One-on-One Interviews: Tradeoff Analysis of Risk, Benefit, and Adherence Claims in Direct to Consumer and HCP Promotion (CDER)
Data to Support Drug Product Communications
Appendix E - Moderator Guides
One-on-One Interviews: Tradeoff Analysis of Risk, Benefit, and Adherence Claims in Direct to Consumer and HCP Promotion (CDER)
OMB: 0910-0695
OMB Control No. 0910-0695
Expiration date: 3/31/2024
Appendix E:
Consumer
Interview Guide
Overview:
The interviewer will provide background information about the project and get consent to proceed (including consent to audio record and livestream).
|
Introduction & Consent
INTERVIEWER: Hello and thank you for making time to talk with me today.
My name is [ ] and I work for RTI International, a not-for-profit research organization located in Research Triangle Park, North Carolina.
The Food and Drug Administration has contracted with RTI to conduct interviews with patients to better understand what’s important to them when they make decisions about medical treatment.
Informed Consent
Before we begin, I would like to review a few items:
Consent Form. I understand that you reviewed the informed consent form when you scheduled your appointment. Just a few highlights:
Participation. Your participation is voluntary and you can stop participating at any time. If at any time you are uncomfortable with any question, you can choose not to answer.
Privacy. Your name and contact information will not be given to anyone else and no one will contact you about this study after our discussion is over. During the interview we will be using your first name only. Please do not tell us anything about yourself that could be used to identify you like your last name or birthday.
Audio Recording. To make sure that we capture everything people say today, we are making an audio recording of this discussion, which will later be transcribed. We will provide the FDA with a transcript (but no audio recording) of our discussion. Your name and any identifying information about you will not be included nor associated with the project or the report in any way.
Observation. Members of our study team, including staff from FDA, may listen and watch today’s discussion via livestream so they can hear your thoughts directly from you.
Reporting. As part of this study, we will write a report for the FDA summarizing what we learned from these interviews. We will not use your name or any identifying information in the report.
Do you have any questions before we begin?
Do I have your consent to participate? [get a verbal “yes” then continue]
Do you agree to have your interview audio recorded and livestreamed? [get a verbal “yes” then continue; If no, remind participant that it is a condition for participation as outlined in the consent-thank them for their time and end call]
Discussion
Background Information About Condition
First, I’d like to get a better idea about your history with [type 2 diabetes/psoriasis].
Please tell me about how you got diagnosed with [type 2 diabetes/psoriasis]?
When did you get diagnosed?
How long did it take for you to get diagnosed from when you first started experiencing symptoms?
What kind of doctor diagnosed you?
Treatment Decisions & Discussion of Attributes: Diabetes Cohort
What treatment(s) have you tried to treat your type 2 diabetes?
How difficult or easy has it been to follow your healthcare provider’s instructions for your treatment? IF DIFFICULT, what makes it difficult?
Thinking about your current treatment for type 2 diabetes, what characteristics of it matter most to you?
How much input did you have in choosing the treatment? [PROBE TO DETERMINE WHETHER THE DOCTOR TOLD THEM WHAT THE TREATMENT WOULD BE VS. SHARED DECISION MAKING]
Have you thought about switching to a new treatment for your type 2 diabetes? IF YES:
[INTERVIEWER WILL HAVE CHECK LIST OF ATTRIBUTES. FOR ANY ATTRIBUTE NOT MENTIONED BY A PARTICIPANT ASK THE FOLLOWING]
I’d like to talk about some other factors that people may consider to be important in a treatment for type 2 diabetes. How important to you is [ATTRIBUTE] in a treatment? Why?
LIST OF ATTRIBUTES FOR TYPE 2 DIABETES
How it is taken (e.g., swallowed in a pill, by injection, etc.)
How often it should be taken
Effectiveness in reducing HbA1c blood glucose levels
Effectiveness in decreasing blood pressure
Impact on life expectancy
Risks of a hypoglycemic event
Side effect: gastrointestinal discomfort
Side effect: changes in body weight
“Serious adverse events” / “Serious side effects”
Cost
Ranking Attributes: Diabetes Cohort
[INTERVIEWER TO SHOW ATTRIBUTE LIST WITH AN “OTHER” CATEGORY ON SCREEN]
Thinking about the different factors that we discussed, which factor is most important to you in a treatment for your type 2 diabetes? Why?
Which factor is the second most important to you in a treatment for your type 2 diabetes?
