COVID-19 Current Events Tracker (CET)
ASPA COVID-19 Public Education Campaign Market Research
ASPA CET Weekly Survey W52 FINAL (2)
COVID-19 Current Events Tracker (CET)
OMB: 0990-0476
ASPA
COVID-19 PUBLIC EDUCATION CAMPAIGN A
campaign to increase vaccine acceptance and reinforce basic
prevention measures


CET – Annotated Questionnaire (Wave 52)
Note: The questions below are the proposed questions for the fifty-second wave of the Weekly Current Events Tracker (CET). Questions highlighted in yellow will be asked every week; questions highlighted in blue will be rotated into the survey on a monthly basis; and questions highlighted in green are meant to be asked in Wave 52 only or are being asked again to update data on a variable of interest. We will be fielding questions about pandemic assessments, vaccine/social distancing/mask mandates, and parents’ vaccination decisions. |
Standard Questions
Modular Questions
One-Time or Repeat Questions Being Asked Again to Update Data
For the next section we would like to talk about current events.
//PROGRAMMING NOTE: RANDOMIZE ORDER OF Q1/Q2
// Page Break //
//BASE: All respondents//
Item #: Q1
Question Type: Single punch
// Soft Prompt: “We would like your response to this question.” //
hhs_trust. How much trust do you have in the U.S. Department of Health and Human Services (HHS) to provide you with accurate information about the coronavirus or COVID-19?
Variable Label: hhs_trust: Trust in HHS
Value |
Value Label |
1 |
None at all |
2 |
Not very much |
3 |
A fair amount |
4 |
A great deal |
99 |
I am not familiar with HHS |
-99 |
Refused |
-100 |
Valid skip |
// Page Break //
//BASE: All respondents//
Item #: Q2
Question Type: Single punch
// Soft Prompt: “We would like your response to this question.” //
cdc_trust. How much trust do you have in the Centers for Disease Control and Prevention (CDC) to provide you with accurate information about the coronavirus or COVID-19?
Variable Label: cdc_trust: Trust in CDC
Value |
Value Label |
1 |
None at all |
2 |
Not very much |
3 |
A fair amount |
4 |
A great deal |
99 |
I am not familiar with the CDC |
-99 |
Refused |
-100 |
Valid skip |
// Page Break //
//BASE: All respondents//
Item #: Q3
Question Type: Single punch
// Soft Prompt: “We would like your response to this question.” //
beh1_cet_r. Have you received a COVID-19 vaccine?
Variable Label: beh1_cet_r: Vaccination behavior
Value |
Value Label |
0 |
No, I have not received a COVID-19 vaccine |
1 |
Yes, but I have only received one shot out of the two required shots |
2 |
Yes, I have received all of the required shots |
-99 |
Refused |
// Page Break //
//BASE: beh1_cet_r=1 or 2//
Item #: Q4
Question Type: Single punch
// Soft Prompt: “We would like your response to this question.” //
vaccine_id. Which COVID-19 vaccine did you receive?
Variable Label: vaccine_id: Vaccine ID
Value |
Value Label |
2 |
Johnson & Johnson/Janssen |
3 |
Moderna |
4 |
Pfizer-BioNTech |
5 |
Other |
99 |
I do not remember |
-99 |
Refused |
-100 |
Valid skip |
// Page Break //
//BASE: beh1_cet_r=2//
Item #: Q5
Question Type: Dropdown menu
// Soft Prompt: “We would like your response to this question.” //
fully_vacc_month. In which month were you considered fully vaccinated (i.e., two weeks after your final COVID-19 vaccine dose)? Final vaccine dose refers to either the second dose of the Pfizer or Moderna vaccine, or the single dose of the Johnson & Johnson vaccine. Please do not consider booster shots for this question. If you do not remember the specific month, give your best guess.
