1 will undergo frequent follow-up testing to document
seroconversion on all tests under investigation, until they become
HIV positive on all tests, have consecutive negative test results on
all tests (indicating reactive Phase 1 tests were false-positive),
or complete 70 days of follow-up.
The
subpopulation to be studied:
The primary study subpopulation will be persons at high risk for or
diagnosed with HIV infection, most of whom will be men who have sex
with men (MSM), transgender women, minorities, and persons who
inject drugs (PWIDs) because the majority of new HIV infections each
year are among these populations.
How
data will be analyzed:
Data will be analyzed using univariate and bivariate statistics and
multivariate regression methods.
A. JUSTIFICATION
A. 1 Circumstances Making the Collection of Information Necessary
The Centers for Disease Control and Prevention (CDC), National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (NCHHSTP), Division of HIV/AIDS Prevention (DHAP) requests a 3-year extension without change of the currently approved “Identification of Behavioral and Clinical Predictors of Early HIV Infection (Project DETECT)” (0920-1100, Expiration date January 31, 2022).
Since the time of the last OMB approval (January 16, 2019), the University of Washington published the findings and protocol for Project DETECT in JMIR Research Protocols (Stekler JD, Violette LR, Clark HA, et al. Prospective Evaluation of HIV Testing Technologies in a Clinical Setting: Protocol for Project DETECT. JMIR Res Protoc 2020;9(1):e16332:1). Between September 2015 and March 2019, there were 14,990 Project DETECT-eligible visits. They enrolled and tested 1,037 people at risk of HIV, 198 with established HIV infection, and 96 with initial or newly diagnosed infection. Based on the testing conducted among these participants, 27 had discordant tests and were enrolled and followed in Phase 2 (see Figure 1.1).
We are requesting a 3-year extension of this OMB approval to collect data from at least 100 persons with discordant HIV results. The study implementers have recently focused efforts on identifying individuals with early infection to achieve the objective of reaching at least 100 persons with discordant results by the completion of the study in 2025.
In 2014, CDC awarded a contract (CDC 200-2014-61285) to the University of Washington (UW) from 2014-19 to conduct Project DETECT.
In 2020, CDC awarded two Cooperative Agreements under RFA-PS-20-001 to the University of Washington (UW) and Johns Hopkins University (JHU) to continue Project DETECT started by the UW contract. The project will be conducted by the University of Washington (UW) at the Public Health Seattle and King County (PHSKC) Sexual Health Clinic and Madison Clinic and at JHU sites (John G. Bartlett Specialty Practice, Baltimore City Health Department STD Clinic, Johns Hopkins Emergency Department) as a 3-year cooperative agreement conducted in three concurrent phases (see Figure 1.1) with information collection at phases 1 and 2.
The project has two goals. The first goal is to characterize the performance of new HIV tests for detecting established and early HIV infection at the point of care (POC), relative to each other and to currently used gold standard tests which are processed in a centralized laboratory rather than at POC. Currently available POC tests are less sensitive than those to be evaluated at detecting early HIV infection. The second goal is to identify behavioral and clinical predictors of early HIV infection. CDC staff will use data collected to update HIV testing guidelines. If differences in behavioral or clinical characteristics can be used to distinguish those most likely to have early infection, CDC will provide this information to HIV test providers to help them choose which HIV tests to use, and to target tests appropriately to persons at different levels of risk.
An estimated 38,000 new HIV infections occur each year in the United States (CDC 2019, reference in Attachment 3). In 2016-2017, most new infections occurred among young Black/African American and Hispanic/Latino men who have sex with men (MSM), and more than half of new HIV infections were reported in southern states and Washington, DC (Fauci et al. 2019, reference in Attachment 3). The first few weeks after HIV infection, referred to as the acute HIV infection stage, comprise the stage when there is no detectable HIV-specific antibody response and HIV infection can only be detected by testing for HIV virus directly. The early detection of HIV infection, particularly during the acute stage, serves a public health need because those with acute infection have large amounts of circulating HIV virus and thereby have an increased risk of transmitting infection (Miller et al 2010, reference in Attachment 3).
HIV testing is one of the four pillars of the Ending the HIV Epidemic in the United States. Early detection allows diagnosed persons to receive treatment to stay healthy, and has also been shown to reduce risk behaviors, thereby decreasing the likelihood of transmitting HIV to others (Cohen et al. 2013, reference in Attachment 3).
