NLRAD ELR SAHO CX Survey - SLT (Revised)
Generic Clearance for the Collection of Qualitative Feedback on Agency Service Delivery - APHIS
NLRAD ELR Survey (Ver 4.1.1)
NLRAD ELR SAHO CX Survey - SLT (Revised)
OMB: 0579-0377
OMB Approved 0579-0377, expires 02/2022
NLRAD Electronic Lab Reporting Project
Questions for State Animal Health Officials
Surveillance Design and Analysis
2150 Centre Ave Bldg B
Fort Collins, CO 80526
USDA APHIS Veterinary Services (VS) is starting a new project to have Laboratories report test findings electronically to satisfy the U.S. National List of Reportable Animal Diseases (NLRAD) system standards. We would like to gather your input about what you would like from this system. To help better this new system please complete this survey by …
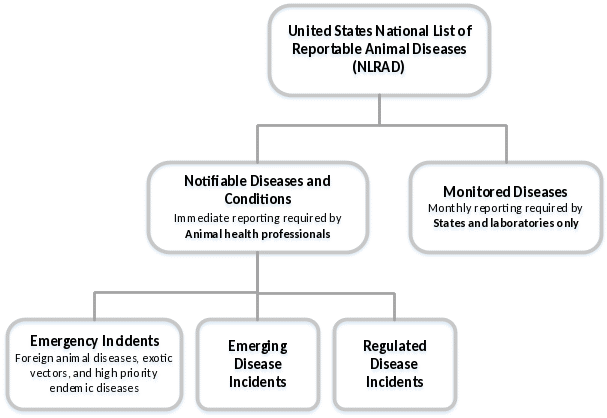
The NLRAD Standards will require information to be collected for two categories: Notifiable Diseases and Conditions and Monitored Diseases (Figure 1). Within the Notifiable Diseases and Conditions category, three sub-categories of diseases have been identified: emergency incidents (such as foreign animal disease investigations, e.g., Rift Valley fever, Virulent Newcastle disease, African swine fever), emerging disease incidents (e.g., SARS-CoV-2/Cov-19 Virus), and regulated disease incidents (e.g., Spring viremia of carp, equine infectious anemia, Pullorum disease). Monitored diseases generally are those that are endemic (present) in the United States (e.g., Bovine viral diarrhea, Tularemia, Enzootic abortion of ewes).
Figure 1. NLRAD disease list structure.
NLRAD - NOTIFIABLE DISEASES
This first section of questions will discuss notifiable diseases. These include emergency incidents, emerging disease incidents, and regulated disease incidents. When answering the questions below please think about your current practices with any laboratories reporting information on notifiable diseases. We understand that each laboratory may have different practices and these practices may be dependent on the disease being reported. Therefore, answer the questions below for any practices done by any laboratories that reports results on any notifiable diseases.
How do you receive results from any laboratory for NOTIFIABLE diseases? Please check all that apply.
Lab electronically messages results directly to a state database or system as an HL7 message
Lab electronically reports results directly to a state database or system as a spreadsheet
Lab manually enters results to a state system and the SAHO downloads or views the report, such as a spreadsheet
Lab calls SAHO’s office with preliminary results
Lab emails preliminary report to SAHO’s office, such as an attached pdf or spreadsheet
Lab calls SAHO’s office with final results
Lab sends final report to SAHO’s office by regular mail
Lab emails final report to SAHO’s office, such as an attached pdf or spreadsheet
Other, Please Specify
In your office, who receives any laboratory results for NOTIFIABLE diseases? Please check all that apply.
Tribal Veterinarian
Assistant Tribal Veterinarian
Field epidemiologists
Office staff
Other, Please Specify
Are there any differences in any laboratory reporting for the three categories of notifiable diseases, emergency incidents (such as foreign animal disease investigations), emerging disease incidents, and regulated disease incidents?
Yes
No
If you answered YES to #3 and you believe there are differences in reporting across labs or across notifiable diseases, please explain in more detail below:
NLRAD - MONITORED DISEASES
This section of questions will focus on monitored diseases. Monitored diseases are generally those that are present in the U.S. and are reported to OIE in 6-month and annual reports. APHIS uses the data it gathers to monitor disease trends. For NLRAD monitored diseases, state veterinarians are required to report disease occurrence (e.g. presence/absence) monthly; however additional information may be requested or provided voluntarily to monitor disease trends and enhance response and control efforts. When answering the questions below please think about your current practices with any laboratories reporting information on monitored diseases. We understand that each laboratory may have different practices and these practices may be dependent on the disease being reported. Therefore, answer the questions below for any practices done by any laboratories that reports results on any monitored diseases.
How do you receive results from any laboratory for MONITORED diseases? Please check all that apply.