Variable Label: fully_vacc_month: Month of vaccination
Value |
Value Label |
1 |
December, 2020 |
2 |
January, 2021 |
3 |
February, 2021 |
4 |
March, 2021 |
5 |
April, 2021 |
6 |
May, 2021 |
7 |
June, 2021 |
8 |
July, 2021 |
9 |
August, 2021 |
10 |
September, 2021 |
11 |
October, 2021 |
12 |
November, 2021 |
13 |
December, 2021 |
14 |
January, 2022 |
15 |
February, 2022 |
16 |
March, 2022 |
-99 |
// Page Break //
//BASE: beh1_cet_r=2//
Item #: Q6
Question Type: Single punch
// Soft Prompt: “We would like your response to this question.” //
booster_uptake3. U.S. health officials and medical experts now recommend COVID-19 vaccine booster shots for people 5 months after their second dose of an mRNA vaccine (Pfizer-BioNTech and Moderna) or 2 months after their dose of the Johnson & Johnson vaccine. Have you received a COVID-19 vaccine booster shot?
Variable Label: booster_uptake3: Booster uptake – January guidance
Value |
Value Label |
0 |
No |
1 |
Yes |
-99 |
Refused |
-100 |
Valid skip |
// Page Break //
//BASE: booster_uptake3=1//
Item #: Q7
Question Type: Dropdown menu
// Soft Prompt: “We would like your response to this question.” //
booster_month. In which month did you receive a COVID-19 booster shot? If you do not remember the specific month, give your best guess.
Variable Label: booster_month: Month received booster shot
Value |
Value Label |
7 |
June, 2021 |
8 |
July, 2021 |
9 |
August, 2021 |
10 |
September, 2021 |
11 |
October, 2021 |
12 |
November, 2021 |
13 |
December, 2021 |
14 |
January, 2022 |
15 |
February, 2022 |
16 |
March, 2022 |
-99 |
Refused |
-100 |
Valid skip |
// Page Break //
//BASE: (booster_uptake3=0 & vaccine_id=2 & fully_vacc_month=1-14) OR (booster_uptake3=0 & vaccine_id=3-4 & fully_vacc_month=1-11) //
Item #: Q8
Question Type: Single punch
// Soft Prompt: “We would like your response to this question.” //
booster_elig_uptake3. You are currently eligible to receive a COVID-19 vaccine booster shot. What is the likelihood that you will get one?
Variable Label: booster_elig_uptake3: Booster uptake likelihood – eligible adults
Value |
Value Label |
1 |
Very unlikely |
2 |
Somewhat unlikely |
3 |
Neither likely nor unlikely |
4 |
Somewhat likely |
5 |
Very likely |
-99 |
Refused |
-100 |
Valid skip |
// Page Break //
//BASE: (booster_uptake3=0 & vaccine_id=2 & fully_vacc_month=15-16) OR (booster_uptake3=0 & vaccine_id=3-4 & fully_vacc_month=12-16)//
Item #: Q9
Question Type: Single punch
// Soft Prompt: “We would like your response to this question.” //
booster_likely_v2. What is the likelihood that you will get a COVID-19 vaccine booster shot when eligible?
Variable Label: booster_likely_v2: Booster uptake likelihood – not yet eligible
Value |
Value Label |
1 |
Very unlikely |
2 |
Somewhat unlikely |
3 |
Neither likely nor unlikely |
4 |
Somewhat likely |
5 |
Very likely |
-99 |
Refused |
-100 |
Valid skip |
// Page Break //
//BASE beh1_cet =2//
Item #: Q10
Question Type: Single punch grid
// Soft Prompt: “We would like your response to this question.” //
booster_time. Scientists are still studying how often booster shots for the COVID-19 vaccines may need to be given to maintain protection against severe illness from COVID-19.
If regular COVID-19 vaccine booster shots were recommended by U.S. public health officials, and they were completely free of cost, how likely would you be to get them at the following intervals?
Variable Label: booster_uptake: Likelihood of getting boosters at intervals
Variable Name |
Variable Text |
Variable Label |
booster_time _1 |
Every six months |
booster_time_1: Six months |
booster_time _2 |
Every year |
booster_time_2: One year |
booster_time _3 |
Every two years |
booster_time_3: Two years |
booster_time _4 |
Every five years |
booster_time_4: Five years |
booster_time _5 |
Every 10 years |
booster_time_5: Ten years |
Value |
Value Label |
1 |
Not at all likely |
2 |
Slightly likely |
3 |
Moderately likely |
4 |
Very likely |
-99 |
Refused |
-100 |
Valid skip |
// Page Break //
//BASE: beh1_cet_r=0 OR -99//
Item #: Q11
Question Type: Single punch
// Soft Prompt: “We would like your response to this question.” //
beh2a_cet. What is the likelihood that you will get a COVID-19 vaccine?