Diagnosing persons during early infection is particularly important as it is during this phase that HIV-infected persons are highly infectious because of the large quantity of virus in their blood. In this early stage of infection, the body has not mounted an antibody response, so those who are recently infected may test negative for HIV antibodies. Many MSM and others at high risk are tested for HIV in settings where POC rapid tests are often used. These tests, which typically are designed to detect HIV antibodies, cannot identify individuals with early infection and can provide false reassurance of HIV-negative status. If these MSM and others at high risk of HIV continue to engage in high-risk behaviors during this early phase of their infection, they may unwittingly be placing their sex partners at very high risk of acquiring HIV infection (Brenner et al., reference in Attachment 3).
Several new HIV tests have recently been approved by the US Food and Drug Administration (FDA), or are expected to be approved soon. These tests can be conducted using blood from a finger stick or oral fluid from a mouth swab. Some of the new tests can detect early infection by identifying the virus (called molecular tests), while other new tests can pick up early antibody response sooner than older HIV tests. Molecular tests are more expensive to conduct compared to currently available tests that only detect antibodies, so the feasibility of using these tests in POC settings may depend on the extent to which these tests can be targeted to those most likely to have early infection.
Although manufacturers seeking approval of HIV tests conduct studies to demonstrate device safety and efficacy, their clinical trials are not designed to evaluate important aspects that determine the public-health impact of these tests (e.g., the implementation logistics and feasibility of using different HIV tests for different populations in POC settings, such as a doctor’s office). In addition, these studies do not compare tests to one another and typically compare performance of new tests to that of diagnostic tests analyzed in centralized laboratories rather than at POC. Therefore, CDC is sponsoring this data collection to assess the performance of these new HIV tests in POC settings among persons at high risk of early HIV infection. This information is expected to be used to guide the efficient application of these new tests to maximize identification of HIV infections and further enhance the effectiveness of disease control efforts. Without this information CDC would not be able to exercise its leadership function with regard to identification and control of HIV infection.
A.2 Purpose and Use of the Information Collected
CDC provides guidelines for HIV testing and diagnosis in the United States, as well as programmatic technical guidance for its grantees. CDC will evaluate HIV laboratory testing recommendations at least every five years and update guidelines when necessary. CDC will use data collected through this project, in conjunction with laboratory evaluations conducted at CDC, to inform HIV testing guidelines. In addition, data collected under this information collection request will provide information to help HIV test providers choose which HIV tests to use and to help them target tests appropriately to persons at different levels of risk.
The data collection will serve three primary purposes: 1) Compare the performance characteristics of new POC HIV tests for detection of early infection, 2) ascertain whether a questionnaire administered at clinic intake can identify persons at highest risk of infection (most likely to have early infection) accurately enough to target the use of POC tests for early infection, and 3) describe the potential impact of earlier diagnosis of infected persons for curtailing HIV transmission, as defined by incidence of specific sexual behaviors and activities.
For this project, it is expected that one of the largest samples to date of persons with early HIV infection will be assembled, providing a unique opportunity to better understand the behavioral and clinical predictors of early infection.
CDC has awarded two Cooperative Agreements under RFA-PS-20-001 to the University of Washington (UW) and Johns Hopkins University (JHU) to continue Project DETECT started by the UW contract. The project will be conducted by the University of Washington (UW) at the Public Health Seattle and King County (PHSKC) Sexual Health Clinic and Madison Clinic and at JHU sites (John G. Bartlett Specialty Practice, Baltimore City Health Department STD Clinic, Johns Hopkins Emergency Department) as a 3-year cooperative agreement conducted in three concurrent phases (see Figure 1.1) with information collection at phases 1 and 2. A pre-study screen based on risk behavior reported on the clinic’s standard intake forms will comprise a phase 0 which is not part of this information collection request . Approximately 12,500 persons per year presenting for an HIV test at the UW and JHU clinics will complete the standard intake form which will be used in this study to limit the evaluation of the new testing technologies in phase 1.
