SABG Report
OMB SABG Report _ Clean 9.21.21_.docx
Community MH Services BG and SAPT BG Application Guidance and Instructions FY 2022-2023
SABG Report
OMB: 0930-0168
Block Grant Reporting Section
CFDA 93.959
Substance Abuse
Prevention and Treatment
Block Grant
(SABG)
U.S. Department of Health and Human Services
Substance Abuse and Mental Health Services Administration
Table of Contents
Introduction
Annual Update
SABG Table 1 Priority Area and Annual Performance Indicators – Progress Report
State Agency Expenditure Reports
SABG Table 2a - State Agency Expenditure Report
SABG Table 2b - COVID-19 Relief Funds Expenditure by Service
SABG Table 3a - Syringe Services Program
SABG Table 3b - Expenditures for Syringe Services Programs (SSPs) Program Information
SABG Table 4 - SABG State Agency Expenditure Compliance Report
SABG Table 5a - SABG Primary Prevention Expenditures
SABG Table 5b - SABG Primary Prevention Targeted Priorities
SABG Table 6 - Systems Development/Non-Direct Service Activities Expenditure
SABG Table 7 - SABG Statewide Entity Inventory
SABG Table 8a - Maintenance of Effort for State Expenditures for Substance Abuse Prevention and Treatment
SABG Table 8b - Base and Maintenance of Effort for Expenditures for Services to Pregnant Women and Women with Dependent Children
Population and Services Reports
SABG Table 9 - Prevention Strategy Report
SABG Table 10 - Treatment Utilization Matrix
SABG Table 11a - Number of Persons Served (Unduplicated Count) for Alcohol and Other Drug Use
SABG Table 11b - COVID-19 Number of Persons Served (Unduplicated Count) for Alcohol and Other Drug Use
SABG Table 11c - Sex, Sexual Orientation, and Gender Identity Number of Persons Served (Unduplicated Count) for Alcohol and Other Drug Use
SABG Table 12 - SABG HIV Early Intervention Services in Designated States
SABG Table 13 - Charitable Choice
E. Performance Data and Outcomes
Treatment Performance Measures
SABG Table 14 - Employment/Education Status
SABG Table 15 - Stability of Housing
SABG Table 16 - Criminal Justice Involvement
SABG Table 17 - Change in Abstinence–Alcohol Use
SABG Table 18 - Change in Abstinence – Other Drug Use
SABG Table 19 - Change in Social Support of Recovery
SABG Table 20 - Retention
Prevention Performance Measures
SABG Table 21 - Reduced Morbidity–Abstinence from Drug Use/Alcohol Use; Measure: 30 Day Use
SABG Table 22 - Reduced Morbidity –Abstinence from Drug Use/Alcohol Use; Measure: Perception of Risk/Harm of Use
SABG Table 23 - Reduced Morbidity–Abstinence from Drug Use/Alcohol Use; Measure: Age of First Use
SABG Table 24 - Reduced Morbidity–Abstinence from Drug Use/Alcohol Use; Measure: Perception of Disapproval/Attitudes
SABG Table 25 - Employment/Education; Measure: Perception of Workplace Policy
SABG Table 26 - Employment/Education; Measure: Average Daily School Attendance Rate
SABG Table 27 - Crime and Criminal Justice; Measure: Alcohol-Related Traffic Fatalities
SABG Table 28 - Crime and Criminal Justice; Measure: Alcohol and Drug Related Arrests
SABG Table 29 - Social Connectedness; Measure: Family Communications around Drug and Alcohol Use
SABG Table 30 - Retention; Measure: Percentage of Youth Seeing, Reading, Watching, or Listening to a Prevention Message
SABG Table 31-35 – Reporting Period – Start and End Dates for Information Reported on Tables 31, 32, 33, 34, and 35
SABG Table 31 - Individual-Based Programs and Strategies; Measure: Number of Persons Served by Age, Gender, Race, and Ethnicity
SABG Table 32 - Population-Based Programs and Strategies; Measure: Number of Persons Served by Age, Gender, Race, and Ethnicity
SABG Table 33 - Number of Persons Served by Type of Intervention
SABG Table 34 - Number of Evidence-Based Programs by Types of Intervention.
SABG Table 35 - Number of Evidence-based Programs and Strategies, and Total SA Block Grant Funds Spent on Evidence-Based
Programs/ Strategies.
Section A. Introduction
Section 1942(a) of Title XIX, Part B, Subpart III of the Public Health Service Act (42 U.S.C. § 300x-52(a)) requires the Secretary of the U.S. Department of Health and Human Services, acting through the Administrator of the Substance Abuse and Mental Health Services Administration (SAMHSA), to determine the extent to which states1 have implemented the State Plan for the preceding fiscal year. The purpose of the Substance Abuse Prevention and Treatment Block Grant (SABG) Annual Report is to provide information to assist the Secretary in making this determination.
States are required to prepare and submit an annual report that includes expenditure summaries for (1) the state fiscal year (SFY) immediately preceding the federal fiscal year for which the state is applying for funds; and (2) the obligation and expenditure period of the Substance Abuse Prevention and Treatment Block Grant (SABG) Notice of Award (NoA) subject to CSAT compliance review (Compliance Award), in the format provided in this guidance. The SABG Annual Report will address the purposes for which the SABG funds were expended, the SABG sub-recipients, and the authorized activities and services purchased with such funds. Particular attention should be given to the progress made toward accomplishing the goals and performance indicators identified in the states’ and jurisdictions’ plans.
All states are required to prepare and submit their respective SABG Annual Reports utilizing SAMHSA’s Web Block Grant Application System (BGAS). Annual reports must be received by SAMHSA not later than December 1 for a state or jurisdiction to receive its fiscal year SABG NoA. If a receipt date falls on a weekend or federal holiday, the receipt date for a report will be the next business day. The following schedule provides specific due dates for the SABG Annual Reports, MHBG Implementation Reports and the Annual Synar Reports:
Due Dates for SA Only and MHBG/SABG Applications |
|||||
FY for which the state is applying for funds |
Application Due |
Plan Due |
Planning Period |
SABG Report Due |
Synar Report Due |
2022 |
9/1/2021 |
Yes |
7/1/21 – 6/30/23 |
12/1/2021 Compliance Year is 2019 |
12/31/2021
|
2023 |
9/1/2022 |
No |
N/A |
12/1/2022 Compliance Year is 2020 |
12/31/2022 |
Due Dates for Reports |
|||
Applicable FY |
SABG Annual Report |
MHBG Implementation Report |
Annual Synar Report |
2022 |
12/01/2021 |
12/01/2021 |
12/31/2021 |
2023 |
12/01/2022 |
12/01/2022 |
12/31/2022 |
States are required to prepare and submit an annual report comprised of the following sections:
Section B: Annual Update - In this section, states are required to provide a brief review of the extent to which their respective plans were implemented, and the progress toward the priorities and goals identified in the SABG plan covering SFY 2022 and 2023. The report should also include a brief review of areas that the state identified in that SABG plan as needing improvement and changes that the state or jurisdiction proposed to achieve the goals established for the priorities.
Section C: State Agency Expenditure Reports - In this section, states must provide information regarding expenditures for authorized activities and services for primary substance use disorder prevention, substance use disorder2 (SUD) treatment, and recovery. The state must provide a description of SABG, COVID-19, and ARP Relief Supplement expenditures for authorized activities to prevent substance misuse and treat SUDs and related services for tuberculosis, and, if it is a “designated state,” a description of SABG and COVID-19 Relief Supplement expenditures for early intervention services for regarding the human immunodeficiency virus (EIS/HIV).
Section D: Populations and Services Reports - In this section, states must provide specific information regarding the number of individuals served with SABG and COVID-19 Relief Supplement funds. In addition, states should provide specific information regarding the services these individuals received.
Section E: Performance Indicators and Accomplishments - In this section of the report, states are required to complete the Performance Indicator tables. Performance indicators should be reported using the table format provided in this document. The purpose of the performance indicator tables is to show progress made over time as measured by SAMHSA’s National Outcome Measures (NOMS) for substance use disorder prevention, SUD treatment, and recovery.
B. Annual Update
The information states enter into SABG Table 1 in the planning section of the 2022/2023 Behavioral Health Assessment and Plan will automatically populate cells 1 - 6 in the progress report table below. States are required to indicate whether each first-year performance target/outcome measurement identified in 6.b below (from the 2022/2023 Plan) was “Achieved” or “Not Achieved” in cell 7, Report of Progress toward Goal Attainment. If a target was not achieved, a detailed explanation must be provided, along with remedial steps proposed to meet the target.
SABG Table 1 - Priority Areas and Annual Performance Indicators – Progress Report
Priority Areas and Annual Performance Indicators
|
1. Priority Area: |
2. Priority Type (SAP, SAT, MHS): |
3. Population(s) (SMI, SED, FEP, PWWDC, PP, PWID (formerly IVDUs), EIS/HIV, TB, OTHER): |
4. Goal of the Priority Area: |
5. Strategies to Attain the Goal: |
6. Annual Performance Indicators to Measure Goal Success: |
Indicator #1: |
|
|
|
|
|
|
Priority Areas and Annual Performance Indicators (continued) |
7. Report of Progress toward Goal Attainment:
First-year target: ____ Achieved _____ Not Achieved (If not achieved, explain why.) |
Reason why target was not achieved and changes proposed to meet target:
|
C. State Agency Expenditure Reports
States are required to provide information regarding SABG, COVID-19 Relief Supplement, American Rescue Plan (ARP), and state funds expended for authorized activities to prevent and treat SUDs and for related public health services, e.g., tuberculosis services (TB) and, if applicable, early intervention services regarding the human immunodeficiency virus (EIS/HIV), as well as expenditures for services to assist states in responding to the COVID-19 pandemic. Please complete the tables described below:
SABG Table 2a - State Agency Expenditure Report. This table provides a report of SABG, Coronavirus Response and Relief Supplement Appropriations Act (COVID-19), 2021 [P.L. 116-260]), the American Rescue Plan Act (ARP), 2021 [P.L. 117-2] and state expenditures by the principal agency of a state, i.e., single state agency (SSA), during the state fiscal year immediately preceding the federal fiscal year for which the state is applying for funds. Expenditures to be reported are for authorized activities to prevent and treat SUDs pursuant to section 1921 of Title XIX, Part B, Subpart II of the Public Health Service (PHS) Act (42 U.S.C. § 300x-21); tuberculosis services; early intervention services regarding the human immunodeficiency virus (EIS/HIV), if applicable; pursuant to section 1924(b) of Title XIX, Part B, Subpart II of the PHS Act (42 U.S.C. § 300x-24(b)); and administration pursuant to section 1931(a)(2) of Title XIX, Part B, Subpart II of the PHS Act (42 U.S.C. § 300x-31(a)(2)). In column A, the applicable federal fiscal years’ SABG funds expended during the state fiscal year should be included. In column H, the applicable federal fiscal years COVID-19 Relief Supplement funds expended during the state fiscal year should be included. NOTE: For FY 2023 this table provides a report of expenditures from the SABG award, the COVID-19 Relief Supplement award, and the ARP Supplement award in column I.
SABG Table 2b - COVID-19 Relief Supplement Funds Expenditure by Service. This table provides a report of COVID-19 Relief Supplement expenditures for services to assist states in responding to the COVID-19 pandemic.
SABG Table 3a - Syringe Services Program. This table provides a report of SABG and COVID-19 Relief Supplement expenditures for elements of syringe services programs carried out by SABG sub-recipients as described in the guidance disseminated by the Office of HIV/AIDS and Infectious Disease Policy, the Centers for Disease Control and Prevention, National Center on HIV/AIDS, Viral Hepatitis, STD and TB Prevention, Division of HIV Prevention and SAMHSA. The authorization to expend federal funds for elements of a syringe services program is subject to an authorization in the annual appropriations’ bill(s). NOTE: For FY 2023 this table provides a report of expenditures from the COVID-19 Relief Supplement and ARP Supplement funding.
SABG Table 3b - Expenditures for Syringe Services Programs (SSPs) Program Information. This table is intended to capture the unduplicated count of persons that received onsite services from an SSP or were referred to services by an SSP including HIV, Hepatitis C, and sexually transmitted disease testing, treatment for substance use conditions, and treatment for physical health. NOTE: For FY 2023 this table provides a report of expenditures from the COVID-19 Relief Supplement and ARP Supplement funding.
SABG Table 4 - SABG State Agency Expenditure Compliance Report. This table provides a report of expenditures for authorized activities to prevent and treat SUDs associated with a SABG Notice of Award (NoA) for the applicable fiscal year. It covers the two-year obligation and expenditure period.
SABG Table 5a - SABG Primary Prevention Expenditures. This table provides a report of primary prevention expenditures associated with a SABG NoA for the applicable fiscal year. It covers the two-year obligation and expenditure period. Table 5a excludes Expenditures for Systems Development/Non-Direct Service Activities (formerly known as Resource Development Expenditures), which are located on SABG Table 6.
SABG Table 5b - SABG Primary Prevention Targeted Priorities. This required table provides a report of actual state primary prevention priorities and special population categories on which the state expended primary prevention funds from the SABG NoA for the applicable fiscal year.
SABG Table 6 - Expenditures for System Development/Non-Direct Service Activities. This table provides a report of expenditures from the SABG NoA for system development and non-direct service activities that were supported by the SABG NoA for the applicable fiscal year.
SABG Table 7 - SABG Statewide Entity Inventory. This table provides a report of the SABG sub-recipients including community and faith-based organizations which provided SUD prevention activities and treatment services, along with intermediaries/administrative service organizations. Table 7 excludes Expenditures for Systems Development/Non-Direct Service Activities (formerly known as Resource Development Expenditures).