Which factor is the third most important in a treatment for your type 2 diabetes?
Which factor is the least important to you in a treatment for your type 2 diabetes? Why?
Treatment Decisions & Discussion of Attributes: Psoriasis Cohort
What treatment(s) have you tried to treat your psoriasis?
How difficult or easy has it been to follow your healthcare provider’s instructions for your treatment? IF DIFFICULT, what makes it difficult?
Thinking about your current treatment for psoriasis, what characteristics of it matter most to you?
How much input did you have in choosing the treatment? [PROBE TO DETERMINE WHETHER THE DOCTOR TOLD THEM WHAT THE TREATMENT WOULD BE VS. SHARED DECISION MAKING]
[INTERVIEWER WILL HAVE CHECK LIST OF ATTRIBUTES. FOR ANY ATTRIBUTE NOT MENTIONED BY A PARTICIPANT ASK THE FOLLOWING]
I’d like to talk about some other factors that people may consider to be important in a treatment for psoriasis. How important to you is [ATTRIBUTE] in a treatment? Why?
LIST OF ATTRIBUTES FOR PSORIASIS
How it is taken (e.g., cream or ointment, swallowed by pill, by injection, etc.)
How often it should be taken
Where you take the treatment, such as at home or at a doctor’s office
Effectiveness in achieving skin clearance
Risk of infection
Side effect: skin cancer
Side effect: liver damage
“Serious adverse events” / “Serious side effects”
Cost
Ranking Attributes: Psoriasis Cohort
[INTERVIEWER TO SHOW ATTRIBUTE LIST WITH AN “OTHER” CATEGORY ON SCREEN]
Thinking about the different factors that we discussed, which factor is most important to you in a treatment for your psoriasis? Why?
Which factor is the second most important to you in a treatment for your psoriasis?
Which factor is the third most important in a treatment for your psoriasis?
Which factor is the least important to you in a treatment for your psoriasis? Why?
Condition-Specific Statements About Attributes: Diabetes Cohort
Efficacy Options: Type 2 Diabetes
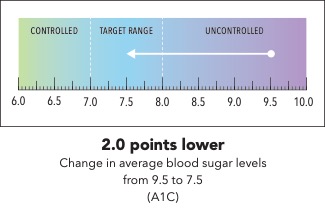
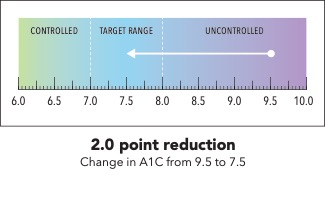
Next, I’m going to ask you to take a minute and look at the graphic I’m going to show on my screen [SHOW IMAGE A].
In your own words, how would you explain the information in the graphic to a friend or family member?
Is there anything confusing or unclear about the information? If so, what? How could it be made clearer?
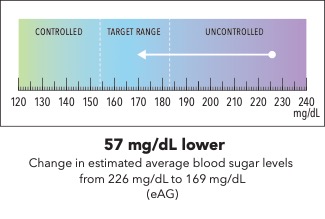
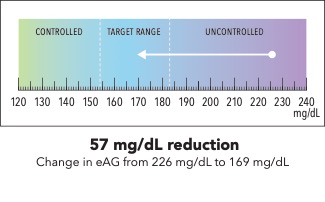
Now I’m going to show you a slightly different graphic. [SHOW IMAGE B ON SCREEN]. This version shows changes in average blood sugar levels in milligrams per deciliter (mg/dL).
How would you explain the information in this graphic to a friend or family member?
Is there anything confusing or unclear about the information? If so, what? How could it be made clearer?
[SHOW IMAGE A and B ON SCREEN].
Which version do you prefer? Why?
IMAGE A
|
IMAGE B
|
Delivery Method and Dose Frequency: Diabetes Cohort
[FOR THIS SECTION: REMIND PARTICIPANTS AS NEEDED THAT WE ARE REFERRING TO TYPE 2 DIABETES]
Route of Administration
Next, I’m going to show you some descriptions of how a treatment may be taken [SHOW SCREEN].
How the treatment is taken
|
Are you familiar with the term “injection with a syringe” as a method for how a treatment is taken? What about “injector pen”?
What, if anything, is the difference between these methods?
Do you think these methods should be listed as two separate categories, or do you think they should be listed as “injection”? Why do you say that?