Phase 1 is limited to up to 200 HIV-infected persons per year (recruited from the UW PHSKC STD clinic and JHU emergency department and STD clinic to increase the sample size for the evaluation of test performance [objective 1]) and up to 1,667 persons at highest risk for HIV infection including MSM, transgender women, minorities, and persons who inject drugs (PWIDs)(recruited from the UW and JHU clinics and emergency department). Therefore, Phase I will include 1,867 respondents (1,667 at high-risk and 200 HIV-positive). In phase 1 of the study we will evaluate test performance (objective 1) by collecting specimens for testing with the HIV testing technologies being evaluated. All test results, as well as results from an additional behavioral survey (Enrollment Survey: Attachment 7), will be reported to the CDC. Phase 1 participants with discordant test results (i.e., those with reactive results on at least one screening test and non-reactive results on another screening test), will be eligible for Phase 2.
In phase 2 we seek to describe the difference in days to detection for the new HIV tests on different specimen types collected (objective 1). Phase 2 participants will undergo frequent follow-up testing until they are positive on all tests being evaluated, or until they have two consecutive visits with negative test results on all tests (indicating reactive phase 1 tests were false-positive), or completion of 70 days of follow-up. At each return visit a Symptom and Care Survey (Attachment 9) will be administered to assess the presence of symptoms during HIV seroconversion (objective 2) and the effects of HIV treatment on test performance (objective 1).
It is expected that up to 50 participants per year will enter phase 2 of the study, of which approximately 16 participants will complete the study with false positive results and up to 32 participants will complete phase 2 follow-up with seroconversion. Based on previous experience in the PHSKC clinic, we expect that approximately 2 participants who begin phase 2 of the study will be lost to follow-up. A follow-up Behavioral Survey (Attachment 8) will be conducted at the end of phase 2 to assess changes in behavior after diagnosis (objective 3). All test results, as well as results from the Symptom and Care Surveys (Attachment 9), and the follow-up Behavioral Survey (Attachment 8) will be reported to the CDC. A non-substantive change request to the ICR was approved on May 8, 2021 (Attachment 6).
Figure 1.1. Description of Study Phases
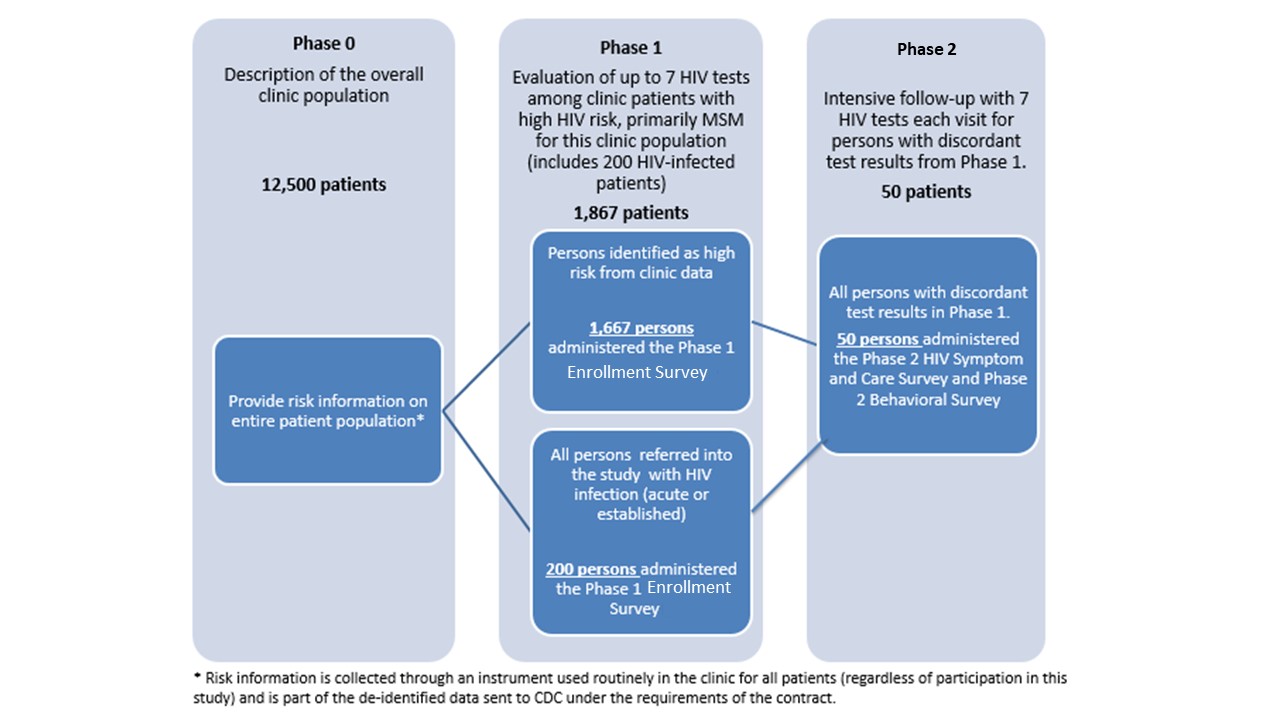
The information from this study will be used to help HIV providers more effectively target the tests designed to detect early HIV infection, which are the most expensive HIV tests, and are most appropriately used to test those at highest risk of infection. To identify predictors of seroconversion, such as differences in sexual and illicit drug use behaviors and clinical signs of early infection, behavioral and clinical characteristics will be compared among uninfected persons, persons with early infection and persons with established infection (objectives 2 and 3).