SABG Table 8a - Maintenance of Effort for State Expenditures for Substance Abuse Prevention and Treatment. This table provides a report of aggregate state expenditures by the SSA for authorized activities to prevent and treat SUDs during the state fiscal year immediately preceding the federal fiscal year for which the state is applying for funds.
SABG Table 8b - Base and Maintenance of Effort for Expenditures for Services to Pregnant Women and Women with Dependent Children. This table provides a report of SABG and/or state funds pursuant to 42 U.S.C. § 300x-22(b) and 45 CFR § 96.124(c)(3) expended to establish new programs or expand the capacity of existing programs designed to serve pregnant women and women with dependent children and the services required pursuant to 45 CFR § 96.124(e) to address the treatment and recovery needs of such women during the state fiscal year immediately preceding the federal fiscal year for which the state is applying for funds.
SABG Table 2a - State Agency Expenditure Report
This table provides a report of SABG and state expenditures by the SSA during the state fiscal year immediately preceding the federal fiscal year for which the state is applying for funds for authorized activities to prevent and treat SUDs. For detailed instructions, refer to those in the Block Grant Application System (BGAS). Please note that this expenditure period is different from that on SABG Table 4.
State Agency Expenditure Report |
|||||||||
SABG Table 2a |
|||||||||
State Identifier: |
|||||||||
Report Period- From: To: |
|||||||||
(Include ONLY funds expended by the executive branch agency administering the Substance Abuse Block Grant) |
|||||||||
Source of Funds |
|||||||||
Activity (See instructions for using Row 1) |
A. SABG |
B. MHBG |
C. Medicaid (Federal, State, and local) |
D. Other Federal Funds (e.g., ACF (TANF), CDC, CMS (Medicare) SAMHSA, etc.) |
E. State Funds |
F. Local Funds (excluding local Medicaid) |
G. Other |
H. COVID – 19 1 |
I. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2. Primary Substance Use Disorder Prevention |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5. Administration (Excluding Program and Provider Level) |
|
|
|
|
|
|
|
|
|
11. Total |
|
|
|
|
|
|
|
|
|
The 24-month expenditure period for the COVID-19 Relief supplemental funding is March 15, 2021 – March 14, 2023, which is different from the expenditure period for the “standard” SABG and MHBG. Per the instructions, the standard SABG expenditures are for the state planned expenditure period of July 1, 2021 – June 30, 2023 for most states.
2 The expenditure period for The American Rescue Plan Act of 2021 (ARP) supplemental funding is September 1, 2021 – September 30, 2025, which is different from the expenditure period for the “standard” MHBG/SABG. Per the instructions, the planning period for standard MHBG/SABG expenditures is July 1, 2021 – June 30, 2023.
3 Prevention other than primary prevention
4 Only designated states as defined in 42 U.S.C. § 300x-24(b)(2) and 45 CFR § 96.128(b) for the applicable federal fiscal year should enter information in this row. This may include a state or states that were previously considered “designated states” during any of the three prior federal fiscal years for which a state was applying for a grant. See EIS/HIV policy change in SABG Annual Report instructions.
Please indicate if the expenditures are actual or estimated.
Actual Estimated
0930-0168 Approved: 04/19/2019 Expires: 04/30/2022
SABG Table 2b – COVID-19 Relief Funds Expenditure by Service
Expenditure Period Start Date: Expenditure Period End Date:
Service |
COVID-19 Expenditures |
Healthcare Home/Physical Health |
$ |
Specialized Outpatient Medical Services |
|
Acute Primary Care |
|
COVID-19 Screening (e.g., temperature checks, symptom questionnaires) |
|
COVID-19 Testing |
|
COVID-19 Vaccination |
|
Comprehensive Care Management |
|
Care Coordination and Health Promotion |
|
Comprehensive Transitional Care |
|
Individual and Family Support |
|
Referral to Community Services Dissemination |
|
Prevention (Including Promotion) |
|
Screening with Evidence-based Tools |
|
Risk Messaging |
|
Access Line/Crisis Phone Line/Warm Line |
|
Purchase of Technical Assistance |
|
COVID-19 Awareness and Education for Persons with SUD |
|
Media Campaigns (Information Dissemination) |
|
Primary Substance Use Disorder Prevention (Education) |
|
Primary Substance Use Disorder Prevention (Alternatives) |
|
Employee Assistance Programs (Problem Identification and Referral) |
|
Primary Substance Use Disorder Prevention (Community-based Processes) |
|
Primary Substance Use Disorder Prevention (Environmental) |
|
Intervention Services |
|
Fentanyl Test Strips |
|
Syringe Services Program |
|
Naloxone |
|
Overdose Kits/Dissemination of Overdose Kits |
|
Engagement Services |
|
Assessment |
|
Specialized Evaluations (Psychological and Neurological) |
|
Services Planning (including crisis planning) |
|
Consumer/Family Education |
|
Outreach (including hiring of outreach workers) |
|
Outpatient Services |
|
Evidence-based Therapies |
|
Group Therapy |
|
Family Therapy |
|
Multi-family Therapy |
|
Consultation to Caregivers |
|
Medication Services |
|
Medication Management |
|
Pharmacotherapy (including MAT) |
|
Laboratory Services |
|
Community Support (Rehabilitative) |
|
Parent/Caregiver Support |
|
Case Management |
|
Behavior Management |
|
Supported Employment |
|
Permanent Supported Housing |
|
Recovery Housing |
|
Recovery Supports |
|
Peer Support |
|
Recovery Support Coaching |
|
Recovery Support Center Services |
|
Supports for Self-Directed Care |
|
Supports (Habilitative) |
|
Personal Care |
|
Respite |
|
Supported Education |
|
Acute Intensive Services |
|
Mobile Crisis |
|
Peer-based Crisis Services |
|
Urgent Care |
|
23-hour Observation Bed |
|
Medically Monitored Intensive Inpatient for SUD |
|
24/7 Crisis Hotline |
|
Other |
|
Smartphone Apps |
|
Personal Protective Equipment |
|
Virtual/Telehealth/Telemedicine Services |
|
Purchase of increased connectivity (e.g., Wi-Fi) |
|
Cost-sharing Assistance (e.g., copayments, coinsurance, and deductibles) |
|
Provider Stabilization Payments |
|
Transportation to COVID-19 Services (e.g., testing, vaccination) |
|
Other (Please List) |
|
SABG Table 3 – Syringe Services Program
Table 3 provides a report of SABG and state expenditures by the SSA during the state fiscal year immediately preceding the federal fiscal year for which the state is applying for funds for authorized activities to prevent and treat SUDs. For detailed instructions, refer to those in the Block Grant Application System (BGAS).
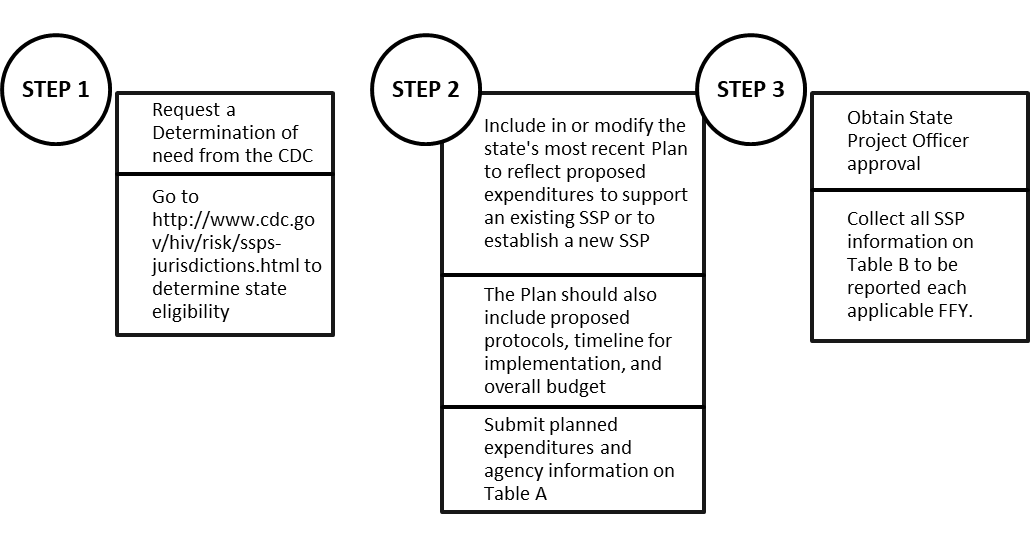
Table 3a SABG – Syringe Services Program
Report Period From: To: |
|||||||||
State Identifier |
|||||||||
Syringe Services Program (SSP) Agency Name |
Main Address of SSP |
Dollar Amount of SABG Funds Expended for SSP |
Dollar Amount of COVID-193 Funds Expended for SSP |
Dollar Amount of ARP4 Funds Expended for SSP |
|
SUD Treatment Provider (Yes or No) |
# of Locations (include any mobile locations) |
Narcan® Provided (Yes or No) |
Fentanyl Test Strips Provided (Yes or No) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0930-0168 Approved: 04/19/2019 Expires: 04/30/2022
Footnotes:

Table 3b SABG - Syringe Services Program
SABG |
||||||||||||||||||||
Syringe Services Program Name |
# of Unique Individuals Served |
HIV Testing (Please enter total number of individuals served) |
Treatment for Substance Use Conditions (Please enter total number of individuals served) |
Treatment for Physical Health (Please enter total number of individuals served) |
STD Testing (Please enter total number of individuals served) |
Hep C (Please enter total number of individuals served) |
||||||||||||||
ONSITE testing |
REFERRAL to testing |
ONSITE treatment |
REFERRAL to treatment |
ONSITE treatment |
REFERRAL to treatment |
ONSITE testing |
REFERRAL to testing |
ONSITE testing |
REFERRAL to testing |
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
COVID-19 |
||||||||||||||||||||
Syringe Services Program Name |
# of Unique Individuals Served |
HIV Testing (Please enter total number of individuals served) |
Treatment for Substance Use Conditions (Please enter total number of individuals served) |
Treatment for Physical Health (Please enter total number of individuals served) |
STD Testing (Please enter total number of individuals served) |
Hep C (Please enter total number of individuals served) |
||||||||||||||
|
|
ONSITE testing |
REFERRAL to testing |
ONSITE treatment |
REFERRAL to treatment |
ONSITE treatment |
REFERRAL to treatment |
ONSITE testing |
REFERRAL to testing |
ONSITE testing |
REFERRAL to testing |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
Syringe Services Program Name |
# of Unique Individuals Served |
HIV Testing (Please enter total number of individuals served) |
Treatment for Substance Use Conditions (Please enter total number of individuals served) |
Treatment for Physical Health (Please enter total number of individuals served) |
STD Testing (Please enter total number of individuals served) |
Hep C (Please enter total number of individuals served) |
|||||
|
|
ONSITE testing |
REFERRAL to testing |
ONSITE treatment |
REFERRAL to treatment |
ONSITE treatment |
REFERRAL to treatment |
ONSITE testing |
REFERRAL to testing |
ONSITE testing |
REFERRAL to testing |
|
|
|
|
|
|
|
|
|
|
|
|
SABG Table 4 - State Agency SABG Expenditure Compliance Report
This table provides a description of SABG expenditures for authorized activities to prevent and treat SUDs. For detailed instructions, refer to those in BGAS. Only one column is to be filled in each year.
State Agency SABG Expenditure Compliance Report |
||
SABG Table 4 |
FY 2019 SA Block Grant Award |
FY 2020 SA Block Grant Award |
State Identifier: |
||
Expenditure Category |
||
|
|
|
|
|
|
2. Primary Substance Use Disorder Prevention |
|
|
|
|
|
|
|
|
5. Administration (excluding program / provider level) |
|
|
6. Total |
|
|
* Prevention other than Primary Prevention
** Only designated states as defined in 42 U.S.C. § 300x-24(b)(2) and 45 CFR § 96.128(b) for the applicable federal fiscal year should enter information in this row. This may include a state or states that were previously considered “designated states” during any of the three prior federal fiscal years for which a state was applying for a grant. See EIS/HIV policy change in SABG Annual Report instructions
SABG Table 5a - Primary Prevention Expenditures
The state or jurisdiction must complete SABG Table 5a. There are six primary prevention strategies typically funded by principal agencies administering the SABG. Expenditures within each of the six strategies or Institute of Medicine Model (IOM) should be directly associated with the cost of completing the activity or task. For example, information dissemination may include the cost of developing pamphlets, the time of participating staff and/or the cost of public service announcements, etc. If a state plans to use strategies not covered by these six categories or the state is unable to calculate expenditures by strategy, please report them under “Other” in Table 5a.
The state or jurisdiction must complete SABG Table 5a Other Strategy if it chooses to report SUD primary prevention activities utilizing the IOM Model of Universal, Selective, and Indicated. Indicate how much funding supported each of the IOM classifications of Universal Direct, Universal Indirect, Selective, or Indicated without specifying the prevention strategy. Include all funding sources (e.g., Centers for Disease Control and Prevention Block Grant, foundations). For detailed instructions, refer to those in the Block Grant Application System (BGAS).
Section 1926 (Synar) - Tobacco: Costs associated with the Synar Program. Pursuant to the January 19, 1996 federal regulation “Tobacco Regulation for Substance Abuse Prevention and Treatment Block Grants, Final Rule” (45 CFR § 96.130) a state may not use the SABG to fund the enforcement of its statute, except that it may expend funds from its primary prevention set aside of its Block Grant allotment under 45 CFR § 96.124(b)(1) for carrying out the administrative aspects of the requirements, such as the development of the sample design and the conducting of the inspections. States should include any non-SABG funds* that were allotted for Synar activities in the appropriate columns under 7 below. Public Law 116-94, signed on December 20, 2019 supersedes this legislation and increased the minimum age for tobacco sales from 18 to 21. SAMHSA revised its guidance to clarify that the prevention set aside may be used to fund revisions to states’ Synar program to comply with PL 116-94. These funds should be reported in the appropriate columns under 7 below.