Do you have any suggestions for other terms for any of these ways that a treatment can be taken that would be easier to understand? If so, what would you suggest?
Are there any other ways that a treatment should be taken that should be added?
Dose Frequency
Now I’m going to show you a list of instructions about how often a treatment should be taken [SHOW SCREEN].
How often the treatment is taken
|
Is there anything confusing or unclear about these categories? If so, what?
Condition Specific Attributes: Psoriasis Cohort
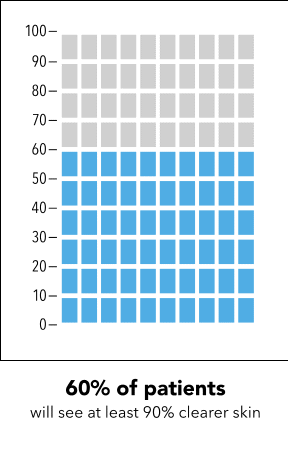
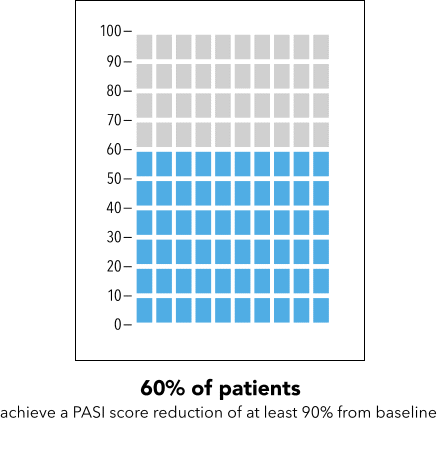
Next, I’m going to ask you to take a minute and look at the graphic I’m going to show on my screen [SHOW IMAGE A].
In your own words, how would you explain the information in the graphic to a friend or family member?
Is there anything confusing or unclear about the information? If so, what? How could it be made clearer?
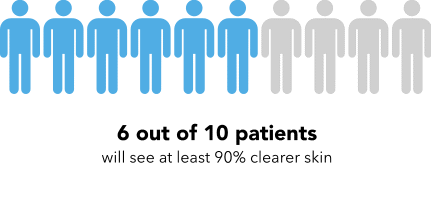
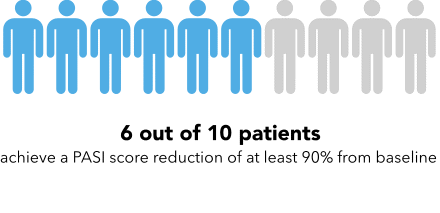
Now I’m going to show you a slightly different graphic. [SHOW IMAGE B ON SCREEN]. This version shows the same information but presented in a slightly different way.
How would you explain the information in this graphic to a friend or family member?
Is there anything confusing or unclear about the information? If so, what? How could it be made clearer?
[SHOW IMAGE A and B ON SCREEN].
Which version do you prefer? Why?
IMAGE A
|
IMAGE B
|
Delivery Method, Dose Frequency, and Dose Setting: Psoriasis Cohort
[FOR THIS SECTION: REMIND PARTICIPANTS AS NEEDED THAT WE ARE REFERRING TO PSORIASIS]
Route of Administration
Next, I’m going to show you some descriptions of how a treatment may be taken [SHOW SCREEN].
How the treatment is taken
|
Are you familiar with the term “injection by needle under the skin” as a method for how a treatment is taken? What about “injector pen”? “Through a needle placed in a vein in your arm”?
What, if anything, is the difference between these methods?
Do you think these methods should be listed as three separate categories, or do you think they should be combined into one? Why do you say that?
Are there other terms you would use for anything on this list to make it easier to understand how to take the treatment? If so, what would you suggest?
Dose Frequency
Now I’m going to show you a list of instructions about how often a treatment should be taken [SHOW SCREEN].
How often the treatment is taken
|
Is there anything confusing or unclear about these categories? If so, what?
Are there other dose frequencies that should be included in this list? If so, what?
Dose Setting
Next, I’m going to show you a list of places where a treatment may be taken [SHOW SCREEN].
Where the treatment is taken
|
Is the wording of any of these options confusing or unclear? If so, what changes would you make?
Are there other options for where a treatment can be taken that should be added? Are there any that should be removed?
Market Claims & Other Statements [Market claims listed here are placeholders]
Market Claim 1: #1 prescribed
SHOW ON SCREEN: “Drug A is the #1 prescribed brand”
As used in this context, what does “#1 prescribed” mean to you?
