Recruitment Letter
PrtApndxA2_HHRecruitmentLetter_20220609.docx
Per- or Polyfluoroalkyl Substances Exposure Assessments (PFAS EAs)
Recruitment Letter
OMB: 0923-0059
Appendix A2: Household Recruitment Letter
PFAS Exposure Assessment, biological sampling
Household Recruitment Letter
Reading Level: 9.9
Dear [Insert Name],
Your household is invited to be a part of an assessment that will measure the levels of per- and polyfluoroalkyl substances (PFAS) in people living in your community. PFAS are a large group of man-made chemicals that have been used in industry and consumer products worldwide since the 1950s. The Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry (CDC/ATSDR) is trying to determine the level of PFAS in the bodies of people who may have consumed contaminated drinking water while living near the [Insert name of city/town/place here]. This exposure assessment is required by Congress to better understand potential exposures to PFAS near current or former military bases.
CDC/ATSDR will conduct this exposure assessment from [insert dates here]. This letter explains the procedures, risks, and benefits of our exposure assessment to help you decide if you would like to participate.
Please be assured that ATSDR will take all necessary steps to protect members of your community from COVID-19. The exposure assessment will be conducted following all state, local, and CDC guidelines in place at the time the exposure assessment is conducted. ATSDR team members will be monitored twice daily for fever and any COVID-19-related symptoms and will wear surgical masks and gloves to ensure the protection of participants. Similarly, participants will be monitored for fever and COVID-19-related symptoms prior to their entry into the testing facility. Participants will be asked to always wear a face covering or mask when interacting with exposure assessment personnel. If you do not have a mask, one will be provided to you before you enter the facility. If you are unable to wear a mask for medical reasons, please let us know.
Within the next week, an ATSDR representative will call you to discuss the exposure assessment, answer any questions you have, and sign you up if you decide to take part. The call will take about 5 minutes of your time. Only households receiving letters are being asked to participate. You can also contact us at [insert contact information].
Here is what you can expect if you choose to participate in the exposure assessment project:
First, when you arrive for your appointment, we will greet you and check you in and will ask you to sign a form agreeing to participate in the testing. At your appointment, we will schedule a convenient time for you and your family to complete a questionnaire by telephone. The questionnaire will ask you to answer a few questions about yourself, your health, and possible exposure to PFAS through water, food, and your environment. The questionnaire should take less than 30 minutes to complete.
We will also ask you to give us a blood sample and urine sample. A phlebotomist will draw a small amount of your blood for testing. On the morning of your blood sampling appointment, you will collect a sample of your first morning urine in a collection cup we will provide. Your samples will be labeled with a unique code. Only the project coordinator will be able to identify whose blood and urine the sample is from.
Your blood and urine will not be tested for HIV, or for the presence of alcohol or drugs. There will be no charge to you for the sample collection or the laboratory analysis. Your blood sample will be sent to the National Center for Environmental Health (NCEH) laboratory in Atlanta, GA. It will be analyzed for twelve PFAS. Some of the urine samples we collect will be analyzed for PFAS – the rest will be saved and stored for analysis in the future, if you give us permission. We will give you the results if we analyze your urine sample in the future.
As soon as test results are available, we will mail them to you at the address you provided on the consent form. If you would like to talk with a healthcare provider about your results, one working on the exposure assessment will be available to you free of charge.
Your PFAS level results (not including any information that would identify you personally) will also be used by ATSDR to improve the understanding of PFAS exposure.
Research to better understand the health effects associated with PFAS exposure is ongoing, but scientists are not currently certain of how PFAS levels in the blood can affect a person’s health. More research is needed to clarify the risks posed by PFAS exposure. It is possible that new tests will be developed in the future that will increase our understanding of how PFAS impact human health. We would like to keep your blood and urine samples so that scientists can test for more things if new tests are developed. To do this, we will need your permission when you give us your samples. We will provide you with the results of future testing.
The Benefits of Participating in Our Exposure Assessment
Your participation in this assessment will give you information about levels of PFAS in your body and potentially help you reduce your exposure. Your participation will provide a better understanding of the extent of exposure to PFAS within your community and will also help scientists understand the range of PFAS exposure and possible exposure sources in your community. Work to better understand the health effects associated with PFAS exposure is ongoing, but scientists are not currently certain of how PFAS levels in the body can affect a person’s health. More work is needed to clarify the risks posed by PFAS exposure. Your participation in this assessment will help advance this research.
We will not be able to tell you if the PFAS levels in your blood or urine will make you sick now or later in life. You will be able to call project staff during and after the exposure assessment if you have any questions about your results. If your doctor has questions about PFAS, he or she may also call project staff or the physician working on the exposure assessment. The names and phone numbers of people to call are listed below.
The Risks of Participating in Our Exposure Assessment
ATSDR will be taking precautions to minimize the risk associated with COVID-19 transmission for both participants and ATSDR/CDC EA team members.
This exposure assessment requires 6 milliliters of blood (which is about 1 teaspoon). You may feel a sharp sting from the needle used to draw your blood. Sometimes a bruise or small blood clot appears at the site. These bruises or clots usually go away on their own. Putting heat on the site can also help the bruise or clot to go away. Although it is not common, the needle could irritate a nerve. This irritation can cause temporary numbness in part of the arm.
Risk of injury from the blood draw is higher for people with bleeding disorders and for anyone on blood thinning medications (such as Coumadin) and other therapies. If you have a bleeding disorder or are taking blood thinning medication, we recommend that you talk to your doctor before participating in this exposure assessment. Another possible risk from the blood draw is infection, which can develop as a result of the puncture through the skin. Much like they do in other healthcare settings such as doctor’s offices and hospitals, our certified phlebotomists will follow standard precautions to minimize this risk. You or your health insurance company would be responsible for any follow-up care if you are injured as a result of the blood draw.