In most cases the total SABG amounts should equal the amounts reported on Plan Table 4, Row 2, Primary Substance Use Disorder Prevention. The one exception is if the state chooses to use a portion of the primary prevention set-aside to fund Non-Direct Services/System Development activities. The total on the Table 6 prevention column combined with the total on Table 5a should equal to expenditure Table 4, Row 2 in most instances.
Institute of Medicine Classification: Universal, Selective, and Indicated:
Prevention strategies may be classified using the IOM Model of Universal, Selective, and Indicated, which classifies preventive interventions by the population targeted. Definitions for these categories appear below:
Universal: Activities targeted to the public or a whole population group that have not been identified based on individual risk.
Universal
Direct: Row 1 - Interventions directly serve an identifiable
group of participants who have not been identified on the basis of
individual risk (e.g., school curriculum, after-school program,
parenting class). This also could include interventions involving
interpersonal and ongoing/repeated contact (e.g., coalitions).
Universal Indirect: Row 2 - Interventions support population-based programs and environmental strategies (e.g., establishing, Alcohol, Tobacco, and Other Drugs (ATOD) policies, modifying ATOD advertising practices). This also could include interventions involving programs and policies implemented by coalitions.
Selective: Activities targeted to individuals or a subgroup of the population whose risk of developing a disorder is significantly higher than average.
Indicated: Activities targeted to individuals in high-risk environments, identified as having minimal but detectable signs or symptoms foreshadowing disorder or having biological markers indicating predisposition for disorder but not meeting diagnostic levels (Adapted from The Institute of Medicine).
States that can report on both the strategy type and the population served (universal, selective, or indicated) should do so. If planned expenditure information is only available by strategy type, then the state should report planned expenditures in the row titled Unspecified (for example, Information Dissemination Unspecified).
Primary Substance Use Disorder Prevention Strategies Defined:
Information Dissemination – This strategy provides knowledge and increases awareness of the nature and extent of alcohol and other drug use, abuse, and addiction, as well as their effects on individuals, families, and communities. It also provides knowledge and increases awareness of available prevention and treatment programs and services. It is characterized by one-way communication from the source to the audience, with limited contact between the two.
Education - This strategy builds skills through structured learning processes. Critical life and social skills include decision making, peer resistance, coping with stress, problem solving, interpersonal communication, and systematic and judgmental abilities. There is more interaction between facilitators and participants than in the information strategy.
Alternatives - This strategy provides participation in activities that exclude alcohol and other drugs. The purpose is to meet the needs filled by alcohol and other drugs with healthy activities and to discourage the use of alcohol and drugs through these activities.
Problem Identification and Referral to Education - This strategy aims at identification of those who have indulged in illegal/age-inappropriate use of tobacco or alcohol and those individuals who have indulged in the first use of illicit drugs in order to assess if their behavior can be reversed through education. It should be noted, however, that this strategy does not include any activity designed to determine if a person needs treatment.
Community-based Process - This strategy provides ongoing networking activities and technical assistance to community groups or agencies. It encompasses neighborhood-based, grassroots empowerment models using action planning and collaborative systems planning.
Environmental - This strategy establishes or changes written and unwritten community standards, codes, and attitudes; thereby, influencing alcohol and other drug use by the general population.
Other - States that plan their primary prevention expenditures using the IOM model of universal, selective, and indicated should use Table 5a to list their FFY 2022 and FFY 2023 SABG planned expenditures in each of these categories.
SABG Primary Prevention Expenditures |
||||||
SABG Table 5a |
||||||
State Identifier: |
||||||
Report Period- From: To: |
||||||
Strategy
|
A. IOM Target |
B. SAPT Block Grant |
C. Other Federal |
D. State |
E. Local |
F. Other |
1. Information Dissemination |
Universal |
$ |
$ |
$ |
$ |
$ |
|
Selective |
$ |
$ |
$ |
$ |
$ |
|
Indicated |
$ |
$ |
$ |
$ |
$ |
|
Unspecified |
$ |
$ |
$ |
$ |
$ |
2. Education |
Universal |
$ |
$ |
$ |
$ |
$ |
|
Selective |
$ |
$ |
$ |
$ |
$ |
|
Indicated |
$ |
$ |
$ |
$ |
$ |
|
Unspecified |
$ |
$ |
$ |
$ |
$ |
3. Alternatives |
Universal |
$ |
$ |
$ |
$ |
$ |
|
Selective |
$ |
$ |
$ |
$ |
$ |
|
Indicated |
$ |
$ |
$ |
$ |
$ |
|
Unspecified |
$ |
$ |
$ |
$ |
$ |
4. Problem Identification and Referral |
Universal |
$ |
$ |
$ |
$ |
$ |
|
Selective |
$ |
$ |
$ |
$ |
$ |
|
Indicated |
$ |
$ |
$ |
$ |
$ |
|
Unspecified |
$ |
$ |
$ |
$ |
$ |
5. Community-Based Processes |
Universal |
$ |
$ |
$ |
$ |
$ |
|
Selective |
$ |
$ |
$ |
$ |
$ |
|
Indicated |
$ |
$ |
$ |
$ |
$ |
|
Unspecified |
$ |
$ |
$ |
$ |
$ |
6. Environmental |
Universal |
$ |
$ |
$ |
$ |
$ |
|
Selective |
$ |
$ |
$ |
$ |
$ |
|
Indicated |
$ |
$ |
$ |
$ |
$ |
|
Unspecified |
$ |
$ |
$ |
$ |
$ |
7. Section 1926 (Synar)-Tobacco |
Universal |
$ |
$ |
$ |
$ |
$ |
|
Selective |
$ |
$ |
$ |
$ |
$ |
|
Indicated |
$ |
$ |
$ |
$ |
$ |
8. Other |
Universal Direct |
$ |
$ |
$ |
$ |
$ |
|
Universal Indirect |
$ |
$ |
$ |
$ |
$ |
|
Selective |
$ |
$ |
$ |
$ |
$ |
|
Indicated |
$ |
$ |
$ |
$ |
$ |
10. Total |
|
$ |
$ |
$ |
$ |
$ |
*Please list all sources, if possible (e.g., Centers for Disease Control and Prevention, Block Grant, foundations, etc.)
SABG Table 5b (Required) - SABG Primary Prevention Targeted Priorities
The purpose of the first table is for the state or jurisdiction to identify the substance and/or categories of substances it identified through its needs assessment and then addressed with primary prevention set-aside dollars from the FY 2021 SABG NoA. The purpose of the second table is to identify each special population the state or jurisdiction selected as a priority for primary prevention set-aside expenditures.
|
SABG Award |
||
Targeted Substances |
|||
Alcohol |
|
||
Tobacco |
|
||
Marijuana |
|
||
Prescription Drugs |
|
||
Cocaine |
|
||
Heroin |
|
||
Inhalants |
|
||
Methamphetamine |
|
||
Synthetic Drugs (i.e. Bath salts, Spice, K2) |
|
||
Targeted Populations |
|||
Students in College |
|
||
Military Families |
|
||
LGBTQ+ |
|
||
American Indians/Alaska Natives |
|
||
African American |
|
||
Hispanic |
|
||
Homeless |
|
||
Native Hawaiian/Other Pacific Islanders |
|
||
Asian |
|
||
Rural |
|
||
Underserved Racial and Ethnic Minorities |
|
||
Footnotes:
SABG/MHBG Table 6 - Non-Direct-Services/System Development
Proposed Categories for Expenditures for System Development/Non-Direct-Service Activities
Expenditures in the following categories of activities may involve the time of state or sub-state personnel, or may be funded through contracts, grants, or agreements with other entities. Expenditures may come from the administrative funds and/or program funds (but may not include the HIV set-aside funds). Please utilize the following categories to describe the types of expenditures your state supports with Block Grant funds, and if the preponderance of the activity fits within a category.
We understand that a particular activity may cross categories but try to identify the primary purpose or goal of the activity. For example, a state may utilize Block Grant funds to train personnel to conduct fidelity assessments of evidence-based practices. While this could fall under either training/education and/or quality assurance/improvement – the primary purpose is to assure the implementation of evidence based practices (EBP), so that expenditure would most likely be captured under quality assurance/improvement.
Information Systems – This includes collecting and analyzing treatment data as well as prevention data under the SABG to monitor performance and outcomes. Costs for electronic health records and other health information technology also fall under this category.
Infrastructure Support – This includes activities that provide the infrastructure to support services but for which there are no individual services delivered. Examples include the development and maintenance of a crisis-response capacity, including hotlines, mobile crisis teams, web-based check-in groups (for medication, treatment, re-entry follow-up), drop-in centers, and respite services.
Partnerships, Community Outreach, and Needs Assessment – This includes state, regional, and local personnel salaries prorated for time and materials to support planning meetings, information collection, analysis, and travel. It also includes the support for partnerships across state and local agencies, and tribal governments. Community/network development activities, such as marketing, communication, and public education, and including the planning and coordination of services, fall into this category, as do needs-assessment projects to identify the scope and magnitude of the problem, resources available, gaps in services, and strategies to close those gaps.
Planning Council Activities – This includes those supports for the performance of a Mental Health Planning Council under the MHBG, a combined Behavioral Health Planning Council, or (OPTIONAL) Advisory Council for the SABG.
Quality Assurance and Improvement – This includes activities to improve the overall quality of services, including those activities to assure conformity to acceptable professional standards, adaptation, and review of implementation of evidence-based practices, identification of areas of technical assistance related to quality outcomes, including feedback. Administrative agency contracts to monitor service-provider quality fall into this category, as do independent peer-review activities.
Research and Evaluation – This includes performance measurement, evaluation, and research, such as services research and demonstration projects to test feasibility and effectiveness of a new approach as well as the dissemination of such information.
Training and Education– This includes skill development and continuing education for personnel employed in local programs as well as partnering agencies, as long as the training relates to either substance use disorder service delivery (prevention, treatment, and recovery) for SABG and services to adults with SMI or children with SED for MHBG. Typical costs include course fees, tuition, and expense reimbursements to employees, trainer(s) and support staff salaries, and certification expenditures.
Please enter the total amount of the block grant expended for each activity.
|
Non-Direct Services/System Development
|
||||
|
SABG Table 6 |
||||
|
State Identifier: |
||||
|
Report Period- From: To: |
||||
Activity
|
A. MHBG |
B. SABG Treatment |
C. SABG Prevention |
D. SABG Integrated * |
|
|
$ |
$ |
$ |
$ |
|
|
$ |
$ |
$ |
$ |
|
|
$ |
$ |
$ |
$ |
|
|
$ |
$ |
$ |
$ |
|
5. Quality Assurance and Improvement |
$ |
$ |
$ |
$ |
|
6. Research and Evaluation |
$ |
$ |
$ |
$ |
|
7. Training and Education |
$ |
$ |
$ |
$ |
|
8. Total |
$ |
$ |
$ |
$ |
|
*
Integrated refers to funds both treatment and prevention portions of
the SABG for overarching activities.
SABG Table 7 – State Entity Inventory
This table provides a report of the sub-recipients of SABG funds including community and faith-based organizations which provided SUD prevention activities and treatment services, as well as intermediaries/administrative service organizations. Table 7 excludes system development/non-direct service expenditures. For detailed instructions, see those in BGAS.
Statewide Entity Inventory |
|||||||||||||
SABG Table 7 |
|||||||||||||
State Identifier: |
|||||||||||||
Report Period- From: To: |
|||||||||||||
|
Source of Funds |
||||||||||||
|
SAPT Block Grant |
||||||||||||
A |
B |
B |
D |
E |
F |
||||||||
Entity Number |
I-BHS ID (formerly I-SATS) |
Area Served (Statewide or Sub-State Planning Area) |
Provider/Program Name |
Street Address |
City |
State |
Zip |
All SA Block Grant Funds |
Prevention (other than Primary Prevention) and Treatment Services |
Pregnant Women and Women with Dependent Children |
Primary Prevention |
Early Intervention Services for HIV |
Syringe Services Program |
|
|
|
|
|
|
|
|
$ |
$ |
$ |
$ |
$ |
$ |
|
|
|
|
|
|
|
|
$ |
$ |
$ |
$ |
$ |
$ |
Total |
|
|
|
|
|
|
|
$ |
$ |
$ |
$ |
$ |
$ |
Description of Calculations for MOE Tables 8a and 8b
Please provide a description of the amounts and methods used to calculate the following:
total Single State Agency (SSA) expenditures for SUD prevention and treatment as required by:
42 U.S.C. § 300x-30 and 45 CFR § 96.124(f)(4)
the base and, for 1994 and subsequent fiscal years, report the federal and state expenditures for services to pregnant women and women with dependent children as required by:
b. 42 U.S.C. § 300x-22(b)(1) and 45 CFR § 96.122(f)(5)(ii)(A)
SABG Table 8a - Maintenance of Effort for State Expenditures for SUD Prevention and Treatment
This Maintenance of Effort table provides a description of non-federal expenditures for authorized activities to prevent and treat substance abuse flowing through the Single State Agency (SSA) during the state fiscal year immediately preceding the federal fiscal year for which the state is applying for funds. Dates given are for the FY 2022 SABG Report. For the FY 2023 SABG report, please increase each year by one. For detailed instructions, see those in BGAS.