The UW and JHU clinical sites are well suited for this work, given the high testing rates and high incidence rates among MSM and at-risk populations in Seattle and Baltimore. Because selected populations in these cities are encouraged to test multiple times per year, the clinics have a high probability of identifying early HIV infection among those who do test positive. Additionally, the participation of Johns Hopkins Emergency Department for this project will allow for the inclusion of a wider variety of sub-populations with different risk factors for HIV infection including transgender women, minorities, and PWIDs. CDC provides guidelines for HIV testing and diagnosis for the United States, as well as technical guidance for its grantees. CDC will use the HIV testing data collected in this project to update these guidance documents to reflect the latest available testing technologies, their performance characteristics, and considerations regarding their use. CDC will also use information collected to describe behavioral and clinical characteristics of persons with early infection to help HIV test providers (including CDC grantees) choose which HIV tests to use and guide them to target tests appropriately to persons at different levels of risk. This information will primarily be disseminated through guidance documents (e.g., guidelines for HIV testing in non-clinical settings) and peer-reviewed journal articles.
A.3 Use of Improved Information Technology and Burden Reduction
One hundred percent of the proposed information collection will be collected via an electronic Computer Assisted Self-Interview (CASI) survey, such as Research Electronic Data Capture (REDCaP). Participants will complete the surveys on an encrypted computer, with the exception of the Phase 2 Symptom and Care Survey, which will be administered by a research assistant and then electronically entered into the CASI system. Use of the CASI minimizes burden by efficiently moving the user through skip patterns automatically and at their own pace. For the Phase 2 survey administered at each follow-up visit, the CASI software will pre-populate some information from the participant’s last clinic visit (e.g., race/ethnicity, age) to further reduce time burden for the participants.
CASI-based data collection methods have additional benefits compared to paper surveys. These include: 1) pre-programmed skip patterns to ensure that respondents are not asked irrelevant questions, and 2) automated validation checks incorporated into the behavioral survey to assist the respondent when incomplete or implausible responses are provided. The latter eliminates the need for data cleaning associated with data entry and the errors listed above, resulting in a reduction in the time between the last interview and the production of a final analysis dataset.
A.4 Efforts to Identify Duplication and Use of Similar Information
We reviewed currently funded programs and did not identify potential areas of duplication. We are not aware of any department or agency that collects data on the association of results from multiple HIV tests in point of care settings with behavioral and clinical predictors of early HIV infection.
A.5 Impact on Small Businesses or Other Small Entities
This data collection will not involve small businesses.
A.6 Consequences of Collecting the Information Less Frequently
The proposed project involves a one-time data collection from Phase 1 participants. Phase 2 participants will be followed up only until their test results are concordant. There are no legal obstacles to reducing burden.
A.7 Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
This request fully complies with regulation 5 CRF 1320.5.
A.8 Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
A 60-day notice to solicit public comments was published in the Federal Register on 07/12/2021, Volume 86, Number 130, Page 36555. No public comments were received.