Variable Label: beh2a: Intention to get vaccinated
Value |
Value Label |
1 |
Very unlikely |
2 |
Somewhat unlikely |
3 |
Neither likely nor unlikely |
4 |
Somewhat likely |
5 |
Very likely |
-99 |
Refused |
-100 |
Valid Skip |
// Page Break //
//BASE: beh1_cet_r=0 OR -99//
Item #: Q12
Question Type: Single punch
// Soft Prompt: “We would like your response to this question.” //
beh3a_cet_r. How soon will you get vaccinated?
Variable Label: beh3a_cet_r: Wait to get vaccinated
Value |
Value Label |
1 |
I will get a vaccine as soon as I can |
2 |
I will wait to get a vaccine for one or more reasons |
3 |
I will never get a COVID-19 vaccine |
-99 |
Refused |
-100 |
// Page Break //
//BASE: All respondents//
Item #: Q13
Question Type: Multi punch
// Soft Prompt: “We would like your response to this question.” //
parent. Are you the parent of a child or children in the following age groups?
Variable Label: parent: Parent of children in following age groups
Value |
Value Label |
1 |
Younger than 6 months old |
2 |
6 months to <2 years old |
3 |
2 to 4 years old |
4 |
5 to 11 years old |
5 |
12 to 15 years old |
6 |
16 to 17 years old |
99 |
None of the above, I do not have children in those age groups [EXCLUSIVE] |
-99 |
Refused |
// Page Break //
//BASE: Parent=4-6//
Item #: Q14
Question Type: Single punch grid
// Soft Prompt: “We would like your response to this question.” //
child_vaxxed_2. Has your child(ren) in the following age group(s) received a COVID-19 vaccine?
Note: If you have more than one child in the same age group, please answer for at least one of them.
Variable Label: child_vaxxed_2: Child vaccinated
Variable Name |
Variable Text |
Variable Label |
child_vaxxed_2_4 |
5 to 11 years old [ONLY SHOW IF parent=4] |
child_vaxxed_2_4: 5 to 11 years old |
child_vaxxed_2_5 |
12 to 15 years old [ONLY SHOW IF parent=5] |
child_vaxxed_2_5: 12 to 15 years old |
child_vaxxed_2_6 |
16 to 17 years old [ONLY SHOW IF parent=6] |
child_vaxxed_2_6: 16 to 17 years old |
Value |
Value Label |
0 |
No, has not received a COVID-19 vaccine |
1 |
Yes, but has only received one shot out of the two required shots |
2 |
Yes, has received all of the required shots |
-99 |
Refused |
-100 |
Valid skip |
// Page Break //
//BASE: child_vaxxed_2_5=2 AND/OR child_vaxxed_2_6=2//
Item #: Q15
Question Type: Single punch grid
// Soft Prompt: “We would like your response to this question.” //
child_boosted. Has your child(ren) in the following age group(s) received a COVID-19 vaccine booster shot?
Note: If you have more than one child in the same age group, please answer for at least one of them.
Variable Label: child_boosted: Child boosted
Variable Name |
Variable Text |
Variable Label |
child_boosted_5 |
12 to 15 years old [ONLY SHOW IF child_vaxxed_2_5=2] |
child_boosted_5: 12 to 15 years old |
child_boosted_6 |
16 to 17 years old [ONLY SHOW IF child_vaxxed_2_6=2] |
child_boosted_6: 16 to 17 years old |
Value |
Value Label |
0 |
No, has not received a COVID-19 vaccine booster shot |
1 |
Yes, has received a COVID-19 vaccine booster shot |
-99 |
Refused |
-100 |
Valid skip |
// Page Break //
//BASE: Parent=1-6//
Item #: Q16
Question Type: Single punch grid
// Soft Prompt: “We would like your response to this question.” //
child_covid_concern. How concerned are you about your child(ren) in the following age groups getting COVID-19?
Note: If you have more than one child in the same age group, please answer for at least one of them.