SABG Table 8a Total Single State Agency (SSA) Expenditures for Substance Abuse Prevention and Treatment |
|
||
State Identifier: |
|
||
Report Period- From: To: |
|
||
Period
(A) |
Expenditures
(B) |
B1 (2018) + B2 (2019) 2
(C) |
|
SFY 2019 (1) |
|
|
|
SFY 2020 (2) |
|
|
|
SFY 2021 (3) |
|
|
|
Are the expenditure amounts reported in Column B “actual” expenditures for the fiscal years involved?
|
Yes |
No |
SFY 2019 |
|
|
SFY 2020 |
|
|
SFY 2021 |
|
|
If any estimated expenditures are provided, please indicate when “actual” expenditure data will be submitted to SAMHSA: ___/___/_____
mm/dd/yyyy
Did the state or jurisdiction have any non-recurring expenditures as described in 42 U.S.C. § 300x-30(b) for a specific purpose which were not included in the MOE calculation?
Yes____ No ___ If yes, specify the amount and the State fiscal year ___________
Did the state or jurisdiction include these funds in previous year MOE calculations? Yes___ No___
When did the State or Jurisdiction submit an official request to SAMHSA to exclude these funds from the MOE calculations? ___/___/_____
mm/dd/yyyy
SABG Table 8b - Expenditures for Services to Pregnant Women and Women with Dependent Children
This table provides a report of all statewide, non-federal funds expended on specialized treatment and related services which meet the SABG requirements for pregnant women and women with dependent children during the state fiscal year immediately preceding the federal fiscal year for which the state is applying for funds. Dates given are for the FY 2022 SABG Report. For the FY 2023 SABG Report, increase each year (other than the base year) by one. For detailed instructions, see those in BGAS.
G Table 8c SABG Table 8c SABG Table 8c
Expenditures for Services to Pregnant Women and Women with Dependent Children |
||
SABG Table 8b |
||
State Identifier: |
||
Report Period- From: To: |
||
Period |
Total Women’s Base (A) |
Total Expenditures (B) |
1994 |
|
|
2019 |
|
|
2020 |
|
|
2021 |
|
|
D. Populations and Services Report
States are required to provide information regarding individuals that are served by the SSA in SABG Tables 9 through 13.
SABG Table 9 - Prevention Strategy Report. This table requires additional information (pursuant to Section 1929 of Title XIX, Part B, Subpart II of the PHS Act (42 U.S.C. § 300x-29) about the primary prevention activities conducted by the entities listed on SABG Table 7, State Entity Inventory, Column D. It seeks further information on the specific strategies and activities being funded by the principal agency of the state which address the sub-populations at risk for ATOD.
SABG Table 10 - Treatment Utilization Matrix. This table is intended to capture the count of persons with initial admissions and subsequent admission(s) to an episode of care as defined in the Behavioral Health Services Information System (BHSIS), formerly known as the Drug and Alcohol Services Information System (DASIS), Treatment Episode Data Set (TEDS) standards (see TEDS data) during the state fiscal year immediately preceding the federal fiscal year for which the state is applying for funds.
SABG Table 11a - Number of Persons Served (Unduplicated Count) for Alcohol and Other Drug Use. This table provides an aggregate profile of the unduplicated number of admissions and persons for services funded through the SABG during the state fiscal year immediately preceding the federal fiscal year for which the state is applying for funds. States are to provide this information on all programs by age, gender, and race/ethnicity. States are to report whether the values reported come from a client-based system(s) with unique client identifiers.
SABG Table 11b - COVID-19 Number of Persons Served (Unduplicated Count) for Alcohol and Other Drug Use. This table provides an aggregate profile of the unduplicated number of admissions and persons for services funded with COVID-19 Relief Supplement funds during the state fiscal year immediately preceding the federal fiscal year for which the state is applying for funds. States are to provide this information on all programs by age, gender, and race/ethnicity. States are to report whether the values reported come from a client-based system(s) with unique client identifiers.
SABG Table 11c - Sex, Sexual Orientation, and Gender Identity Number of Persons Served (Unduplicated Count) for Alcohol and Other Drug Use. This table provides an aggregate profile of the unduplicated number of admissions and persons for services funded through the SABG during the state fiscal year immediately preceding the federal fiscal year for which the state is applying for funds by sex, sexual orientation, and gender identity. States are to report whether the values reported come from a client-based system(s) with unique client identifiers.
SABG Table 12 - SABG Designated States and Early Intervention Services Regarding the Human Immunodeficiency Virus. This table requires designated states as defined in section 1924(b)(2) of Title XIX, Part B, Subpart II of the PHS Act (42 U.S.C. § 300x-24(b)(2)), to provide information on Early Intervention Services Regarding the Human Immunodeficiency Virus (EIS/HIV) provided during the state fiscal year immediately preceding the federal fiscal year for which the state is applying for funds.
SABG Table 13 - Charitable Choice. This table requires states to provide information regarding compliance with section 1955 of Title XIX, Part B, Subpart III of the PHS Act (42 U.S.C. § 300x-65) and the Charitable Choice Provisions and Regulations; Final Rule (42 CFR Part 54) during the state fiscal year immediately preceding the federal fiscal year for which the state is applying for funds. Each section of this table requires that the state respond appropriately to identify the way they have complied with the requirements related to authorizing legislation and implementing regulation. States should report on the number of clients referred due to religious objection from faith and community-based programs to appropriate alternative providers. If no alternate referrals were made, enter zero.
SABG Table 9 - Prevention Strategy Report
This table requires additional information (pursuant to Section 1929 of Title XIX, Part B, Subpart II of the PHS Act (42 U.S.C.§ 300x-29) about the primary prevention activities conducted by the entities listed on SABG Table 7. For detailed instructions, see those in BGAS. Prevention Strategy Report Risk-Strategies |
||
SABG Table 9 |
||
Report Period- From: To: |
||
State Identifier |
||
Column A (Risks) |
Column B (Strategies) |
Column C (Providers) |
|
|
|
Pregnant Women/Teens [2] |
|
|
Drop-Outs [3] |
|
|
Violent and Delinquent Behavior [4] |
|
|
Mental Health Problems [5] |
|
|
Economically Disadvantaged [6] |
|
|
Physically Disabled [7] |
|
|
Abuse Victims [8] |
|
|
Already Using Substances [9] |
|
|
Homeless and/or Runaway Youth [10] |
|
|
Other- Specify [11] |
|
|
|
|
|
SABG Table 10 – Treatment Utilization Matrix
This table is intended to capture the count of persons with initial admissions and subsequent admission(s) to an episode of care. For detailed instructions, see those in BGAS.
SABG Table 10 |
|
|
|
|||||||||||||
Report Period- From: To: |
|
Treatment Utilization Matrix |
|
|||||||||||||
|
SABG Number of Admissions ≥ Number of Persons Served |
COVID-19 Number of Admissions ≥ Number of Persons Served 1 |
ARP Number of Admissions ≥ Number of Persons Served 2 |
SABG Costs per Person (C+D+E) |
COVID-19 Costs per Person (C+D+E) 1 |
ARP Costs per Person (C+D+E)2 |
||||||||||
Level of Care |
Number of Admissions (A) |
Number of Persons Served (B) |
Number of Admissions
(A) |
Number of Persons Served (B) |
Number of Admissions (A) |
Number of Persons Served (B) |
Mean Cost of Services (C) |
Median Cost of Services (D) |
Standard Deviation of Cost (E) |
Mean Cost of Services (C) |
Median Cost of Services (D) |
Standard Deviation of Cost (E) |
Mean Cost of Services (C) |
Median Cost of Services (D) |
Standard Deviation of Cost (E) |
|
Detoxification (24-Hour Care) |
||||||||||||||||
1. Hospital Inpatient |
|
|
|
|
|
|
$ |
$ |
$ |
$ |
$ |
$ |
$ |
$ |
$ |
|
2. Free-Standing Residential |
|
|
|
|
|
|
$ |
$ |
$ |
$ |
$ |
$ |
$ |
$ |
$ |
|
Rehabilitation/Residential |
||||||||||||||||
3. Hospital Inpatient |
|
|
|
|
|
|
$ |
$ |
$ |
$ |
$ |
$ |
$ |
$ |
$ |
|
4. Short-term (up to 30 days) |
|
|
|
|
|
|
$ |
$ |
$ |
$ |
$ |
$ |
$ |
$ |
$ |
|
5. Long-term (over 30 days) |
|
|
|
|
|
|
$ |
$ |
$ |
$ |
$ |
$ |
$ |
$ |
$ |
|
Ambulatory (Outpatient) |
||||||||||||||||
6. Outpatient |
|
|
|
|
|
|
$ |
$ |
$ |
$ |
$ |
$ |
$ |
$ |
$ |
|
7. Intensive Outpatient |
|
|
|
|
|
|
$ |
$ |
$ |
$ |
$ |
$ |
$ |
$ |
$ |
|
8. Detoxification |
|
|
|
|
|
|
$ |
$ |
$ |
$ |
$ |
$ |
$ |
$ |
$ |
|
Opioid Replacement Therapy5 |
||||||||||||||||
9. OUT Medication-Assisted Detoxification |
|
|
|
|
|
|
$ |
$ |
$ |
$ |
$ |
$ |
$ |
$ |
$ |
|
10. OUD Medication-Assisted Treatment Outpatient |
|
|
|
|
|
|
$ |
$ |
$ |
$ |
$ |
$ |
$ |
$ |
$ |
|
0930-0168 Approved: 04/19/2019 Expires: 04/30/2022

The 24-month expenditure period for the COVID-19 Relief supplemental funding is March 15, 2021 – March 14, 2023, which is different from the expenditure period for the “standard” SABG and MHBG. Per the instructions, the standard SABG expenditures are for the state planned expenditure period of July 1, 2021 – June 30, 2023, for most states.
2 The expenditure period for The American Rescue Plan Act of 2021 (ARP) supplemental funding is September 1, 2021 – September 30, 2025, which is different from the expenditure period for the “standard” MHBG/SABG. Per the instructions, the planning period for standard MHBG/SABG expenditures is July 1, 2021 – June 30, 2023.
SABG Table 11a - Unduplicated Count of Persons Served for Alcohol and Other Drug Use
This table provides an aggregate profile of the unduplicated number of admissions and persons for services funded through the SABG. For detailed instructions, see those in BGAS.
Report Period- From: To: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
State Identifier: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Age |
A. Total |
B. White |
C. Black or African American |
D. Native Hawaiian/ Other Pacific Islander |
E. Asian |
F. American Indian/Alaskan Native |
G. More Than One Race Reported |
H. Unknown |
I. Not Hispanic or Latino |
J. Hispanic or Latino |
||||||||||
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
|||
1. 17 and under |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5. 65 and over |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
6. Total
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
7. Pregnant Women |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Numbers of Persons Served who were admitted in a Period Prior to the 12-month reporting Period |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
Number of persons served outside of the levels of care described on SABG Table 10 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
Are the values reported in this table generated from a client-based system with unique client identifiers? |
Yes |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
No |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
0930-0168 Approved: 04/19/2019 Expires: 04/30/2022
Table 11b- COVID-19 Number of Persons Served (Unduplicated Count) for Alcohol and Other Drug Use
Report Period- From: To: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
State Identifier: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Age |
A. Total |
B. White |
C. Black or African American |
D. Native Hawaiian/ Other Pacific Islander |
E. Asian |
F. American Indian/Alaskan Native |
G. More Than One Race Reported |
H. Unknown |
I. Not Hispanic or Latino |
J. Hispanic or Latino |
||||||||||
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
|||
1. 17 and under |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5. 65 and over |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
6. Total
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
7. Pregnant Women |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TABLE 11c: Sex, Sexual Orientation, and Gender Identity Unduplicated Count of Persons Served for Alcohol and Other Drugs
|
|||||||||||
A. AGE |
B. Cisgender Male |
C. Cisgender Female |
D. Transgender Man /Transman /Female-To-Man |
E. Transgender Woman/ Transwoman/ Male-To-Female |
F. Genderqueer/ Gender Non-Conforming/ Neither Exclusively Male nor Female |
G. Additional Gender Category (or Other) |
H. Straight or Heterosexual |
I. Gay or Lesbian |
J. Bisexual |
K. Queer, Pansexual, and/or Questioning |
L. Something Else? Please Specify |
17 and Under |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
18-24 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
25-44 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
45-64 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
65 and over |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SABG Table 12 - SABG Early Intervention Services Regarding the Human Immunodeficiency Virus (EIS/HIV) in Designated States
For detailed instructions, see those in BGAS.
SABG Table 12 |
|
|
Report Period- From: To: |
|
|
State Identifier: |
|
|
Early Intervention Services Regarding the Human Immunodeficiency Virus (EIS/HIV) |
||
1. Number of EIS/HIV projects among SABG sub-recipients in the state: |
Statewide:_____ |
Rural:_____ |
2. Total number of individuals tested through SABG sub-recipient EIS/HIV projects: |
|
|
3. Total number of HIV tests conducted with SABG EIS/HIV funds: |
|
|
4. Total number of tests that were positive for HIV: |
|
|
5. Total number of individuals who prior to the 12-month reporting period were unaware of their HIV infection: |
|
|
6. Total number of HIV infected individuals who were diagnosed and referred into treatment and care during the 12-month reporting period |
|
|
Identify barriers, including state laws and regulations, that exist in carrying out HIV testing services:
|
||
SABG Table 13 - Charitable Choice – Required
Under Charitable Choice Provisions; Final Rule (42 CFR Part 54), states, local governments, and religious organizations, such as SAMHSA grant recipients, must: (1) ensure that religious organizations that are providers provide to all potential and actual program beneficiaries (services recipients) notice of their right to alternative services; (2) ensure that religious organizations that are providers refer program beneficiaries to alternative services; and (3) fund and/or provide alternative services. The term “alternative services” means services determined by the state to be accessible and comparable and provided within a reasonable period of time from another substance abuse provider (“alternative provider”) to which the program beneficiary (services recipient) has no religious objection. The purpose of this table is to document how the state is complying with these provisions.