Consultations were conducted in March 2014 with HIV testing facilities serving MSM in different regions of the United States. All names, affiliations and contact information are included in Table 8-A-1. The consultations were conducted to assess the feasibility of the proposed evaluation of HIV tests and behavioral data collection for the project. In addition, experts provided feedback on the behavioral and clinical indicators that would be most relevant to collect for this project.
Table A-8-1: Persons Consulted in the Development of Project DETECT
Los Angeles Gay and Lesbian Center
Risa Flynn, Research Program Manager Bob Bolan, Medical Director and Director of Clinical Research 1625 N. Schrader Blvd Los Angeles, CA 90028-6213 323-993-7400
|
Whitman Walker Clinic
Dr. Rick Elion, Director of Clinical Research Meghan Davies, Director of Community Health Justin Schmandt, Research Manager Megan Coleman, Research Coordinator/Nurse Practitioner
1701
14th St, NW 202-745-7000
|
Callen-Lorde Clinic Anita Radix, Director of Clinical Research 356 W 18th St
New
York, NY 10011
|
Howard Brown Clinic Daniel Pohl, Director of HIV/STI Prevention David Munar, President and CEO Kristin Keglovitz, Associate Medical Director
4025
N. Sheridan Road
|
A.9 Explanation of Any Payment or Gift to Respondents
Recruiting participants with early HIV infection and retaining them is central to the success of the proposed research project. To promote recruitment and retention, given the intensive follow-up process and discomfort of specimen collection, tokens of appreciation will be provided to respondents.
Tokens of appreciation for respondents have been shown to increase response rates, which in turn improves the validity and reliability of the data (Abreu and Winters 1999; Shettle and Mooney 1999; full references in Attachment 3). A meta-analysis of survey methodologies (Church 1993; reference in Attachment 3) found that cross-sectional studies using prepaid monetary tokens of appreciation yielded an average increase in response rates of 19.1 percentage points, representing a 65% average increase in response. Edwards et al. (2002, reference in Attachment 3) reported similar results in a subsequent meta-analysis. With very few exceptions, reports of more recent experiments are consistent with results reported by Church and Edwards et al. These results support the use of tokens of appreciation in phase 1 of the proposed study, which has a cross-sectional design. Jackle and Lynn (2008, reference in Attachment 3) found that tokens of appreciation at multiple visits in a longitudinal study decreased attrition at all visits. In addition, other federal surveys use tokens of appreciation for respondents. For example, the National Health and Nutrition Examination Survey (NHANES, OMB No. 0920-0950, exp. 12/31/2019), which combines questionnaire responses and physical examinations, as for Phase 2 of the proposed project, has used tokens of appreciation since it began in the 1960s.
For the proposed data collection, UW and JHU will provide up to $40 to participants for the Phase 1 study visit and up to $50 per study visit for participants followed longitudinally in Phase 2. The token amounts in this study are consistent with an HIV testing study conducted by UW among MSM in the Seattle metropolitan area (Stekler et al 2013, reference in Attachment 3). This study differs from the 2013 UW study in that the previous study consisted of a one-time clinic visit without collection of any type of blood or oral fluid specimen. The current study is substantially more intrusive as it involves:
study visits with specimen collection procedures that can be uncomfortable (e.g., oral swabs and a venous blood draw for Phase 1, and for Phase 2, oral swabs, 6 finger stick blood draws and a venous blood draw every few days for up to 70 days – which though not dangerous are painful and medically unnecessary);
requests for sensitive information about participants’ behavior during each visit (Enrollment Survey in Phase 1; and for Phase 2, 5 minutes for the Symptom and Care Survey and 30 minutes for the Behavioral Survey).
repeated travel to the clinic every few days to undergo study procedures which is inconvenient as the clinic does not have extended hours.
Without providing the tokens of appreciation, UW and JHU would not be able to recruit and retain the required number of individuals necessary to meet the goals of the study in the required timeframe.
A.10 Protection of the Privacy and Confidentiality of Information Provided by Respondents
The CDC Privacy Officer had assessed this package for applicability of 5 U.S.C. § 552a and has determined that the Privacy Act is not applicable because personally identifiable information (PII) is not being collected under this CDC funded activity. Any PII is collected as part of standard clinic intake forms that are not collected exclusively for this study and only de-identified data are sent to CDC. Therefore, a signed PIA is not required for this submission.