What do you think about drug A when you hear it is the “#1 prescribed” brand?
Does hearing that the drug is the “#1 prescribed” brand make you think it is safer or less safe than other drugs? More or less effective than other drugs? Why?
Market Claim 2: New (as in new product)
SHOW ON SCREEN: “Drug B is new”
What do you think of a drug that is described as “new”?
Does hearing that the drug is “new” make you think it is safer or less safe than other drugs? More or less effective than other drugs? Why?
Market Claim 3: First and only
SHOW ON SCREEN: “Drug C is the first and only drug to...”
As used in this context, what does the phrase “first and only” mean to you?
What do you think about drug C when you hear that it is the “first and only” drug to…?
Does hearing the drug is the “first and only drug to…” make you think is better or more effective than other drugs? Why?
Specific vs. Nonspecific Side Effect
SHOW ON SCREEN: “30 out of 100 people who use this drug experience a side effect.”
What do you think of when you read this statement? [IF NEEDED: What kind of side effect(s) are you thinking about?]
SHOW ON SCREEN: Statement A: “30 out of 100 people who use this drug experience a side effect.”
Statement B: “30 out of 100 people who use this drug experience gastrointestinal discomfort.”
If you were interested in learning about a new drug, which statement would be most helpful to you? Why?
Conclusion
These are all of my questions. Is there anything else you would like to share before we wrap up? Thank you so much for your time today. Your input has been very helpful.
You will receive your incentive through L&E within 2-3 business days.
Appendix F:
Physician
Interview Guide
Overview:
The interviewer will provide background information about the project and get consent to proceed (including consent to audio record and livestream).
|
Introduction & Consent
INTERVIEWER: Hello and thank you for making time to talk with me today.
My name is [ ] and I work for RTI International, a not-for-profit research organization located in Research Triangle Park, North Carolina.
The Food and Drug Administration has contracted with RTI to conduct interviews with physicians to better understand how treatment decisions are made.
Informed Consent
Before we begin, I would like to review a few items:
Consent Form. I understand that you reviewed the informed consent form when you scheduled your appointment. Just a few highlights:
Participation. Your participation is voluntary and you can stop participating at any time. If at any time you are uncomfortable with any question, you can choose not to answer.
Privacy. Your name and contact information will not be given to anyone else and no one will contact you about this study after our discussion is over. During the interview we will be using your first name only. Please do not tell us anything about yourself that could be used to identify you or your medical practice.
Audio Recording. To make sure that we capture everything people say today, we are making an audio recording of this discussion, which will later be transcribed. We will provide the FDA with a transcript (but no audio recording) of our discussion. Your name and any identifying information about you will not be included nor associated with the project or the report in any way.
Observation. Members of our study team, including staff from FDA, may listen and watch today’s discussion via livestream so they can hear your thoughts directly from you.
Reporting. As part of this study, we will write a report for the FDA summarizing what we learned from these interviews. We will not use your name or any identifying information in the report.
Do you have any questions before we begin?
Do I have your consent to participate? [get a verbal “yes” then continue]
Do you agree to have your interview audio-recorded and livestreamed? [get a verbal “yes” then continue; If no, remind participant that it is a condition for participation as outlined in the consent-thank them for their time and end call]
Discussion
Background on Medical Practice
First, I’d like to get some background information on your medical practice.
Can you tell me a little bit about your practice?
Probe as needed on setting—e.g., solo, group, academic center, etc.
What are the most common medical problems that you treat?
Treatment Decisions & Discussion of Attributes: Diabetes Cohort (main condition)
Next, I’d like to talk about how you make treatment decisions for your patients who have type 2 diabetes.
What are your main considerations in recommending a treatment for a patient who has type 2 diabetes?
Does your recommendation differ depending on:
Time since diagnosis? If so, how?
The patient’s prior experience with prescription drugs? If so, how?
The patient’s age? If so, how?
The patient’s likelihood of adherence with the treatment? If so, how?
The patient’s preference for one treatment over another?
[FOR ANY ATTRIBUTE NOT MENTIONED BY A PARTICIPANT ASK THE FOLLOWING]
I’d like to talk about some other factors that healthcare providers may consider when recommending a specific treatment for a patient. How important is [ATTRIBUTE] to you when choosing which treatment to recommend? Why?