Notice to Program Beneficiaries – Check all that apply:
Used model notice provided in final regulations.
Used notice developed by state (please attach a copy to the Report).
State has disseminated notice to religious organizations that are providers.
State requires these religious organizations to give notice to all potential beneficiaries.
Referrals to Alternative Services – Check all that apply:
State has developed specific referral system for this requirement.
State has incorporated this requirement into existing referral system(s).
SAMHSA’s Behavioral Health Treatment Locator is used to help identify providers.
Other networks and information systems are used to help identify providers.
State maintains record of referrals made by religious organizations that are providers.
Enter the total number of referrals to other substance abuse providers (“alternative providers”) necessitated by religious objection, as defined above, made during the state fiscal year immediately preceding the federal fiscal year for which the state is applying for funds. Provide the total only. No information on specific referrals is required. If no alternative referrals were made, enter zero.

E. Performance Data and Outcomes
SAMHSA is interested in demonstrating program accountability and efficacy through the National Outcome Measures (NOMs). The NOMs are intended to document the performance of federally supported programs and systems of care.
Treatment Performance Measures
SABG Table 14 - Employment/Education Status. This table describes the status of adult clients served by the public SUD treatment service systems in terms of employment and education status. The Employment/Education Status Form seeks information on clients who are employed or who are students (full-time or part-time within the prior 30 days) at admission and discharge.
SABG Table 15 - Stability of Housing. This table requests information regarding the number of individuals in a stable living environment as collected by the most recent assessment in the reporting period. Specifically, information is collected on the individual’s last known living situation.
SABG Table 16 - Criminal Justice Involvement. This table requests information regarding the clients’ involvement in the criminal justice system. Specifically, the table requests information to measure the change in number of arrests over time.
SABG Table 17 - Change in Abstinence - Alcohol Use. This table seeks information regarding alcohol abstinence. Specifically, information is collected on the number of clients with no alcohol use (all clients regardless of primary problem) at admission and discharge.
SABG Table 18 - Change in Abstinence - Other Drug Use. This table collects information regarding clients’ change in abstinence with drugs of abuse other than alcohol. This table seeks to collect information on clients with no other drug use (all clients regardless of primary problem) at admission and discharge.
SABG Table 19 - Change in Social Support of Recovery. This table seeks to measure the change in clients’ social support of recovery. Specifically, this form collects information regarding the number of clients participating in self-help groups at admission and discharge.
SABG Table 20 - Retention. This table collects information regarding retention. Specifically, this table collects information regarding the length of stay of clients completing treatment.
Prevention Performance Measures
SABG Table 21 - Reduced Morbidity –Abstinence from Drug Use/Alcohol Use; Measure: 30 Day Use. This table seeks information regarding 30-day use of alcohol, tobacco, and other drugs.
SABG Table 22 - Reduced Morbidity –Abstinence from Drug Use/Alcohol Use; Measure: Perception of Risk/Harm of Use. This table seeks information regarding the individuals’ perceived risk of harming themselves with alcohol, tobacco, and other drugs.
SABG Table 23 - Reduced Morbidity–Abstinence from Drug Use/Alcohol Use; Measure: Age of First Use. This table seeks information regarding the age of first use of alcohol, cigarettes, and other drugs.
SABG Table 24 - Reduced Morbidity–Abstinence from Drug Use/Alcohol Use; Measure: Perception of Disapproval/Attitudes. This table seeks information regarding public perception or attitude regarding use of alcohol, cigarettes, and other drugs.
SABG Table 25 - Employment/Education; Measure: Perception of Workplace Policy. This table reports the percent of individuals who would be more likely to work for an employer conducting random drug and alcohol tests.
SABG Table 26 - Employment/Education; Measure: Average Daily School Attendance Rate. This table collects information regarding the average daily school attendance.
SABG Table 27 - Crime and Criminal Justice; Measure: Alcohol-Related Traffic Fatalities. This table collects information regarding the number of alcohol-related traffic fatalities divided by the total number of traffic fatalities and multiplied by 100.
SABG Table 28 - Crime and Criminal Justice; Measure: Alcohol and Drug Related Arrests. This table collects information regarding alcohol- and drug-related arrests.
SABG Table 29 - Social Connectedness; Measure: Family Communications around Drug and Alcohol Use. This table provides information regarding the percent of youth reporting having talked with a parent and the percent of parents reporting that they have talked to their child about alcohol and drug use.
SABG Table 30 - Retention; Measure: Percentage of Youth Seeing, Reading, Watching, or Listening to a Prevention Message. This table collects information regarding the percent of youth reporting having been exposed to prevention messaging.
SABG Table 31-35 – Reporting Period – Start and End Dates for Information Reported on Tables 31, 32, 33, 34, and 35. This table provides information regarding the corresponding reporting period dates for Table 31 through Table 35. States also must describe their data collection system regarding prevention NOMS reporting.
SABG Table 31 - Individual-Based Programs and Strategies; Measure: Number of Persons Served by Age, Gender, Race, and Ethnicity. This table provides information on the number of persons served by individual-based programs and strategies. This includes practices and strategies with identifiable goals designed to change behavioral outcomes among a definable population or within a definable geographic area.
SABG Table 32 - Population-Based Programs and Strategies; Measure: Number of Persons Served by Age, Gender, Race, and Ethnicity. This table provides information regarding the number of persons by age, gender, race, and ethnicity that participated in population-based programs. Population-based programs and strategies include planned and deliberate goal-oriented practices, procedures, processes, or activities that have identifiable outcomes achieved with a sequence of steps subject to monitoring and modification.
SABG Table 33 - Number of Persons Served by Type of Intervention. This table seeks to measure information on access and capacity of intervention programs. Specifically, this form collects information on the number of persons served by type of intervention. Interventions include activities, practices, procedures, processes, programs, services, and strategies.
SABG Table 34 - Number of Evidence-Based Programs by Types of Intervention. This table collects information on the number of evidence-based programs and strategies by type of intervention.
SABG Table 35 - Number of Evidence-based Programs and Strategies, and Total SABG Funds Spent on Evidence-Based Programs/ Strategies. This table collects information on the number of Evidence-Based Programs and Strategies funded by the type of Institute of Medicine (IOM) intervention (e.g. Universal, Selective, and Indicated). In addition, the state must indicate the amount of SABG funds spent on the Evidence-Based interventions.
SABG Table 14 – Treatment Performance Measure: Employment /Education Status
(From Admission to Discharge)
Most recent year for which data are available: _____________
Employment/Education Status – Clients employed or student (full-time or part-time) (prior 30 days) at admission vs. discharge |
Admission Clients (T1) |
Discharge Clients (T2) |
Number of clients employed or student (full-time and part-time) [numerator] |
|
|
Total number of clients with non-missing values on employment/student status [denominator] |
|
|
Percent of clients employed or student (full-time and part-time) |
|
|
(SABG Table 14) (continued) State Description of Employment/Education Status Data Collection
STATE CONFORMANCE TO INTERIM STANDARD |
State Description of Employment/Education Data Collection (SABG Table 14): States should detail exactly how this information is collected. Where data and methods vary from interim standard, variance should be described.
|
DATA SOURCE |
What is the source of data for SABG Table 14 (select all that apply): □ Client self-report □ Client self-report confirmed by another source→ □ collateral source □ Administrative data source □ Other Specify ___________________
|
EPISODE OF CARE |
How is the admission/discharge basis defined for SABG Table 14 (Select one) □ Admission is on the first date of service, prior to which no service has been received for 30 days AND discharge is on the last date of service, subsequent to which no service has been received for 30 days □ Admission is on the first date of service in a Program/Service Delivery Unit and Discharge is on the last date of service in a Program/Service Delivery Unit □ Other Specify ___________________________________________ _________________________________________________________ |
DISCHARGE DATA COLLECTION |
How was discharge data collected for SABG Table 14 (select all that apply) □ Not applicable, data reported on form is collected at time period other than discharge→ Specify: □ In-treatment data ___ days post-admission, OR □ Follow-up data ___ (specify) months Post- □ admission □ discharge □ other ______ □ Discharge data are collected for the census of all (or almost all) clients who were admitted to treatment □ Discharge data are collected for a sample or all clients who were admitted to treatment □ Discharge records are directly collected (or in the case of early dropouts) are created for all (or almost all) clients who were admitted to treatment □ Discharge records are not collected for approximately ___ % of clients who were admitted for treatment |
RECORD LINKING |
Was the admission and discharge data linked for table 14 (select all that apply): □ Yes, all clients at admission were linked with discharge data using a Unique Client Identifier (UCID) Select type of UCID □ Master Client Index or Master Patient Index, centrally assigned □ Social Security Number (SSN) □ Unique client ID based on fixed client characteristics (such as date of birth, gender, partial SSN, etc.) □ Some other Statewide unique ID □ Provider-entity-specific unique ID □ No, State Management Information System does not utilize UCID that allows comparison of admission and discharge data on a client specific basis (data developed on a cohorts basis) or State relied on other data sources for post admission data □ No, admission and discharge records were matched using probabilistic record matching. |
IF DATA ARE UNAVAILABLE |
If data are not reported, why is State unable to report (select all that apply): □ Information is not collected at admission □ Information is not collected at discharge □ Information is not collected by the categories requested □ State collects information on the indicator area but utilizes a different measure. |
DATA PLANS IF DATA ARE NOT AVAILABLE |
State must provide time-framed plans for capturing employment\student status data on all clients if data are not currently available. Plans should also discuss barriers, resource needs and estimates of cost.
|
SABG Table 15 – Treatment Performance Measure: Stability of Housing
(From Admission to Discharge)
Most recent year for which data are available: _____________
Clients living in a stable living situation (prior 30 days) at admission vs. discharge |
Admission Clients (T1) |
Discharge Clients (T2) |
Number of clients living in a stable situation [numerator] |
|
|
Total number of clients with non-missing values on living arrangements [denominator] |
|
|
Percent of clients in a stable living situation |
|
|
SABG Table 15 – State Description of Stability in Housing Data Collection
STATE CONFORMANCE TO INTERIM STANDARD |
State Description of Stability in Housing Data Collection (SABG Table 15): States should detail exactly how this information is collected. Where data and methods vary from interim standard, variance should be described.
|
DATA SOURCE |
What is the source of data for SABG Table 15 (select all that apply): □ Client self-report □ Client self-report confirmed by another source→ □ collateral source □ Administrative data source □ Other Specify ___________________ |
EPISODE OF CARE |
How is the admission/discharge basis defined for SABG Table 15 (Select one) □ Admission is on the first date of service, prior to which no service has been received for 30 days AND discharge is on the last date of service, subsequent to which no service has been received for 30 days □ Admission is on the first date of service in a Program/Service Delivery Unit and Discharge is on the last date of service in a Program/Service Delivery Unit □ Other Specify ___________________________________________ _________________________________________________________
|
DISCHARGE DATA COLLECTION |
How was discharge data collected for SABG Table 15 (select all that apply) □ Not applicable, data reported on form is collected at time period other than discharge→ Specify: □ In-treatment data ___ days post-admission, OR □ Follow-up data ___ (specify) months Post- □ admission □ discharge □ other ______ □ Discharge data are collected for the census of all (or almost all) clients who were admitted to treatment □ Discharge data are collected for a sample or all clients who were admitted to treatment □ Discharge records are directly collected (or in the case of early dropouts) are created for all (or almost all) clients who were admitted to treatment □ Discharge records are not collected for approximately ___ % of clients who were admitted for treatment |
RECORD LINKING |
Was the admission and discharge data linked for SABG Table 15 (select all that apply): □ Yes, all clients at admission were linked with discharge data using a Unique Client Identifier (UCID) Select type of UCID □ Master Client Index or Master Patient Index, centrally assigned □ Social Security Number (SSN) □ Unique client ID based on fixed client characteristics (such as date of birth, gender, partial SSN, etc.) □ Some other Statewide unique ID □ Provider-entity-specific unique ID □ No, State Management Information System does not utilize UCID that allows comparison of admission and discharge data on a client specific basis (data developed on a cohorts basis) or State relied on other data sources for post admission data □ No, admission and discharge records were matched using probabilistic record matching.
|
IF DATA ARE UNAVAILABLE |
If data are not reported, why is the state unable to report (select all that apply): □ Information is not collected at admission □ Information is not collected at discharge □ Information is not collected by the categories requested □ State collects information on the indicator area but utilizes a different measure. |
DATA PLANS IF DATA ARE NOT AVAILABLE |
State must provide time-framed plans for capturing criminal justice involvement status data on all clients if data are not currently available. Plans should also discuss barriers, resource needs and estimates of cost. |
SABG Table 16 – Treatment Performance Measure Criminal Justice Involvement
(From Admission to Discharge)
Most recent year for which data are available: _____________
Clients without arrests (any charge) (prior 30 days) at admission vs. discharge |
Admission Clients (T1) |
Discharge Clients (T2) |
Number of Clients without arrests [numerator] |
|
|
Total number of clients with non-missing values on arrests [denominator] |
|
|
Percent of clients without arrests |
|
|
State Description of Criminal Involvement Data Collection (SABG Table 16)
STATE CONFORMANCE TO INTERIM STANDARD |
State Description of Criminal Involvement Data Collection (SABG Table 16): States should detail exactly how this information is collected. Where data and methods vary from interim standard, variance should be described.