CDC provides a Certificate of Confidentiality automatically and therefore, the sites will not need to apply for one. The de-identified, but sensitive information from the behavioral surveys will be transmitted monthly to the CDC via an encrypted File Transfer Protocol (FTP) site. At no time will CDC receive any identifying information such as names; instead, CDC will receive datasets containing a unique identification number (ID) for each participant.
A.11 Institutional Review Board (IRB) and Justification for Sensitive Questions
IRB Approval
The protocol for Project DETECT has been reviewed and approved by UW’s and JHU’s Institutional Review Board (IRB). The approval letters are included as Attachment 4. The IRB-approved questionnaires are included as Attachments 7, 8 and 9 and the approved consent forms are included as Attachments 10 and 11.
The objectives of Project DETECT and its goal to inform HIV testing guidelines and HIV test providers regarding diagnosing early HIV infection cannot be accomplished without the collection of sensitive information regarding HIV risk, such as sexual behavior, drug use behavior (including injection drug use), as well as information on HIV/AIDS status, medical history and sexual orientation. Collection of these data will be used to identify predictors of early HIV infection, which can help HIV test providers more effectively use the tests designed to detect early HIV infection, which are the most expensive HIV tests.
Sensitive Questions
The context in which questions will be asked helps to overcome their potential sensitivity and to emphasize to the respondent the legitimate need for the information:
Nearly all questions allow for responses of “don’t know” or “refuse to answer.”
Consent forms make it clear that the survey is sponsored by CDC and implemented by UW and JHU and that the information will be put to important uses (Attachments 10 and 11).
Local phone numbers are provided if the participant has questions about the survey.
The questionnaires (except for the HIV Symptom and Care Survey in Phase 2) are self-administered and carefully organized to lead smoothly from one topic to another. Transitions are made clear to participants and the need for the information explained.
Assurances about the privacy of the data are reiterated.
A.12 Estimates of Annualized Burden Hours and Costs
In the previous Project DETECT, UW did not enroll the estimated number of respondents and the estimated burden was not achieved, therefore, for this request we are decreasing the estimated burden from 2,110 to 1,594 hours. It is estimated that UW and JHU together will recruit and enroll the same number of participants as reflected in the current burden Table (Exhibit 12.A) with a shorter length of time for survey completion (based on the findings from the initial Project DETECT).
The estimate of annualized burden hours for this data collection is 1,594 hours; details are provided in exhibit 12.A. For the proposed information collection, approximately 2,334 persons will be recruited annually into the study and undergo the consent process (Attachment 10a and 10b). The participant will take approximately 15 minutes to read the Phase 1 consent form.
We estimate that 20% of persons approached and consented will not be interested in completing the HIV testing and behavioral survey. Therefore, it is estimated that 1,867 will participate in Phase 1 of the study during each 12-month period. Of these 1,867 participants, 1,667 identified as high risk per clinic data will be recruited from the UW and JHU sites and will complete the Phase 1-Enrollment Survey, and 200 with HIV infection will be referred from other clinics and will also complete the Phase 1 – Enrollment Survey (Attachment 7a and 7b). New information from the experience implementing these Surveys provided an updated estimated average of 30 minutes for the Enrollment Survey.
Among these 1,867 participants from Phase 1, an estimated maximum of 50 persons will participate annually in Phase 2 of the study. Reading the Phase 2 consent form (Attachment 11a and 11b) is estimated to take 15 minutes. Completion of the Phase 2 HIV Symptom and Care Survey (Attachment 9a and 9b) is estimated to take 5 minutes for each of up to 9 follow-up visits. The Phase 2 Behavioral Survey (Attachment 8a and 8b) will be completed at the end of follow-up and is estimated to take 30 minutes.
