NLRAD ELR Lab Requirements Survey - SLT
Generic Clearance for the Collection of Qualitative Feedback on Agency Service Delivery - APHIS
0377 NLRAD ELR Lab Requirements Survey
NLRAD ELR Lab Requirements Survey - SLT
OMB: 0579-0377
OMB Approved 0579-0377, exp. 02/2022
NLRAD Electronic Lab Reporting Project
Questions for Laboratories
Surveillance Design and Analysis
2150 Centre Ave Bldg B
Fort Collins, CO 80526
USDA APHIS Veterinary Services (VS) is starting a new project to have Laboratories report test findings electronically to satisfy the U.S. National List of Reportable Animal Diseases (NLRAD) system standards. We would like to gather your input about what you would like from this system.
The NLRAD Standards will require information to be collected for two categories: Notifiable Diseases and Conditions and Monitored Diseases (Figure 1). Within the Notifiable Diseases and Conditions category, three sub-categories of diseases have been identified: emergency incidents (such as foreign animal disease investigations, e.g., Rift Valley fever, Virulent Newcastle disease, African swine fever), emerging disease incidents (e.g., SARS-CoV-2/Cov-19 Virus), and regulated disease incidents (e.g., Spring viremia of carp, equine infectious anemia, Pullorum disease). Monitored diseases generally are those that are endemic (present) in the United States (e.g., Bovine viral diarrhea, Tularemia, Enzootic abortion of ewes).
Figure 1. NLRAD disease list structure.
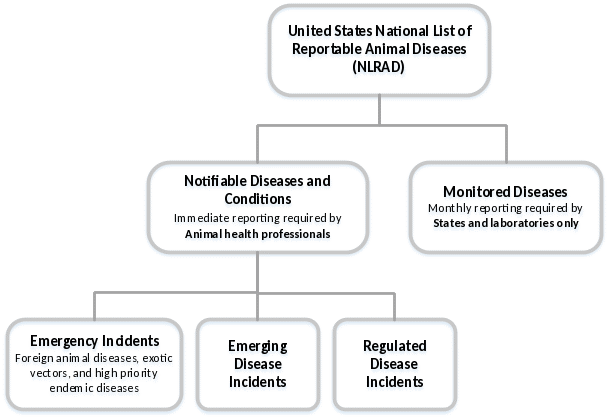
NLRAD - NOTIFIABLE DISEASES
This first section of questions will discuss notifiable diseases. These include emergency, emerging disease, and regulated disease incidents. When answering the questions below please think about your current practices with reporting test findings on notifiable diseases to any State (SAHO), Tribal (TAHO), or Federal Animal Health Official. We understand that your current practices with reporting information on notifiable diseases may be dependent on the disease or preferences of each animal health official. Therefore, answer the questions below for any reporting practice performed across diseases and animal health officials.
How does your lab report results to any SAHO, TAHO, or Federal Animal Health Official for NOTIFIABLE diseases? Please check all that apply.
Lab electronically messages results directly to a state, tribal, or APHIS database or system as an HL7 message
Lab electronically reports results directly to a state, tribal, or APHIS database or system as a spreadsheet
Lab manually enters results to a state, tribal, or APHIS system and the animal health official downloads or views the report, such as a spreadsheet
Lab calls Animal Health Official’s office with preliminary results
Lab emails preliminary report to Animal Health Official’s office, such as an attached pdf or spreadsheet
Lab calls Animal Health Official’s office with final results
Lab sends final report to Animal Health Official’s office by regular mail
Lab emails final report to Animal Health Official’s office, such as an attached pdf or spreadsheet
Other, Please Specify
Are there any differences in laboratory reporting for the three categories of notifiable diseases: emergency incidents (such as foreign animal disease investigations), emerging disease incidents, and regulated disease incidents?
Yes
No
If you answered YES to #2 and you believe there are differences in reporting across notifiable diseases, please explain in more detail below:
NLRAD - MONITORED DISEASES
This section of questions will focus on monitored diseases. Monitored diseases are generally those that are present in the U.S. and are reported to OIE in 6-month and annual reports. APHIS uses the data it gathers to monitor disease trends. For NLRAD monitored diseases, SAHOs are required to report disease occurrence (e.g. presence/absence) monthly; however additional information may be requested or provided voluntarily to monitor disease trends and enhance response and control efforts. When answering the questions below please think about your current practices with reporting results on monitored diseases to SAHOs or AHPIS. We understand that your current practices with reporting results may be dependent on the disease being reported or preferences of the SAHO or APHIS. Therefore, answer the questions below for any reporting practice performed across diseases and SAHOs or APHIS.
How does your lab report results to any SAHO or to APHIS for MONITORED diseases? Please check all that apply.