|
DATA SOURCE |
What is the source of data for SABG Table 16 (select all that apply): □ Client self-report □ Client self-report confirmed by another source→ □ collateral source □ Administrative data source □ Other Specify ___________________ |
EPISODE OF CARE |
How is the admission/discharge basis defined for SABG Table 16 (Select one) □ Admission is on the first date of service, prior to which no service has been received for 30 days AND discharge is on the last date of service, subsequent to which no service has been received for 30 days □ Admission is on the first date of service in a Program/Service Delivery Unit and Discharge is on the last date of service in a Program/Service Delivery Unit □ Other Specify ___________________________________________ _________________________________________________________
|
DISCHARGE DATA COLLECTION |
How was discharge data collected for SABG Table 16 (select all that apply) □ Not applicable, data reported on form is collected at time period other than discharge→ Specify: □ In-treatment data ___ days post-admission, OR □ Follow-up data ___ (specify) months Post- □ admission □ discharge □ other ______ □ Discharge data are collected for the census of all (or almost all) clients who were admitted to treatment □ Discharge data are collected for a sample or all clients who were admitted to treatment □ Discharge records are directly collected (or in the case of early dropouts) are created for all (or almost all) clients who were admitted to treatment □ Discharge records are not collected for approximately ___ % of clients who were admitted for treatment |
RECORD LINKING |
Was the admission and discharge data linked for SABG Table 16 (select all that apply): □ Yes, all clients at admission were linked with discharge data using a Unique Client Identifier (UCID) Select type of UCID □ Master Client Index or Master Patient Index, centrally assigned □ Social Security Number (SSN) □ Unique client ID based on fixed client characteristics (such as date of birth, gender, partial SSN, etc.) □ Some other Statewide unique ID □ Provider-entity-specific unique ID □ No, State Management Information System does not utilize UCID that allows comparison of admission and discharge data on a client specific basis (data developed on a cohorts basis) or State relied on other data sources for post admission data □ No, admission and discharge records were matched using probabilistic record matching.
|
IF DATA ARE UNAVAILABLE |
If data are not reported, why is State unable to report (select all that apply): □ Information is not collected at admission □ Information is not collected at discharge □ Information is not collected by the categories requested □ State collects information on the indicator area but utilizes a different measure. |
DATA PLANS IF DATA ARE NOT AVAILABLE |
State must provide time-framed plans for capturing criminal justice involvement status data on all clients if data are not currently available. Plans should also discuss barriers, resource needs and estimates of cost. |
SABG Table 17– Treatment Performance Measure: Change in Abstinence – Alcohol Use
(From Admission to Discharge)
Most recent year for which data are available: _____________
Alcohol Abstinence – Clients with no alcohol use (all clients regardless of primary problem) (use Alcohol Use in last 30 days field) at admission vs. discharge. |
Admission Clients (T1) |
Discharge Clients (T2) |
Number of clients abstinent from alcohol [numerator] |
|
|
Total number of clients with non-missing values on “used any alcohol” variable [denominator] |
|
|
Percent of clients abstinent from alcohol |
|
|
(1) If State does not have a "used any alcohol" variable, calculate instead using frequency of use variables for all primary, secondary, or tertiary problem codes in which the coded problem is Alcohol (e.g., TEDS Code 01) |
|
|
State Description of Alcohol Use Data Collection (SABG Table 17)
STATE CONFORMANCE TO INTERIM STANDARD |
State Description of Alcohol Use Data Collection (SABG Table 17): State should detail exactly how this information is collected. Where data and methods vary from interim standard, variance should be described.
|
DATA SOURCE |
What is the source of data for SABG Table 17 (select all that apply): □ Client self-report □ Client self-report confirmed by another source→ □ urinalysis, blood test or other biological assay □ collateral source □ Administrative data source □ Other Specify ___________________ |
EPISODE OF CARE |
How is the admission/discharge basis defined for SABG Table 17 (Select one) □ Admission is on the first date of service, prior to which no service has been received for 30 days AND discharge is on the last date of service, subsequent to which no service has been received for 30 days □ Admission is on the first date of service in a Program/Service Delivery Unit and Discharge is on the last date of service in a Program/Service Delivery Unit □ Other Specify ___________________________________________ |
DISCHARGE DATA COLLECTION |
How was discharge data collected for SABG Table 17 (select all that apply) □ Not applicable, data reported on form is collected at time period other than discharge→ Specify: □ In-treatment data ___ days post-admission, OR □ Follow-up data ___ (specify) months Post- □ admission □ discharge □ other ______ □ Discharge data are collected for the census of all (or almost all) clients who were admitted to treatment □ Discharge data are collected for a sample or all clients who were admitted to treatment □ Discharge records are directly collected (or in the case of early dropouts) are created for all (or almost all) clients who were admitted to treatment □ Discharge records are not collected for approximately ___ % of clients who were admitted for treatment |
RECORD LINKING |
Was the admission and discharge data linked for SABG Table 17 (select all that apply): □ Yes, all clients at admission were linked with discharge data using a Unique Client Identifier (UCID) Select type of UCID □ Master Client Index or Master Patient Index, centrally assigned □ Social Security Number (SSN) □ Unique client ID based on fixed client characteristics (such as date of birth, gender, partial SSN, etc.) □ Some other Statewide unique ID □ Provider-entity-specific unique ID □ No, State Management Information System does not utilize UCID that allows comparison of admission and discharge data on a client specific basis (data developed on a cohorts basis) or State relied on other data sources for post admission data □ No, admission and discharge records were matched using probabilistic record matching.
|
IF DATA ARE UNAVAILABLE |
If data are not reported, why is State unable to report (select all that apply): □ Information is not collected at admission □ Information is not collected at discharge □ Information is not collected by the categories requested □ State collects information on the indicator area but utilizes a different measure. |
DATA PLANS IF DATA ARE NOT AVAILABLE |
State must provide time-framed plans for capturing abstinence - alcohol use status data on all clients if data are not currently available. Plans should also discuss barriers, resource needs and estimates of cost. |
SABG Table 18 – Performance Measure: Change in Abstinence – Other Drug Use (From Admission to Discharge) Most recent year for which data are available: _____________
Drug Abstinence – Clients with no drug use (all clients regardless of primary problem) (use Any Drug Use in last 30 days field) at admission vs. discharge. |
Admission Clients (T1) |
Discharge Clients (T2) |
Number of Clients abstinent from illegal drugs [numerator] |
|
|
Total number of clients with non-missing values on “used any drug” variable [denominator] * |
|
|
Percent of clients abstinent from drugs |
|
|
*If State does not have a "used any drug" variable, calculate instead using frequency of use variables for all primary, secondary, or tertiary problem codes in which the coded problem is drugs (e.g., TEDS Codes 01-04) |
||
SABG Table 18 – State Description of Other Drug Use Data Collection
STATE CONFORMANCE TO INTERIM STANDARD |
State Description of Other Drug Use Data Collection (SABG Table 18): States should detail exactly how this information is collected. Where data and methods vary from interim standard, variance should be described.
|
DATA SOURCE |
What is the source of data for SABG Table 18 (select all that apply): □ Client self-report □ Client self-report confirmed by another source→ □ urinalysis, blood test or other biological assay □ collateral source □ Administrative data source □ Other Specify ___________________ |
EPISODE OF CARE |
How is the admission/discharge basis defined for SABG Table 18 (Select one) □ Admission is on the first date of service, prior to which no service has been received for 30 days AND discharge is on the last date of service, subsequent to which no service has been received for 30 days □ Admission is on the first date of service in a Program/Service Delivery Unit and Discharge is on the last date of service in a Program/Service Delivery Unit □ Other Specify ___________________________________________ |
DISCHARGE DATA COLLECTION |
How was discharge data collected for SABG Table 18 (select all that apply) □ Not applicable, data reported on form is collected at time period other than discharge→ Specify: □ In-treatment data ___ days post-admission, OR □ Follow-up data ___ (specify) months Post- □ admission □ discharge □ other ______ □ Discharge data are collected for the census of all (or almost all) clients who were admitted to treatment □ Discharge data are collected for a sample or all clients who were admitted to treatment □ Discharge records are directly collected (or in the case of early dropouts) are created for all (or almost all) clients who were admitted to treatment □ Discharge records are not collected for approximately ___ % of clients who were admitted for treatment |
RECORD LINKING |
Was the admission and discharge data linked for SABG Table 18 (select all that apply): □ Yes, all clients at admission were linked with discharge data using a Unique Client Identifier (UCID) Select type of UCID □ Master Client Index or Master Patient Index, centrally assigned □ Social Security Number (SSN) □ Unique client ID based on fixed client characteristics (such as date of birth, gender, partial SSN, etc.) □ Some other Statewide unique ID □ Provider-entity-specific unique ID □ No, State Management Information System does not utilize UCID that allows comparison of admission and discharge data on a client specific basis (data developed on a cohorts basis) or State relied on other data sources for post admission data □ No, admission and discharge records were matched using probabilistic record matching.
|
IF DATA ARE UNAVAILABLE |
If data are not reported, why is State unable to report (select all that apply): □ Information is not collected at admission □ Information is not collected at discharge □ Information is not collected by the categories requested □ State collects information on the indicator area but utilizes a different measure. |
DATA PLANS IF DATA ARE NOT AVAILABLE |
State must provide time-framed plans for capturing abstinence – drug use status data on all clients if data are not currently available. Plans should also discuss barriers, resource needs and estimates of cost. |
SABG Table 19 – Performance Measure: Change in Social Support of Recovery
(From Admission to Discharge)
Most recent year for which data are available: _____________
Social Support of Recovery – Clients participating in self-help groups (e.g., AA, NA, etc.) (prior 30 days) at admission vs. discharge |
Admission Clients (T1) |
Discharge Clients (T2) |
Number of clients participating in self-help (AA NA meetings attended, etc.) [numerator] |
|
|
Total number of Admission and Discharge clients with non-missing values on self-help activities [denominator] |
|
|
Percent of clients participating in self-help activities |
|
|
SABG Table 19 – State Description of Social Support of Recovery Data Collection
STATE CONFORMANCE TO INTERIM STANDARD |
State Description of Social Support of Recovery Data Collection (SABG Table 19): States should detail exactly how this information is collected. Where data and methods vary from interim standard, variance should be described.
|
DATA SOURCE |
What is the source of data for SABG Table 19 (select all that apply): □ Client self-report □ Client self-report confirmed by another source→ □ collateral source □ Administrative data source □ Other Specify ___________________ |
EPISODE OF CARE |
How is the admission/discharge basis defined for SABG Table 19 (Select one) □ Admission is on the first date of service, prior to which no service has been received for 30 days AND discharge is on the last date of service, subsequent to which no service has been received for 30 days □ Admission is on the first date of service in a Program/Service Delivery Unit and Discharge is on the last date of service in a Program/Service Delivery Unit □ Other Specify ___________________________________________ |
DISCHARGE DATA COLLECTION |
How was discharge data collected for SABG Table 19 (select all that apply) □ Not applicable, data reported on form is collected at time period other than discharge→ Specify: □ In-treatment data ___ days post-admission, OR □ Follow-up data ___ (specify) months Post- □ admission □ discharge □ other ______ □ Discharge data are collected for the census of all (or almost all) clients who were admitted to treatment □ Discharge data are collected for a sample or all clients who were admitted to treatment □ Discharge records are directly collected (or in the case of early dropouts) are created for all (or almost all) clients who were admitted to treatment □ Discharge records are not collected for approximately ___ % of clients who were admitted for treatment |
RECORD LINKING |
Was the admission and discharge data linked for SABG Table 19 (select all that apply): □ Yes, all clients at admission were linked with discharge data using a Unique Client Identifier (UCID) Select type of UCID □ Master Client Index or Master Patient Index, centrally assigned □ Social Security Number (SSN) □ Unique client ID based on fixed client characteristics (such as date of birth, gender, partial SSN, etc.) □ Some other Statewide unique ID □ Provider-entity-specific unique ID □ No, State Management Information System does not utilize UCID that allows comparison of admission and discharge data on a client specific basis (data developed on a cohorts basis) or State relied on other data sources for post admission data □ No, admission and discharge records were matched using probabilistic record matching. |
IF DATA ARE UNAVAILABLE |
If data are not reported, why is State unable to report (select all that apply): □ Information is not collected at admission □ Information is not collected at discharge □ Information is not collected by the categories requested □ State collects information on the indicator area but utilizes a different measure.
|
DATA PLANS IF DATA ARE NOT AVAILABLE |
State must provide time-framed plans for capturing self-help participation status data on all clients if data are not currently available. Plans should also discuss barriers, resource needs and estimates of cost. |
SABG Table 20 – Retention; Length of Stay (in Days) of Clients Completing Treatment
Most recent year for which data are available: _____________
Length of Stay |
|||
Level of Care |
Median |
Interquartile Range |
|
Detoxification (24-hour care) |
|||
1. Hospital Inpatient |
|
|
|
2. Free-Standing Residential |
|
|
|
Rehabilitation/Residential |
|||
3. Hospital Inpatient |
|
|
|
4. Short-term (up to 30 days) |
|
|
|
5. Long-term (over 30 days) |
|
|
|
Ambulatory (Outpatient) |
|||
6. Outpatient |
|
|
|
7. Intensive Outpatient |
|
|
|
8. Detoxification |
|
|
|
Opioid Replacement Therapy |
|||
9. ORT Detox |
|
|
|
10. Opioid Replacement Therapy |
|
|
|
Section V: Performance Indicators and Accomplishments
Tables 21 – 36: Prevention Performance Measures
Tables 21 – 30: Prevention Performance Measures
Introduction:
The National Outcome Measures (NOMs) are a set of domains and measures that the Substance Abuse and Mental Health Services Administration (SAMHSA) uses to accomplish its vision and to meet all of its federal reporting requirements, thus reducing burden and redundancy for grantees.