LIST OF ATTRIBUTES FOR TYPE 2 DIABETES
How it is administered (e.g., swallowed in a pill, by injection, etc.)
How frequently it needs to be administered
Effectiveness in reducing HbA1c blood glucose levels
Effectiveness in decreasing blood pressure
Impact on life expectancy
Risks of a hypoglycemic event
Side effect: gastrointestinal discomfort
Side effect: changes in body weight
“Serious adverse events” / “Serious side effects”
Cost
Ranking Attributes: Diabetes Cohort (main condition)
[INTERVIEWER TO SHOW ATTRIBUTE LIST WITH AN “OTHER” CATEGORY ON SCREEN]
Thinking about the different factors that we discussed, which factor is most important to you when choosing a treatment to recommend? Why?
Which factor is the second most important to you when choosing which treatment to recommend?
Which factor is the third most important consideration when choosing a treatment to recommend?
Which factor is the least important to you when choosing a treatment to recommend? Why?
Treatment Decisions & Discussion of Attributes: Psoriasis Cohort (main condition)
Next, I’d like to talk about how you make treatment decisions for your patients who have psoriasis.
What are your main considerations in recommending a treatment for a patient who has psoriasis?
Does your recommendation differ depending on:
Time since diagnosis? If so, how?
The patient’s prior experience with prescription drugs? If so, how?
The patient’s age? If so, how?
The patient’s likelihood of adherence with the treatment? If so, how?
The patient’s preference for one treatment over another?
[FOR ANY ATTRIBUTE NOT MENTIONED BY A PARTICIPANT ASK THE FOLLOWING]
I’d like to talk about some other factors that healthcare providers may consider when recommending a specific treatment for a patient. How important is [ATTRIBUTE] to you when choosing which treatment to recommend? Why?
LIST OF ATTRIBUTES FOR PSORIASIS
How it is administered (e.g., topical, tablet, injection/intravenous infusion, etc.)
How frequently it needs to be administered
Dose setting (e.g., where the treatment is administered, such as at the patient’s home or at a doctor’s office)
Effectiveness in achieving skin clearance
Risk of infection
Side effect: skin cancer
Side effect: liver damage
“Serious adverse events” / “Serious side effects”
Cost
Ranking Attributes: Psoriasis Cohort (main condition)
[INTERVIEWER TO SHOW ATTRIBUTE LIST WITH AN “OTHER” CATEGORY ON SCREEN]
Thinking about the different factors that we discussed, which factor is most important to you when choosing a treatment to recommend? Why?
Which factor is the second most important to you when choosing which treatment to recommend?
Which factor is the third most important consideration when choosing a treatment to recommend?
Which factor is the least important to you when choosing a treatment to recommend? Why?
Treatment Decisions & Discussion of Attributes: Psoriasis Cohort (type 2 diabetes secondary condition)
(Only for HCPs who treat patients for both medical conditions who focused on psoriasis for their main condition)
I’d like to switch now to another condition, type 2 diabetes.
What are your main considerations in recommending a treatment for a patient who has type 2 diabetes?
[IF MORE THAN ONE] Which factor is most important in your decision on which treatment to recommend?
How does your decision making differ when recommending a treatment for type 2 diabetes vs. psoriasis?
Treatment Decisions & Discussion of Attributes: Diabetes Cohort (psoriasis secondary condition)
(Only for HCPs who treat patients for both medical conditions who focused on type 2 diabetes for their main condition)
What are your main considerations in recommending a treatment for a patient who has psoriasis?
[IF MORE THAN ONE] Which factor is most important in your decision on which treatment to recommend?
How does your decision making differ when recommending a treatment for psoriasis vs. type 2 diabetes?
Condition-Specific Statements About Attributes: Diabetes Cohort
(Only for HCPs who focused on type 2 diabetes for their main condition)
Efficacy Options: Type 2 Diabetes
Next, I’m going to ask you to take a minute and look at the graphic I’m going to show on my screen [SHOW IMAGE A].
Is there anything confusing or unclear about the information? If so, what? How could it be made clearer?
Are there any changes that you would make to this graphic? If so what?
Now I’m going to show you a slightly different graphic. [SHOW IMAGE B ON SCREEN]. This version shows changes in average blood sugar levels in milligrams per deciliter (mg/dL).
Is there anything confusing or unclear about the information? If so, what? How could it be made clearer?
Are there any changes that you would make to this graphic? If so, what?