Variable Label: child_covid_concern: Concern about child(ren)’s COVID-19 risk
//PROGRAMMING NOTE: PIPE 1-6 responses from parent//
Variable Name |
Variable Text |
Variable Label |
child_covid_concern_1 |
Younger than 6 months old |
child_covid_concern_1: Younger than 6 months old |
child_covid_concern_2 |
6 months to <2 years old |
child_covid_concern_2: 6 months to <2 years old |
child_covid_concern_3 |
2 to 4 years old |
child_covid_concern_3: 2 to 4 years old |
child_covid_concern_4 |
5 to 11 years old |
child_covid_concern_4: 5 to 11 years old |
child_covid_concern_5 |
12 to 15 years old |
child_covid_concern_5: 12 to 15 years old |
child_covid_concern_6 |
16 to 17 years old |
child_covid_concern_6: 16 to 17 years old |
Value |
Value Label |
1 |
Not concerned |
2 |
Slightly concerned |
3 |
Somewhat concerned |
4 |
Very concerned |
5 |
Child has already had COVID |
-99 |
Refused |
// Page Break //
//BASE: Parent=1-6//
Item #: Q17
Question Type: Single punch grid
// Soft Prompt: “We would like your response to this question.” //
vacc_child_parent. If a COVID-19 vaccine was authorized and available for children in the following age groups, how likely would you be to get your child(ren) vaccinated?
Note: COVID-19 vaccines have now been authorized and are available for use in children as young as 5 years old. If you have more than one child in the same age group, please answer for at least one of them.
Variable Label: vacc_child_parent: Parent likelihood to get child(ren) vaccinated
//PROGRAMMING NOTE: PIPE 1-6 responses from parent.//
Variable Name |
Variable Text |
Variable Label |
vacc_child_parent_6m |
Younger than 6 months old |
vacc_child_parent_6m: Younger than 6-months-old |
vacc_child_parent_6mto2 |
6 months to <2 years old |
vacc_child_parent_6mto2: 6 months- to 2-years-old |
vacc_child_parent_2to4 |
2 to 4 years old |
vacc_child_parent_2to4: 2- to 4-years-old |
vacc_child_parent_5to11 |
5 to 11 years old [ONLY SHOW IF child_vaxxed_2_4=0 or 99] |
vacc_child_parent_5to11: 5- to 11-years-old |
vacc_child_parent_12to15 |
12 to 15 years old [ONLY SHOW IF child_vaxxed_2_5=0 or 99] |
vacc_child_parent_12to15: 12- to 15-years-old |
vacc_child_parent_16to17 |
16 to 17 years old [ONLY SHOW IF child_vaxxed_2_6=0 or 99] |
vacc_child_parent_16to17: 16- to 17-years-old |
Value |
Value Label |
1 |
Very unlikely |
2 |
Somewhat unlikely |
3 |
Neither likely nor unlikely |
4 |
Somewhat likely |
5 |
Very likely |
-99 |
Refused |
-100 |
Valid Skip |
// Page Break //
//BASE: Parent=1-6//
Item #: Q18
Question Type: Single punch grid
// Soft Prompt: “We would like your response to this question.” //
child_vaccine_concern. How concerned are you about your child(ren) in the following age groups having any side effects from a COVID-19 vaccine?
Note: If you have more than one child in the same age group, please answer for at least one of them.
Variable Label: child_vaccine_concern: Concern about child(ren)’s vaccine risk
//PROGRAMMING NOTE: PIPE 1-6 responses from parent//
Variable Name |
Variable Text |
Variable Label |
child_vaccine_concern_1 |
Younger than 6 months old |
child_vaccine_concern_1: Younger than 6 months old |
child_vaccine_concern_2 |
6 months to <2 years old |
child_vaccine_concern_2: 6 months to <2 years old |
child_vaccine_concern_3 |
2 to 4 years old |
child_vaccine_concern_3: 2 to 4 years old |
child_vaccine_concern_4 |
5 to 11 years old [ONLY SHOW IF child_vaxxed_2_4=0 or 99] |
child_vaccine_concern_4: 5 to 11 years old |
child_vaccine_concern_5 |
12 to 15 years old [ONLY SHOW IF child_vaxxed_2_5=0 or 99] |
child_vaccine_concern_5: 12 to 15 years old |
child_vaccine_concern_6 |
16 to 17 years old [ONLY SHOW IF child_vaxxed_2_6=0 or 99] |
child_vaccine_concern_6: 16 to 17 years old |
Value |
Value Label |
1 |
Not at all concerned |
2 |
Slightly concerned |
3 |
Somewhat concerned |
4 |
Very concerned |
-99 |
Refused |
-100 |
Valid skip |
// Page Break //
//BASE: Parent=1-6//
Item #: Q19
Question Type: Single punch grid
// Soft Prompt: “We would like your response to this question.” //
child_ped. Does your child(ren) in the following age group(s) currently have a pediatrician?