The NOMs Data Collection and Reporting tables are to be completed as part of the state's annual SABG application. For Tables 21-25 and 27-30, the compliance year is calendar year (CY) 2019 (note that pre-populated NOMs from the National Survey on Drug Use and Health (NSDUH) reflect pooled data from CYs 2018-2019. For substance abuse prevention NOMs Table 26, the compliance year is School Year 2019.
For purposes of this section, unless otherwise noted, the term "state" refers to states, territories, and the one Native American tribe that receive SABG funding.
Tables 21 through 30 - Information
A. Pre-populated Data
CSAP and the states have agreed that the state-level reporting requirement for the NOMs listed in Tables 21-30 will be fulfilled through the use of extant data from sources including the National Survey on Drug Use and Health (NSDUH), the Fatality Analysis Reporting System (FARS) of the National Highway Traffic Safety Administration, the Uniform Crime Report (UCR) of the Federal Bureau of Investigation, and the National Center for Education Statistics (NCES) of the U.S. Department of Education. These pre-populated state-level NOMs will meet most of the state-level NOMs reporting requirements for the prevention portion of the SABG funding. These data will be pre-populated into the data tables by CSAP.
NOMs Domain - Reduced Morbidity Abstinence from Drug Use/Alcohol Use
• Table 21: 30-Day Use
• Table 22: Perception of Risk/Harm of Use
• Table 23: Age of First Use
• Table 24: Perception of Disapproval/Attitudes
NOMs Domain - Employment/Education
• Table 25: Perception of Workplace Policy
• Table 26: Average Daily School Attendance Rate
NOMs Domain - Crime and Criminal Justice
• Table 27: Alcohol-Related Traffic Fatalities
• Table 28: Alcohol- and Drug-Related Arrests
NOMs Domain - Social Connectedness
• Table 29: Family Communications Around Drug and Alcohol Use
NOMs Domain - Retention
• Table 30: Youth Seeing, Reading, Watching, or Listening to a Prevention Message
In this block grant application pre-populated data are automatically provided to fulfill the majority of the reporting requirements.
Territories and Native American tribes for which there are no NSDUH, FARS, UCR, and/or NCES data will not be required to report on those measures, but will be encouraged to provide substitute data in Column D.
B. Supplemental Data
States may also wish to provide additional data related to the NOMs. The data can be included in the block grant appendix. When describing the supplemental data, states should provide any relevant Web addresses (URLs) that provide links to specific state data sources.
D. Instructions for Completing Forms
Column A: Measure - The SAMHSA defined measure for the domain listed.
Column B: Question/Response
• Source Survey Item: For Tables 21-25, 29, and 30, the source is the NSDUH. For Tables 26-28 other "archival" sources are identified. The specific language used for each item is provided.
• Response Option: The range of responses that are provided for the survey item.
• Outcome Reported: The specific responses that are included in the calculation provided for the item.
• Age: The age range for which the responses are provided.
Column C: Pre-populated Data - Pre-populated data are provided; see letter A, Pre-populated data.
Column D: Approved Substitute Data – Grantees for which there are no NSDUH, FARS, UCR and/or NCES data will be able to voluntarily enter data for the items in this column. Substitute data are not allowed for grantees with pre-populated data.
SAGB Table 21 – Primary Substance Abuse Use Disorder Prevention NOMs Domain: Reduced Morbidity – Abstinence from Drug Use/Alcohol Use
Measure: 30-Day Use
A. Measure |
B. Question/Response |
C. Pre-populated Data |
D. Supplemental Data, if any |
1. 30-day Alcohol Use |
Source Survey Item: NSDUH Questionnaire. “Think specifically about the past 30 days, that is, from [DATEFILL] through today. During the past 30 days, on how many days did you drink one or more drinks of an alcoholic beverage?” [Response option: Write in a number between 0 and 30.] Outcome Reported: Percent who reported having used alcohol during the past 30 days. |
|
|
Ages 12–20 – CY 2018-2019 |
|
|
|
Ages 21+ - CY 2018-2019 |
|
|
|
2. 30-day Cigarette Use |
Source Survey Item: NSDUH Questionnaire: “During the past 30 days, that is, since [DATEFILL], on how many days did you smoke part or all of a cigarette?” [Response option: Write in a number between 0 and 30.] Outcome Reported: Percent who reported having smoked a cigarette during the past 30 days. |
|
|
Ages 12–17 - CY 2018-2019 |
|
|
|
Ages 18+ - CY 2018-2019 |
|
|
|
3. 30-day Use of Other Tobacco Products |
Source Survey Item: NSDUH Questionnaire: “During the past 30 days, that is, since [DATEFILL], on how many days did you use [other tobacco products]†?” [Response option: Write in a number between 0 and 30.] Outcome Reported: Percent who reported having used a tobacco product other than cigarettes during the past 30 days, calculated by combining responses to questions about individual tobacco products (cigars, smokeless tobacco, pipe tobacco). |
|
|
Ages 12–17 - CY 2018-2019 |
|
|
|
Ages 18+ - CY 2018-2019 |
|
|
|
4. 30-day Use of Marijuana |
Source Survey Item: NSDUH Questionnaire: “Think specifically about the past 30 days, from [DATEFILL] up to and including today. During the past 30 days, on how many days did you use marijuana or hashish?” [Response option: Write in a number between 0 and 30.] Outcome Reported: Percent who reported having used marijuana or hashish during the past 30 days. |
|
|
Ages 12–17 - CY 2018-2019 |
|
|
|
Ages 18+ - CY 2018-2019 |
|
|
|
5. 30-day Use of Illicit Drugs Other Than Marijuana |
Source Survey Item: NSDUH Questionnaire: “Think specifically about the past 30 days, from [DATEFILL] up to and including today. During the past 30 days, on how many days did you use [any other illegal drug]‡?” Outcome Reported: Percent who reported having used illegal drugs other than marijuana or hashish during the past 30 days, calculated by combining responses to questions about individual drugs (heroin, cocaine, hallucinogens, inhalants, methamphetamine, and misuse of prescription drugs). |
|
|
Ages 12–17 - CY 2018-2019 |
|
|
|
Ages 18+ - CY 2018-2019 |
|
|
† NSDUH asks separate questions for each tobacco product. The number provided combines responses to all questions about tobacco products other than cigarettes.
‡ NSDUH asks separate questions for each illegal drug. The number provided combines responses to all questions about illegal drugs other than marijuana or hashish.
SAGB Table 22 – Primary Substance Abuse Use Disorder Prevention NOMs Domain: Reduced Morbidity – Abstinence from Drug Use/Alcohol Use
Measure: Perception of Risk/Harm of Use
A. Measure |
B. Question/Response |
C. Pre-populated Data |
D. Supplemental Data if any |
1. Perception of Risk from Alcohol |
Source Survey Item: NSDUH Questionnaire: “How much do people risk harming themselves physically and in other ways when they have five or more drinks of an alcoholic beverage once or twice a week?” [Response options: No risk, slight risk, moderate risk, great risk] Outcome Reported: Percent reporting moderate or great risk. |
|
|
Ages 12–20 - CY 2018-2019 |
|
|
|
Ages 21+ - CY 2018-2019 |
|
|
|
2. Perception of Risk from Cigarettes |
Source Survey Item: NSDUH Questionnaire: “How much do people risk harming themselves physically and in other ways when they smoke one or more packs of cigarettes per day?” [Response options: No risk, slight risk, moderate risk, great risk] Outcome Reported: Percent reporting moderate or great risk. |
|
|
Ages 12–17 - CY 2018-2019 |
|
|
|
Ages 18+ - CY 2018-2019 |
|
|
|
3. Perception of Risk from Marijuana |
Source Survey Item: NSDUH Questionnaire: “How much do people risk harming themselves physically and in other ways when they smoke marijuana once or twice a week?” [Response options: No risk, slight risk, moderate risk, great risk] Outcome Reported: Percent reporting moderate or great risk. |
|
|
Ages 12–17 - CY 2018-2019 |
|
|
|
Ages 18+ - CY 2018-2019 |
|
|
SAGB Table 23 – Primary Substance Abuse Use Disorder Prevention NOMs Domain: Reduced Morbidity – Abstinence from Drug Use/Alcohol Use
Measure: Age of First Use
A. Measure |
B. Question/Response |
C. Pre-populated Data |
D. Supplemental Data if any |
1. Age at First Use of Alcohol |
Source Survey Item: NSDUH Questionnaire: “Think about the first time you had a drink of an alcoholic beverage. How old were you the first time you had a drink of an alcoholic beverage? Please do not include any time when you only had a sip or two from a drink.” [Response option: Write in age at first use.] Outcome Reported: Average age at first use of alcohol. |
|
|
Ages 12–20 - CY 2018-2019 |
|
|
|
Ages 21+ - CY 2018-2019 |
|
|
|
2. Age at First Use of Cigarettes |
Source Survey Item: NSDUH Questionnaire: “How old were you the first time you smoked part or all of a cigarette?” [Response option: Write in age at first use.] Outcome Reported: Average age at first use of cigarettes. |
|
|
Ages 12–17 - CY 2018-2019 |
|
|
|
Ages 18+ - CY 2018-2019 |
|
|
|
3. Age at First Use of Tobacco Products Other Than Cigarettes |
Source Survey Item: NSDUH Questionnaire: “How old were you the first time you used [any other tobacco product]†?” [Response option: Write in age at first use.] Outcome Reported: Average age at first use of tobacco products other than cigarettes. |
|
|
Ages 12–17 - CY 2018-2019 |
|
|
|
Ages 18+ CY 2018-2019 |
|
|
|
4. Age at First Use of Marijuana or Hashish |
Source Survey Item: NSDUH Questionnaire: “How old were you the first time you used marijuana or hashish?” [Response option: Write in age at first use.] Outcome Reported: Average age at first use of marijuana or hashish. |
|
|
Ages 12–17 - CY 2018-2019 |
|
|
|
Ages 18+ - CY 2018-2019 |
|
|
|
5. Age at First Use of Heroin |
Source Survey Item: NSDUH Questionnaire: “How old were you the first time you used heroin?” [Response option: Write in age at first use.] Outcome Reported: Average age at first use of heroin. |
|
|
Ages 12–17 - CY 2018-2019 |
|
|
|
Ages 18+ - CY 2018-2019 |
|
|
|
6. Age at First Misuse of Prescription Pain Relievers Among Past Year Initiates |
Source Survey Item: NSDUH Questionnaire: “How old were you the first time you used [specific pain reliever]‡ in a way a doctor did not direct you to use it?” [Response option: Write in age at first use.] Outcome Reported: Average age at first misuse of prescription pain relievers among those who first misused prescription pain relievers in the last 12 months. |
|
|
Ages 12–17 - CY 2018-2019 |
|
|
|
Ages 18+ - CY 2018-2019 |
|
|
† The question was asked about each tobacco product separately, and the youngest age at first use was taken as the measure.
‡ The question was asked about each drug in this category separately, and the youngest age at first use was taken as the measure.