Exhibit A12A. Estimate of Annualized Burden Hours |
|||||
Type of Respondent |
Form Name |
Number of Respondents |
Number of Responses per Respondent |
Average Minutes Per Response |
Total Response Burden (Hours) |
Persons eligible for study |
Phase 1 Consent |
2,334 |
1 |
15/60 |
584 |
Enrolled participants
|
Phase 1 Enrollment Survey |
1,867 |
1 |
30/60 |
934 |
Phase 2 Consent |
50 |
1 |
15/60 |
13 |
|
Phase 2 HIV Symptom and Care survey |
50 |
9 |
5/60 |
38 |
|
Phase 2 Behavioral Survey |
50 |
1 |
30/60 |
25 |
|
Total |
|
|
|
|
1,594 |
A.12.B. Estimated Annualized Costs
The annualized cost to respondents for the burden hours is estimated to be $40,998; details are provided in Exhibit A.12.B. The estimates of hourly wages were based on mean wages for all occupations National Compensation Survey: Occupational Wages in the United States May 2019, “U.S. Department of Labor, Bureau of Labor Statistics.” Available at: https://www.bls.gov/oes/current/oes_nat.htm
Exhibit A12B. Annualized Cost to Respondents |
||||
Type of Respondent |
Form Name |
Total Burden Hours |
Hourly wage rate |
Total respondent costs |
Persons eligible for study |
Phase 1 Consent |
584 |
$25.72 |
$15,020 |
Enrolled participants |
Phase 1 Enrollment Survey |
934 |
$25.72 |
$24,022 |
Enrolled participants |
Phase 2 Consent |
13 |
$25.72 |
$334 |
Enrolled participants |
Phase 2 HIV symptom and care survey |
38 |
$25.72 |
$977 |
Enrolled participants |
Phase 2 Behavioral Survey |
25 |
$25.72 |
$643 |
Total |
|
|
|
$40,998 |
A.13 Estimates of Other Total Annual Cost Burden to Respondents and Record Keepers
There are no other costs to respondents associated with this proposed collection of information.
A.14 Annualized Cost to the Federal Government
The annualized cost to the government is $1,258,676.
-
Exhibit 14.A Estimated Cost to the Government
Expense Type
(Based on FY2020 dollars)
Expense Explanation
Annual Costs (dollars)
Direct Costs to the Federal Government
DETECT Personnel
Epidemiologist-13 (1) 100%
$112,138
Epidemiologist-14 (1) 25%
$33,127
Site Visit (2 trips x 2 staff)
$6,000
Total direct costs to federal government
$151,265
Contractor and Other Expenses*
Cooperative Agreement
#RFA-PS-20-001
Salary and Wages
$402,892
Fringe benefits
$131,960
Equipment
$15,354
Supplies
$123,374
Other
$125,731
Indirect costs
$308,101
Total contractor and other expenses
$1,107,411
TOTAL COST TO THE GOVERNMENT
$1,258,676
*Salary estimates were obtained from the US Office of Personnel Management salary scale at https://www.opm.gov/policy-data-oversight/pay-leave/salaries-wages/salary-tables/20Tables/html/ATL.aspx
The personnel related to the Project DETECT data collection include project officers (epidemiologists) at the GS-13 and 14 levels.
A.15 Explanation for Program Changes or Adjustments
Burden has decreased from the burden shown in the current inventory.
New information from implementing surveys during the first DETECT project provided an updated estimate of 30 minutes for Phase 1 Enrollment Survey. This change decreases the burden hours and the estimated annualized cost to the respondents.
A.16 Plans for Tabulation and Publication and Project Time Schedule
Data collection was conducted during the 3-year period after OMB approval. It was expected that the project would take 6 years to complete and the investigators submitted an extension request after 3 years. With the addition of the JHU site we are requesting an additional extension for 3 more years. Data analysis will occur within 12 months of final data collection. The following is a brief overview of the DETECT Timeline. Data collection is ongoing under the current OMB approval (exp. 1/31/2022) using the information collection request approved in the non-substantive change request (Attachment 6) on 5/8/2021.
Exhibit 16.A Project Time Schedule |
|
Activity |
Time Schedule |
Initiate recruitment |
Immediately after OMB approval |
Conduct Phase 1 |
1 month – 3 years after OMB approval |
Conduct Phase 2 |
2 months – 3 years after OMB approval |
Data management |
1 month – 3 years after OMB approval |
Analysis |
Within 6 months of project completion |
Publication |
Within 12 months of project completion |
A.17 Reasons(s) Display of OMB Expiration Data is Inappropriate
The OMB Expiration Date will be displayed. No exception is requested.
A.18 Exceptions to Certification for Paperwork Reduction Act Submission
There are no exceptions to the certification.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | CDC User |
| File Modified | 0000-00-00 |
| File Created | 2021-12-08 |
© 2026 OMB.report | Privacy Policy