Note: If you have more than one child in the same age group, please answer for at least one of them.
Variable Label: child_ped: Child currently has pediatrician
Variable Name |
Variable Text |
Variable Label |
child_ped_1 |
Younger than 6 months old [ONLY SHOW IF parent=1] |
child_ped_1: <6months has pediatrician |
child_ped_2 |
6 months to <2 years old [ONLY SHOW IF parent=2] |
child_ped_2: 6 months to <2yo has pediatrician |
child_ped_3 |
2 to 4 years old [ONLY SHOW IF parent=3] |
child_ped_3: 2-4yo has pediatrician |
child_ped_4 |
5 to 11 years old [ONLY SHOW IF parent=4] |
child_ped_4: 5-11yo has pediatrician |
child_ped_5 |
12 to 15 years old [ONLY SHOW IF parent=5] |
child_ped_5: 12-15yo has pediatrician |
child_ped_6 |
16 to 17 years old [ONLY SHOW IF parent=6] |
child_ped_6: 16-17yo has pediatrician |
Value |
Value Label |
0 |
No, this child does not currently have a pediatrician |
1 |
Yes, this child currently has a pediatrician |
-99 |
Refused |
// Page Break //
//BASE: child_ped_1-child_ped_6=1//
Item #: Q20
Question Type: Single punch grid
// Soft Prompt: “We would like your response to this question.” //
pedi_vax. Thinking about your youngest child who has a pediatrician, please answer yes or no to the following statements.
//PROGRAMMING NOTE: randomize variables in grid//
Variable Name |
Variable Text |
Variable Label |
pedi_vax_1 |
I have talked to my child’s pediatrician about COVID vaccination. [ANCHOR] |
pedi_vax_1: Not spoken about COVID vaccination |
pedi_vax_2 |
My child’s pediatrician recommended that my child receive a COVID vaccine. |
pedi_vax_2: Recommended COVID vacc |
pedi_vax_3 |
My child’s pediatrician recommended that my child not receive a COVID vaccine. |
pedi_vax_3: Recommended against vaccination |
pedi_vax_4 |
My child’s pediatrician has shared information about children’s COVID vaccines outside of a visit (such as through an email or letter). |
pedi_vax_4: Shared info outside of visit |
pedi_vax_5 |
My child’s pediatrician has shared information about children’s COVID vaccines during a visit. |
pedi_vax_5: Shared info during visit |
pedi_vax_6 |
My child has underlying health conditions and I have talked to my child’s pediatrician about whether a COVID vaccine is safe for them. |
pedi_vax_6: Underlying conditions |
pedi_vax_7 |
I have talked to my child’s pediatrician about the short-term side effects of a COVID vaccine. |
pedi_vax_7: Talked about short-term side effects |
pedi_vax_8 |
I have talked to my child’s pediatrician about the long-term side effects of a COVID vaccine. |
pedi_vax_8: Talked about long-term side effects |
Value |
Value Label |
0 |
No |
1 |
Yes |
99 |
I do not remember |
-99 |
Refused |
-100 |
Valid skip |
// Page Break //
//BASE: All respondents//
Item #: Q21
Question Type: Single punch
// Soft Prompt: “We would like your response to this question.” //
pbeuadelay_know. True or false: Pfizer-BioNTech recently postponed its application to the FDA for authorization to expand use of its COVID-19 vaccine for children ages 6 months to 4 years.
Variable Label: pbeuadelay_know: Awareness of delay in EUA for under 5
Value |
Value Label |
1 |
True |
2 |
False |
99 |
I don’t know |
-99 |
Refused |
// Page Break //
//BASE: All respondents//
Item#: Q22
Question Type: Single punch grid
// Soft Prompt: “We would like your response to this question.” //
pbeuadelay_perc: This is true. In mid-February, Pfizer-BioNTech postponed their application for FDA authorization to expand use of their COVID-19 vaccine in children ages 6 months to 4 years until early April, when more data on the effectiveness of a third dose will be available.