SABG Table 24 – Primary Substance Abuse Use Disorder Prevention NOMs Domain: Reduced Morbidity – Abstinence from Drug Use/Alcohol Use
Measure: Perception of Disapproval/Attitudes
A. Measure |
B. Question/Response |
C. Pre-populated Data |
D. Supplemental Data if any |
1. Disapproval of Cigarettes |
Source Survey Item: NSDUH Questionnaire: “How do you feel about someone your age smoking one or more packs of cigarettes a day?” [Response options: Neither approve nor disapprove, somewhat disapprove, strongly disapprove] Outcome Reported: Percent somewhat or strongly disapproving. |
|
|
Ages 12–17 - CY 2018-2019 |
|
|
|
2. Perception of Peer Disapproval of Cigarettes |
Source Survey Item: NSDUH Questionnaire: “How do you think your close friends would feel about you smoking one or more packs of cigarettes a day?” [Response options: Neither approve nor disapprove, somewhat disapprove, strongly disapprove] Outcome Reported: Percent reporting that their friends would somewhat or strongly disapprove. |
|
|
Ages 12–17 - CY 2018-2019 |
|
|
|
3. Disapproval of Using Marijuana Experimentally |
Source Survey Item: NSDUH Questionnaire: “How do you feel about someone your age trying marijuana or hashish once or twice?” [Response options: Neither approve nor disapprove, somewhat disapprove, strongly disapprove] Outcome Reported: Percent somewhat or strongly disapproving. |
|
|
Ages 12–17 - CY 2018-2019 |
|
|
|
4. Disapproval of Using Marijuana Regularly |
Source Survey Item: NSDUH Questionnaire: “How do you feel about someone your age using marijuana once a month or more?” [Response options: Neither approve nor disapprove, somewhat disapprove, strongly disapprove] Outcome Reported: Percent somewhat or strongly disapproving. |
|
|
Ages 12–17 - CY 2018-2019 |
|
|
|
5. Disapproval of Alcohol |
Source Survey Item: NSDUH Questionnaire: “How do you feel about someone your age having one or two drinks of an alcoholic beverage nearly every day?” [Response options: Neither approve nor disapprove, somewhat disapprove, strongly disapprove] Outcome Reported: Percent somewhat or strongly disapproving. |
|
|
Ages 12–20 - CY 2018-2019 |
|
|
SAGB Table 25 – Primary Substance Abuse Use Disorder Prevention NOMs Domain: Reduced Morbidity – Abstinence from Drug Use/Alcohol Use
Measure: Perception of Workplace Policy
A. Measure |
B. Question/Response |
C. Pre-populated Data |
D. Supplemental Data, if any |
Perception of Workplace Policy |
Source Survey Item: NSDUH Questionnaire: “Would you be more or less likely to want to work for an employer that tests its employees for drug or alcohol use on a random basis? Would you say more likely, less likely, or would it make no difference to you?” [Response options: More likely, less likely, would make no difference] Outcome Reported: Percent reporting that they would be more likely to work for an employer conducting random drug and alcohol tests. |
|
|
Ages 15–17 - CY 2018-2019 |
|
|
|
Ages 18+ - CY 2018-2019 |
|
|
SAGB Table 26 – Primary Substance Abuse Use Disorder Prevention NOMs Domain: Reduced Morbidity – Abstinence from Drug Use/Alcohol Use
Measure: Average Daily School Attendance Rate
A. Measure |
B. Source |
C. Pre-populated Data |
D. Supplemental Data, if any |
Average Daily School Attendance Rate |
Source: National Center for Education Statistics, Common Core of Data: The National Public Education Finance Survey available for download at http://nces.ed.gov/ccd/stfis.asp Measure calculation: Average daily attendance (NCES defined) divided by total enrollment and multiplied by 100. |
|
|
CY 2019 |
|
|
SAGB Table 27 – Primary Substance Abuse Use Disorder Prevention NOMs Domain: Crime and Criminal Justice
Measure: Alcohol Related Fatalities
A. Measure |
B. Source |
C. Pre-populated Data |
D. Supplemental Data if any |
Alcohol-Related Traffic Fatalities |
Source: National Highway Traffic Safety Administration Fatality Analysis Reporting System Measure calculation: The number of alcohol-related traffic fatalities divided by the total number of traffic fatalities and multiplied by 100. |
|
|
CY 2019 |
|
|
SAGB Table 28 – Primary Substance Abuse Use Disorder Prevention NOMs Domain: Crime and Criminal Justice
Measure: Alcohol and Drug-Related Arrests
A. Measure |
B. Source |
C. Pre-populated Data |
D. Supplemental Data if any |
Alcohol- and Drug-Related Arrests |
Source: Federal Bureau of Investigation Uniform Crime Reports Measure calculation: The number of alcohol- and drug-related arrests divided by the total number of arrests and multiplied by 100. |
|
|
CY 2019 |
|
|
SAGB Table 29 – Primary Substance Abuse Use Disorder Prevention NOMs Domain: Social Connectedness
Measure: Family Communications Around Drug and Alcohol Use
A. Measure |
B. Question/Response |
C. Pre-populated Data |
D. Supplemental Data if any |
1. Family Communications Around Drug and Alcohol Use (Youth) |
Source Survey Item: NSDUH Questionnaire: “Now think about the past 12 months, that is, from [DATEFILL] through today. During the past 12 months, have you talked with at least one of your parents about the dangers of tobacco, alcohol, or drug use? By parents, we mean either your biological parents, adoptive parents, stepparents, or adult guardians, whether or not they live with you.” [Response options: Yes, No] Outcome Reported: Percent reporting having talked with a parent. |
|
|
Ages 12–17 - CY 2018-2019 |
|
|
|
2. Family Communications Around Drug and Alcohol Use (Parents of children aged 12–17) |
Source Survey Item: NSDUH Questionnaire: “During the past 12 months, how many times have you talked with your child about the dangers or problems associated with the use of tobacco, alcohol, or other drugs?”† [Response options: 0 times, 1 to 2 times, a few times, many times] Outcome Reported: Percent of parents reporting that they have talked to their child. |
|
|
Ages 18+ - CY 2018-2019 |
|
|
† NSDUH does not ask this question of all sampled parents. It is a validation question posed to parents of 12- to 17-year-old survey respondents. Therefore, the responses are not representative of the population of parents in a State. The sample sizes are often too small for valid reporting.
SAGB Table 30 – Primary Substance Abuse Use Disorder Prevention NOMs Domain: Retention
Measure: Percentage of Youth Seeing, or Listening to a Prevention Message
Measure |
Question/Response |
Pre-populated Data
|
Supplemental Data if any |
Exposure to Prevention Messages |
Source Survey Item: NSDUH Questionnaire: “During the past 12 months, do you recall [hearing, reading, or watching an advertisement about the prevention of substance use]†?” Outcome Reported: Percent reporting having been exposed to prevention message. |
|
|
Ages 12–17 - CY 2018-2019 |
|
|
† This is a summary of four separate NSDUH questions each asking about a specific type of prevention message delivered within a specific context
SABG Tables 31-35 – Reporting Period
Start and End Dates for Information Reported on SABG Tables 31, 32, 33, 34, and 35.
The following chart is for collecting information on the reporting periods for the data entered in Tables 31-35. Please note that the correct reporting period for Tables 31-34 is the Calendar Year (CY) which coincides with the reporting period for the pre-populated prevention NOMs in Tables 21-30. We understand that some states have reported on the state fiscal year (SFY) or federal fiscal year (FFY) for these tables in past SABG Reports. If your state is unable to report on the calendar year, please indicate in this footnote why you are unable to report on the calendar year and the steps the state intends to take to make calendar year reporting possible in future years. Note that the correct reporting period for Table 35 is the SABG compliance period that coincides with the reporting period for Tables 4, 5a, 5b, 6 and 7.
Rows 1 through 5 each correspond to a single form in the current year application among the following five tables: 31, 32, 33, 34 and 35.
Column A: Enter the reporting period start date.
Column B: Enter the reporting period end date.
The date format to be entered in columns A and B should be month/day/year, as follows.
• Month: enter 2 digits (e.g. January = 01; December = 12)
• Day: enter 2 digits (e.g. 1st of the month = 01; 15th of the month = 15)
• Year: enter all 4 digits (e.g. 2012, 2013)
Reporting Period Start and End Dates for Information Reported on
SABG Tables 31, 32, 33, 34 and 35
Please indicate the reporting period for each of the following NOMS.
Tables |
A. Reporting Period Start Date |
B. Reporting Period End Date |
Individual-Based Programs and Strategies – Number of Persons Served by Age, Gender, Race, and Ethnicity |
mm/dd/yyyy |
mm/dd/yyyy |
Population-Based Programs and Strategies – Number of Persons Served by Age, Gender, Race, and Ethnicity |
mm/dd/yyyy |
mm/dd/yyyy |
Number of Persons Served by Type of Intervention |
mm/dd/yyyy |
mm/dd/yyyy |
Number of Evidence-Based Programs and Strategies by Type of Intervention |
mm/dd/yyyy |
mm/dd/yyyy |
Total Number of Evidence-Based Programs and Total SABG Dollars Spent on Evidence-Based Programs/Strategies |
mm/dd/yyyy |
mm/dd/yyyy |
General Questions Regarding Prevention NOMS Reporting
Question 1: Describe the data collection system you used to collect the NOMs data (e.g., MDS, DbB, KIT Solutions, manual process).
|
Question 2: Describe how your State’s data collection and reporting processes record a participant’s race, specifically for participants who are more than one race.
Indicate whether the State added those participants to the number for each applicable racial category or whether the State added all those participants to the More Than One Race subcategory.
|
SABG Table 31 – Primary Substance Use Disorder Prevention Individual-Based Programs and Strategies – Number of Persons Served by Age, Gender, Race, and Ethnicity
Category |
Total |
A. Age |
|
0–4 |
|
5–11 |
|
12–14 |
|
15–17 |
|
18–20 |
|
21–24 |
|
25–44 |
|
45–64 |
|
65 and Over |
|
Age Not Known |
|
B. Gender |
|
Male |
|
Female |
|
Gender Not Known |
|
|
|
C. Ethnicity |
|
Hispanic or Latino |
|
Not Hispanic or Latino |
|
Ethnicity Unknown |
|
D. Race |
|
White |
|
Black or African American |
|
Native Hawaiian/Other Pacific Islander |
|
Asian |
|
American Indian/Alaska Native |
|
More Than One Race (not OMB required) |
|
Race Not Known or Other (not OMB required) |
|
SABG Table 32 – Primary Substance Use Disorder Prevention Population-Based Programs and Strategies – Number of Persons Served by Age, Gender, Race, and Ethnicity
Category |
Total |
A. Age |
|
0–4 |
|
5–11 |
|
12–14 |
|
15–17 |
|
18–20 |
|
21–24 |
|
25–44 |
|
45–64 |
|
65 and Over |
|
Age Not Known |
|
B. Gender |
|
Male |
|
Female |
|
Gender Not Known |
|
C. Race |
|
White |
|
Black or African American |
|
Native Hawaiian/Other Pacific Islander |
|
Asian |
|
American Indian/Alaska Native |
|
More Than One Race (not OMB required) |
|
Race Not Known or Other (not OMB required) |
|
D. Ethnicity |
|
Hispanic or Latino |
|
Not Hispanic or Latino |
|
Ethnicity unknown |
|
SABG Table 33 (Optional) – Primary Substance Use Disorder Prevention Number of Persons Served by Type of Intervention
Intervention Type |
Number of Persons Served by Individual- or Population-Based Program or Strategy |
|
A. Individual-Based Programs and Strategies |
B. Population-Based Programs and Strategies |
|
1. Universal Direct |
|
|
2. Universal Indirect |
|
|
3. Selective |
|
|
4. Indicated |
|
|
5. Total |
|
|
SABG Table 34 – Primary Substance Use Disorder Prevention
Evidence-Based Programs and Strategies by Type of Intervention
Definition of Evidence-Based Programs and Strategies: The guidance document for the Strategic Prevention Framework State Incentive Grant, Identifying and Selection Evidence-based Interventions, provides the following definition for evidence-based programs:
Inclusion in a Federal List or Registry of evidence-based interventions
Being Reported (with positive effects) in a peer-reviewed journal
Documentation of effectiveness based on the following guidelines:
Guideline 1: The intervention is based on a theory of change that is documented in a clear logic or conceptual model; and
Guideline 2: The intervention is similar in content and structure to interventions that appear in registries and/or the peer-reviewed literature; and
Guideline 3: The intervention is supported by documentation that it has been effectively implemented in the past, and multiple times, in a manner attentive to Identifying and Selecting Evidence-Based Interventions scientific standards of evidence and with results that show a consistent pattern of credible and positive effects; and
Guideline 4: The intervention is reviewed and deemed appropriate by a panel of informed prevention experts that includes: well-qualified prevention researchers who are experienced in evaluating prevention interventions similar to those under review; local prevention practitioners; and key community leaders as appropriate, e.g., officials from law enforcement and education sectors or elders within indigenous cultures.
|
|
|
|
SABG Table 34 – Primary Substance Use Disorder Prevention Number of Evidence-Based Programs and Strategies by Type of Intervention
|
Number of Programs and Strategies by Type of Intervention |
|||||
A. Universal Direct |
B. Universal Indirect |
C. Universal Total |
D. Selective |
E. Indicated |
F. Total |
|
1. Number of Evidence-Based Programs and Strategies Funded |
|
|
|
|
|
|
2. Total number of Programs and Strategies Funded |
|
|
|
|
|
|
3. Percent of Evidence-Based Programs and Strategies |
|
|
|
|
|
|
SABG Table 35 – Total Primary Substance Use Disorder Prevention Number of Evidence Based Programs/Strategies and Total SABG Dollars Spent on Primary Substance Use Disorder Prevention Evidence-Based Programs/Strategies
Total Number of Evidence-Based Programs/Strategies for IOM Category below: |
Total SAPT Block Grant $Dollars Spent on evidence-based Programs/Strategies |
|
Universal Direct |
Total # |
$ |
Universal Indirect |
Total # |
$ |
Selective |
Total # |
$ |
Indicated |
Total # |
$ |
Unspecified |
Total# |
$ |
|
Total EBPs: |
Total Dollars Spent: $ |
1 The term “state” means each of the several states, the District of Columbia and each of the territories of the United States. The term “territories of the United States” means each of the Commonwealth of Puerto Rico, Virgin Islands, American Samoa, Commonwealth of the Northern Marianas Islands, Federated States of Micronesia, Guam, Republic of the Marshall Islands, and the Republic of Palau.
2 The term “substance use disorder” means substance-related and addictive disorders as described in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Arlington, VA, American Psychiatric Association, 2013.
5 In FY 2020 SAMHSA modified the “Level of Care" (LOC)” and "Type of Treatment Service/Setting" to “Medication-Assisted Treatment” and “Medication- Assisted Treatment,” respectively. In prior SABG Reports, the LOC was entitled "Opioid Replacement Therapy" and the Type of Treatment Service/Setting included "Opioid Replacement Therapy," Row 9 and "ORT Outpatient," Row 10. The changes inadvertently created a barrier for data analysis as one-to-one mapping of the data submitted in the FY 2020 Table 10 to the data submitted in prior Reports is not possible. In the current and future SABG Reports, the LOC is "OUD Medication Assisted Treatment" and the Types of Treatment Service/Setting will include "OUD Medication-Assisted Treatment Detoxification," Row 9 and "OUD Medication Assisted Treatment Outpatient," Row 10. OUD Medication-Assisted Treatment Detoxification includes hospital detoxification, residential detoxification, or ambulatory detoxification services/settings AND Opioid Medication-Assisted Treatment. OUD Medication Assisted Treatment Outpatient includes outpatient services/settings AND Opioid Medication-Assisted Treatment. The change was made to better align with language that reflects not all medications used to treat opioid use disorder (OUD) are opioid-based and more importantly convey that medications do not merely substitute one drug for another.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | TABLE 5A – Planned Primary Prevention Targeted Priorities – FY 2014 |
| Author | DHHS |
| File Modified | 0000-00-00 |
| File Created | 2022-01-07 |
© 2026 OMB.report | Privacy Policy