Lab electronically messages results directly to a tribal database or system as an HL7 message
Lab electronically reports results directly to a tribal database or system as a spreadsheet
Lab manually enters results to a tribal system and the SAHO downloads or views the report, such as a spreadsheet
Lab calls SAHO’s office with preliminary results
Lab emails preliminary report to SAHO’s office, such as an attached pdf or spreadsheet
Lab calls SAHO’s office with final results
Lab sends final report to SAHO’s office by regular mail
Lab emails final report to SAHO’s office, such as an attached pdf or spreadsheet
Other, Please Specify
In your office, who receives any laboratory results for MONITORED diseases? Please check all that apply.
Tribal Veterinarian
Assistant Tribal Veterinarian
Field epidemiologists
Office staff
Other, Please Specify
Current Reporting
Please provide responses regarding your current reporting system for both notifiable and monitored diseases.
What system(s) do you use now to summarize information about notifiable and monitored diseases that you report to USDA? Please check all that apply.
Tribal-owned instance of CoreOne
Surveillance Collaboration Services (SCS)
USAHERDS
Other, Please Specify
Do you have any challenges in the way lab information is received and transferred into the NAHRS/NLRAD reporting system?
Yes
No
If you answered YES to #8 and you believe there are challenges in the way lab information is received and transferred into the NAHRS/NLRAD reporting system, please explain in more detail below:
Future Reporting
The goal of this project is to improve NLRAD reporting to both tribal veterinary offices and to APHIS. For the next set of questions, please provide responses regarding how you would like to report information in the future.
In the future, what information do you need from the labs, that you cannot get from other sources, to support decision making on NOTIFIABLE diseases? Please check all that apply.
-
Data Field
Data Field
Animal ID
Sample ID assigned by sample collector
Animal species
Sample type (example: nasal swab, lung, etc.)
Animal Location: Premises ID
Date Sample was collected
Animal Location: State
Lab accession number
Animal Location: Zip Code
Sample ID assigned by laboratory
Animal Location: Address
Diagnostic Test: Type
Animal age
Diagnostic Test: Date performed
Clinical signs
Diagnostic Test: Result
Case/herd history
Diagnostic Test: Interpretation
Herd type
Other, please specify
Vaccination history
Owner: Name
Owner: Address
Reason for sample submission
In the future, what information do you need from the labs, that you cannot get from other sources, to support decision making on MONITORED diseases? Please check all that apply.
-
Data Field
Data Field
Animal ID
Sample ID assigned by sample collector
Animal species
Sample type (example: nasal swab, lung, etc.)
Animal Location: Premises ID
Date Sample was collected
Animal Location: State
Lab accession number
Animal Location: Zip Code
Sample ID assigned by laboratory
Animal Location: Address
Diagnostic Test: Type
Animal age
Diagnostic Test: Date performed
Clinical signs
Diagnostic Test: Result
Case/herd history
Diagnostic Test: Interpretation
Herd type
Monthly number of diagnostic tests conducted
Vaccination history
Monthly number of confirmed cases
Owner: Name
Monthly number of susceptible animals
Owner: Address
Other, please specify
Reason for sample submission
For the options listed below, please rank the top 3 ways you would like to see future reporting of NLRAD information to APHIS with the selection placed in the top position being your highest priority
___ |
No change to the current process: have lab send results directly to your office, your office reports NLRAD results to APHIS via current reporting process |
___ |
Lab electronically messages results to APHIS which would be routed to your tribe’s system and your office reports via NAHRS |
___ |
Lab electronically messages results to APHIS, but results would only be available to APHIS after your office has reviewed and approved for release |
___ |
Lab electronically messages results to APHIS, with results accessible to your office and to APHIS simultaneously |
Is there any other option you would prefer for reporting of NLRAD information to APHIS that is not listed above?
In the future, how would you like to be notified that results are available for review? Please check all that apply.
Text
Other, Please Specify
Is there anything else that would help you with report NLRAD information to APHIS?
Yes
No
If you answered YES to #15, and have suggestions on how to improve the reporting of NLRAD information to APHIS, please provide your suggestions below:
If you would like to contact us with any additional questions or input, below are contact names and information |
|
Leah Estberg, DVM, MPVM, PhD VMO Veterinary Informaticist Center for Informatics (CFI) Strategy and Policy USDA APHIS Veterinary Services Mobile: 970-631-6517 Email: [email protected] |
Jane A. Rooney, D.V.M. Veterinary Medical Officer (Epidemiology) NLRAD Coordinator Center for Epidemiology and Animal Health Strategy and Policy USDA APHIS Veterinary Services Ph: 970-494-7397 Mobile: 301-789-3064 Email: [email protected] |
Paperwork Reduction Act Notice
According to the Paperwork Reduction Act of 1995, an agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a valid OMB control number. The valid OMB control number for this information collection is 0579-0377. The time required to complete this information collection is estimated to average 15 minutes per response, including the time to review instructions, search existing data resources, gather the data needed, and complete and review the information collected.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Harris, Beth N - APHIS |
| File Modified | 0000-00-00 |
| File Created | 2022-02-09 |
© 2026 OMB.report | Privacy Policy