How much do you agree or disagree with the following statements?
//PROGRAMMING NOTE: RANDOMIZE options //
pbeuadelay_perc_1 |
The delay increases my confidence in the safety and effectiveness of FDA-authorized vaccines. |
pbeuadelay_perc_1: Delay increases confidence in vaccines |
pbeuadelay_perc_2 |
The delay decreases my confidence in the safety and effectiveness of FDA-authorized vaccines. |
pbeuadelay_perc_2: Delay decreases confidence in vaccines |
pbeuadelay_perc_3 |
The delay makes me more likely to vaccinate my child(ren) under the age of 5, when the vaccine is authorized and available for them. [ONLY SHOW IF parent=1, 2, or 3] |
pbeuadelay_perc_3: Delay increases likelihood to vaccine child under 5 |
pbeuadelay_perc_4 |
The delay makes me less likely to vaccinate my child(ren) under the age of 5, when the vaccine is authorized and available for them. [ONLY SHOW IF parent=1, 2, or 3] |
pbeuadelay_perc_4: Delay decreases likelihood to vaccine child under 5 |
Value |
Value Label |
1 |
Strongly disagree |
2 |
Somewhat disagree |
3 |
Neither agree nor disagree |
4 |
Somewhat agree |
5 |
Strongly agree |
99 |
I don’t know |
-99 |
Refused |
-100 |
Valid skip |
//BASE: All respondents//
Item#: Q23-Q24
Question Type: Single punch grid
// Soft Prompt: “We would like your response to this question.” //
cdc_commlevels: The CDC relaxed its mask guidance last week, dropping its recommendation for universal masking in areas of low or medium risk of COVID-19. They also unveiled a new method for calculating risk in each U.S. county, called COVID-19 Community Levels, which focuses on the impact of severe COVID-19 disease on local hospitals. The below map depicts COVID-19 Community Levels across the United States as of late February.
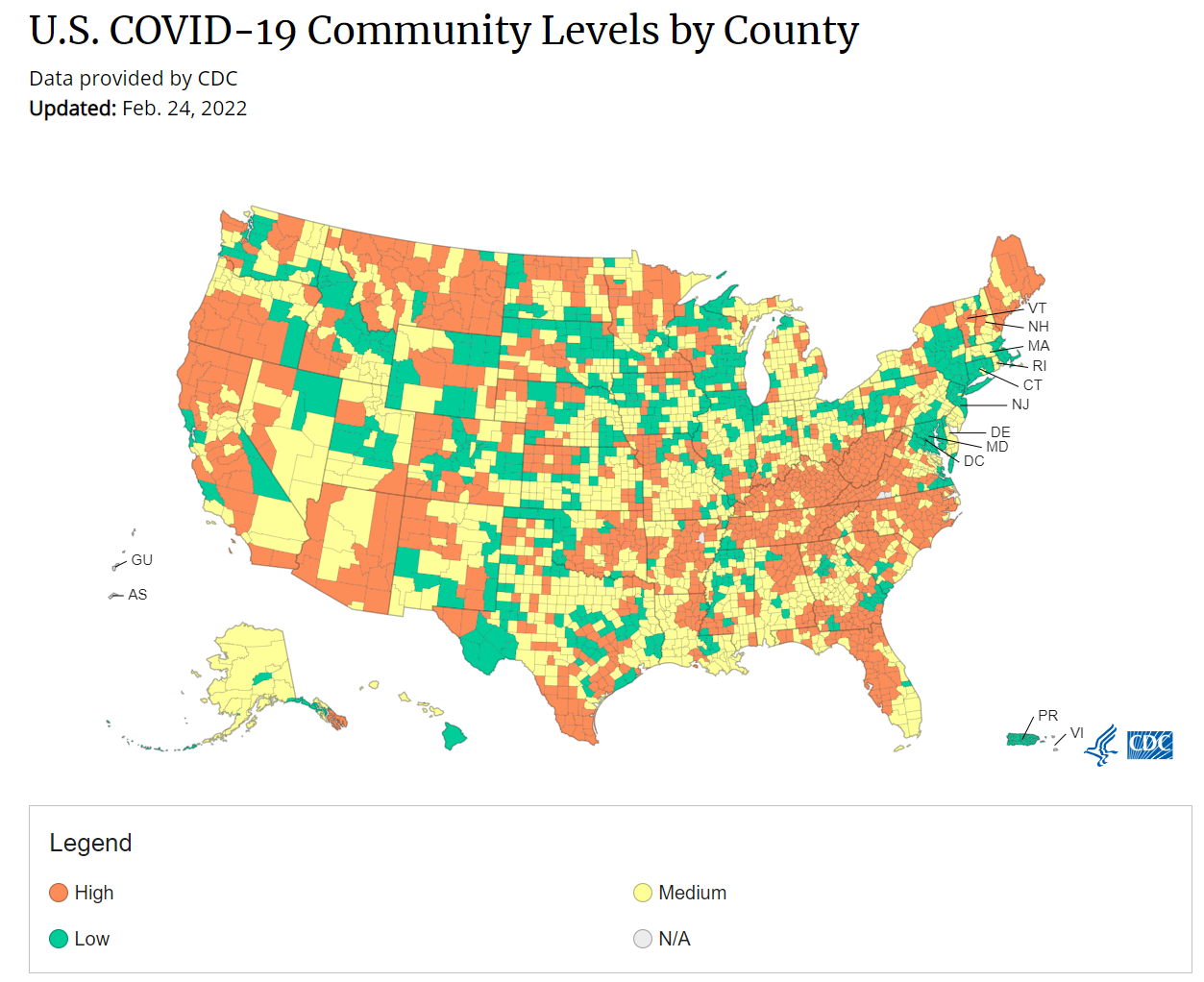
How much do you agree or disagree with the following statements about the updated CDC masking guidance?
//PROGRAMMING NOTE: RANDOMIZE options //
cdc_commlevels _1 |
I have heard about the CDC’s new COVID-19 Community Levels tool. |
cdc_commlevels_1: Have heard of Community Levels |
cdc_commlevels _2 |