Lab electronically messages results directly to a state or APHIS database or system as an HL7 message
Lab electronically reports results directly to a state or APHIS database or system as a spreadsheet
Lab manually enters results to a state or APHIS system and the state or APHIS Animal Health Official downloads or views the report, such as a spreadsheet
Lab calls SAHO’s office with preliminary results
Lab emails preliminary report to SAHO’s office, such as an attached pdf or spreadsheet
Lab calls SAHO’s office with final results
Lab sends final report to SAHO’s office by regular mail
Lab emails final report to SAHO’s office, such as an attached pdf or spreadsheet
Other, Please Specify
Current Reporting
Please provide responses regarding your current reporting system for both notifiable and monitored diseases.
What system do you use now to manage the laboratory data (e.g. Laboratory Information Management System (LIMS) or equivalent)?
☐USALIMS
☐VADDS
☐UVIS
☐VetView
☐CoreOne
☐StarLIMS
☐Custom built LIMS
☐Don’t have a LIMS
☐Don’t know
☐Other, Please Specify
Do you have any current challenges with the way lab results are reported and your ability to transfer this data into a state or APHIS system?
Yes
No
If you answered YES to # 6 and you believe there are challenges with the way lab information is reported and your ability to transfer this data into a state or APHIS system, please explain in more detail below:
Future Reporting
The goal of this project is to improve NLRAD test result reporting to APHIS and Animal Health Official’s IT systems. For the next set of questions, please provide responses regarding how you would like to report information in the future.
In the future, what information could your lab potentially supply by way of HL7 messaging, transfer of spreadsheets, or manual data entry to support decision making on NOTIFIABLE diseases? Please check all that apply.
-
Data Field
Data Field
Animal ID
Sample ID assigned by sample collector
Animal species
Sample type (example: nasal swab, lung, etc.)
Animal Location: Premises ID
Date Sample was collected
Animal Location: State
Lab accession number
Animal Location: Zip Code
Sample ID assigned by laboratory
Animal Location: Address
Diagnostic Test: Type
Animal age
Diagnostic Test: Date performed
Clinical signs
Diagnostic Test: Result
Case/herd history
Diagnostic Test: Interpretation
Herd type
Other, please specify
Vaccination history
Owner: Name
Owner: Address
Reason for sample submission
In the future, what information could your lab potentially supply by way of HL7 messaging, transfer of spreadsheets, or manual data entry to support decision making on MONITORED diseases? Please check all that apply.
|
Data Field |
|
|
Data Field |
Animal ID |
|
Sample ID assigned by sample collector |
||
Animal species |
|
Sample type (example: nasal swab, lung, etc.) |
||
Animal Location: Premises ID |
|
Date Sample was collected |
||
Animal Location: State |
|
Lab accession number |
||
Animal Location: Zip Code |
|
Sample ID assigned by laboratory |
||
Animal Location: Address |
|
Diagnostic Test: Type |
||
Animal age |
|
Diagnostic Test: Date performed |
||
Clinical signs |
|
Diagnostic Test: Result |
||
Case/herd history |
|
Diagnostic Test: Interpretation |
||
Herd type |
|
Monthly number of diagnostic tests conducted |
||
Vaccination history |
|
Monthly number of confirmed cases |
||
Owner: Name |
|
Monthly number of susceptible animals |
||
Owner: Address |
|
Other, please specify |
||
Reason for sample submission |
|
|
|
For the options listed below, please rank the top 3 ways you would like to see future reporting of NLRAD lab results with the selection placed in the top position being your highest priority
___ |
No change to the current process: continue to have your lab send results to State and Federal Animal Health Officials |
___ |
Provide results on a spreadsheet to APHIS which will then then route results to the state system or make them available to State and Federal Officials based on their preference |
___ |
Manually enter results into an APHIS system which will then route results to the state system or make them available to State and Federal Officials based on their preference |
___ |
Have your lab electronically message (i.e. HL7) results to APHIS which will then route results to the state system or make them available to State and Federal Officials based on their preference |
Is there any other option you would prefer for reporting of NLRAD lab results that is not listed above?
Is there anything else that would help you with reporting NLRAD lab results?
Yes
No
If you answered YES to #12, and have suggestions on how to improve the reporting of NLRAD results, please provide your suggestions below:
If you would like to contact us with any additional questions or input below are contact names and information
Leah Estberg, DVM, MPVM, PhD VMO Veterinary Informaticist Center for Informatics (CFI) Strategy and Policy USDA APHIS Veterinary Services Mobile: 970-631-6517 Email: [email protected] |
Jane A. Rooney, D.V.M. Veterinary Medical Officer (Epidemiology) NLRAD Coordinator Center for Epidemiology and Animal Health Strategy and Policy USDA APHIS Veterinary Services Ph: 970-494-7397 Mobile: 301-789-3064 Email: [email protected] |
According to the Paperwork Reduction Act of 1995, an agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a valid OMB control number. The valid OMB control number for this information collection is 0579-0377. The time required to complete this information collection is estimated to average 15 minutes per response, including the time to review instructions, search existing data resources, gather the data needed, and complete and review the information collected.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Harris, Beth N - APHIS |
| File Modified | 0000-00-00 |
| File Created | 2022-02-09 |
© 2026 OMB.report | Privacy Policy