[SHOW IMAGE A and B ON SCREEN].
Which version do you prefer? Why?
IMAGE A
|
IMAGE B
|
Delivery Method and Dose Frequency: Diabetes Cohort (main condition)
[FOR THIS SECTION: REMIND PARTICIPANTS AS NEEDED THAT WE ARE REFERRING TO TYPE 2 DIABETES]
Route of Administration
Next, I’m going to show you some descriptions of routes of administration [SHOW SCREEN].
Route of administration
|
Do you think “subcutaneous injection with a syringe” and “subcutaneous injection with a pre-filled pen” should be listed separately or combined into one category: “subcutaneous injection”? Why do you say that?
Do you have any suggestions for other terms for any of these routes of administration that would be easier to understand? If so, what would you suggest?
Are there any other routes of administration that should be added?
Dose Frequency
Now I’m going to show you some categories of dose frequencies [SHOW SCREEN].
Dose frequency
|
Is there anything confusing or unclear about these categories? If so, what?
Are there other dose frequencies that should be included in this list? If so, what?
Condition-Specific Statements About Attributes: Psoriasis Cohort (main condition)
(Only for HCPs who focused on psoriasis for their main condition)
Next, I’m going to ask you to take a minute and look at the graphic I’m going to show on my screen [SHOW IMAGE A].
Is there anything confusing or unclear about the information? If so, what? How could it be made clearer?
Are there any changes that you would make to this graphic? If so, what?
Now I’m going to show you a slightly different graphic. [SHOW IMAGE B ON SCREEN]. This version shows the same information but presented in a slightly different way.
Is there anything confusing or unclear about the information? If so, what? How could it be made clearer?
Are there any changes that you would make to this graphic? If so, what?
[SHOW IMAGE A and B ON SCREEN].
Which version do you prefer? Why?
IMAGE A
|
IMAGE B
|
Delivery Method, Dose Frequency, and Dose Setting: Psoriasis Cohort (main condition)
[FOR THIS SECTION: REMIND PARTICIPANTS AS NEEDED THAT WE ARE REFERRING TO PSORIASIS]
Route of Administration
Next, I’m going to show you some descriptions of routes of administration [SHOW SCREEN].
Route of administration
|
Do you think “subcutaneous injection with a syringe” and “subcutaneous injection with a pre-filled pen” should be listed separately or combined into one category: “subcutaneous injection”? Why do you say that?
Do you have any suggestions for other terms for any of these routes of administration that would be easier to understand? If so, what would you suggest?
Are there any other routes of administration that should be added?
Dose Frequency
Now I’m going to show you some categories of dose frequencies [SHOW SCREEN].
Dose Frequency
|
Is there anything confusing or unclear about these categories? If so, what?
Are there other dose frequencies that should be included in this list? If so, what?
Dose Setting
Next, I’m going to show you some categories of dose settings [SHOW SCREEN].
Dose setting
|
Is the wording of any of these options confusing or unclear? If so, what changes would you make?
Are there other options for dose setting that should be added? Are there any that should be removed?
Market Claims [Market claims listed here are placeholders]
Market Claim 1: #1 prescribed
SHOW ON SCREEN: “Drug A is the #1 prescribed brand”
As used in this context, what does “#1 prescribed” mean to you?
What do you think about drug A when you hear it is the “#1 prescribed” brand?
Does hearing that the drug is the “#1 prescribed” brand make you think it is safer or less safe than other drugs? More or less effective than other drugs? Why?
Market Claim 2: New (as in new product)
SHOW ON SCREEN: “Drug B is new”
What do you think of a drug that is described as “new”?
Does hearing that the drug is “new” make you think it is safer or less safe than other drugs? More or less effective than other drugs? Why?
Market Claim 3: First and only
SHOW ON SCREEN: “Drug C is the first and only drug to...”
As used in this context, what does the phrase “first and only” mean to you?
What do you think about drug C when you hear that it is the “first and only” drug to…?
Does hearing the drug is the “first and only drug to…” make you think is better or more effective than other drugs? Why?
Conclusion
These are all of my questions. Is there anything else you would like to share before we wrap up? Thank you so much for your time today. Your input has been very helpful.
You will receive your incentive through L&E within 2-3 business days.
E-
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Squire, Claudia |
| File Modified | 0000-00-00 |
| File Created | 2022-07-01 |
© 2026 OMB.report | Privacy Policy