I understand the CDC’s updated masking guidance. |
cdc_commlevels_2: Understand new masking guidance |
cdc_commlevels _3 |
I have visited the CDC’s website to find out more about masking guidance for my area. |
cdc_commlevels_3: Visited CDC website |
cdc_commlevels _4 |
I know how to find the COVID-19 Community Level for my area. |
cdc_commlevels_4: Know how to find Community Level |
cdc_commlevels _5 |
I know how to determine whether masking is recommended in my area based on the CDC’s COVID-19 Community Levels. |
cdc_commlevels_5: Know how to determine masking guidance |
cdc_commlevels _6 |
I plan to continue masking indoors in public, regardless of the CDC’s guidance for my community. |
cdc_commlevels_6: Plan to continue masking |
cdc_commlevels_7 |
I do not plan on masking indoors in public, even if my COVID-19 Community Level is rated as High. |
cdc_commlevels_7: Do not plan to continue masking |
cdc_commlevels_8 |
I plan to use the CDC guidance for my community when deciding whether to wear a mask indoors in public. |
cdc_commlevels_8: Plan to use CDC guidance |
Value |
Value Label |
1 |
Strongly disagree |
2 |
Somewhat disagree |
3 |
Neither agree nor disagree |
4 |
Somewhat agree |
5 |
Strongly agree |
-99 |
Refused |
// Page Break //
//BASE: All respondents//
Item
#: Q25
Question
Type: Single
punch grid
//
Soft Prompt: “We would like your response to this
question.”//
ptn_w52.
We are interested in your opinion of a few messages about COVID-19
vaccines or boosters.
For each of the below messages, please indicate how much you agree or disagree with the following statement:
“I would share the information in the message with a friend or family member who wants to know more about COVID-19 vaccines or boosters.”
//PROGRAMMING NOTE: randomize variables in grid//
Value |
Value Label |
1 |
Strongly disagree |
2 |
Disagree |
3 |
Neither agree nor disagree |
4 |
Agree |
5 |
Strongly agree |
-99 |
Refused |
For
internal communications only

| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Lindsey Strausser |
| File Modified | 0000-00-00 |
| File Created | 2022-06-13 |
© 2026 OMB.report | Privacy Policy