Additional Information and Privacy Act Statement:
Results: We will send you a letter with your PFAS test results along with how they compare to levels in other people in the United States. We do not yet know enough to say whether there are levels in the blood or urine that are safe or unsafe. This assessment will only tell you how much PFAS are currently in your body. It will not tell you when or for how long you were exposed.
Privacy: All personally identifiable information (such as name, address, date of birth) gathered for the exposure assessment is private. This information is protected to the extent possible by (insert name of state here) and federal laws related to privacy protection. Only trained and authorized project staff will have access to information that can identify you, and we will keep all of the information in a secure, locked database or file at all times. Aside from the exposure assessment team, you are the only one who will receive your individual results. In accordance with CDC/ATSDR’s policy regarding data access, sampling results that do not include PII may be used by public health researchers for approved research purposes.
Voluntary Participation: Participation in this exposure assessment is completely voluntary. Your choice will not affect your current or future relationships with groups that are part of the exposure assessment. Even if you decide to participate, you are free to quit the exposure assessment at any time. If project staff decide it is in your best interest, or if you fail to meet the exposure assessment criteria, you may be removed from the exposure assessment without your consent.
The enclosed fact sheets provide more information about PFAS.
You can expect a call within the next week from [INSERT CALL DETAILS] inviting you to participate in the exposure assessment.
If you would like to indicate your willingness to be a part of this exposure assessment now, please contact us [INSERT PHONE NUMBER and HOURS OF OPERATION]. Remember, only households receiving an invitation letter are eligible to participate.
If you have any additional questions, please feel free to contact [insert contact information].
Thank you,
Enclosures:
PFAS Frequently Asked Questions Fact Sheet
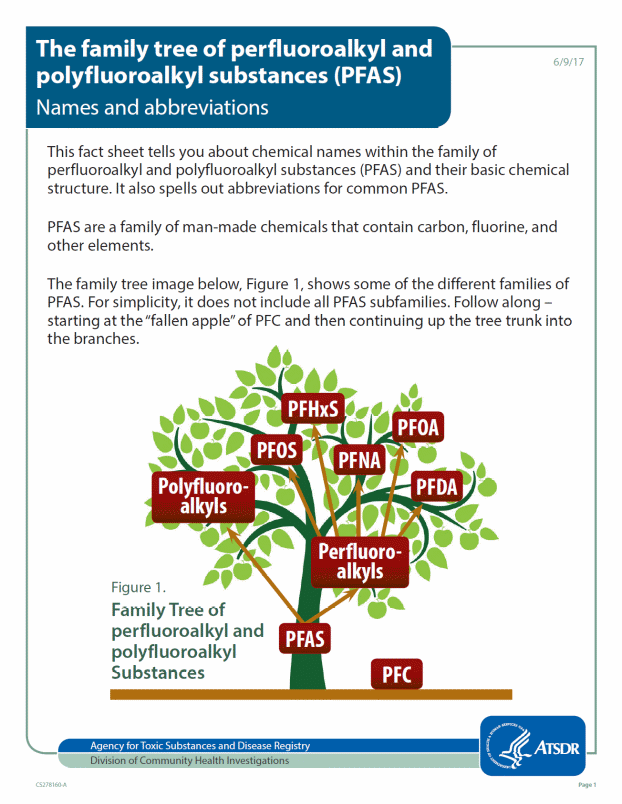
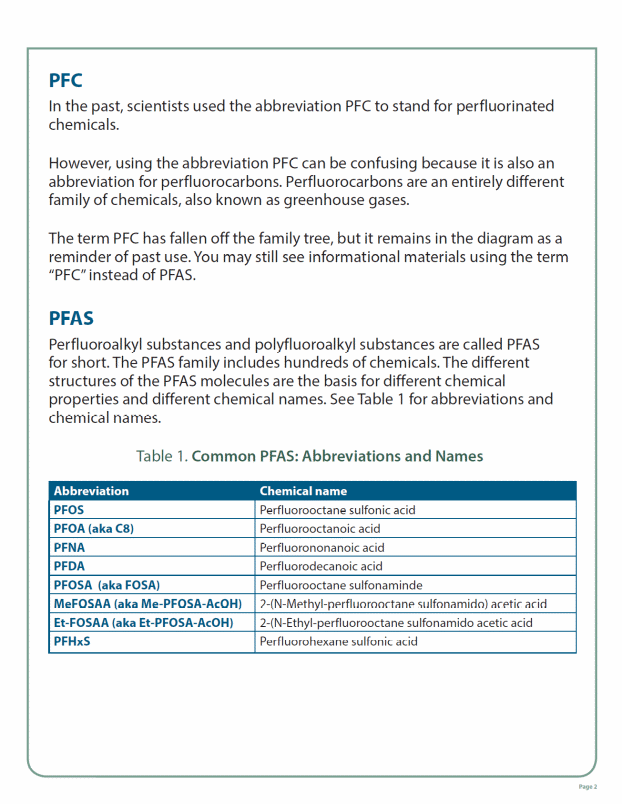
PFAS Family Tree Fact Sheet
PFAS Exposure Assessment Fact Sheet
From: https://www.atsdr.cdc.gov/pfas/additional_resources.html

From: https://www.atsdr.cdc.gov/pfas/additional_resources.html
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Scruton, Karen M. (ATSDR/DCHI/SSB) |
| File Modified | 0000-00-00 |
| File Created | 2022-06-20 |
© 2026 OMB.report | Privacy Policy