CMS-10569 Supporting Statement A EQRS CY 2023 (10-20-2022) (3)
CMS-10569 Supporting Statement A EQRS CY 2023 (10-20-2022) (3).docx
Data Collection for Quality Measures Using the Consolidated Renal Operations in a Web-Enabled Network (CROWNWeb) (CMS-10569)
OMB: 0938-1289
Supporting Statement – Part A
Data Collection for Quality Measures Using the End-Stage Renal Disease Quality Reporting System (EQRS)
Background
Pursuant to section 1881(h) of the Social Security Act (the Act) as amended by section 153(h) of the Medicare Improvements for Patients and Providers Act (MIPPA) the Centers for Medicare and Medicaid Services (CMS) established the End-Stage Renal Disease (ESRD) Quality Incentive Program (QIP) starting in 2011. The ESRD QIP is the first value-based purchasing program established by CMS, and it is aimed at promoting patient health by providing a financial incentive for renal dialysis facilities to deliver high-quality care.
In implementing the ESRD QIP, CMS believes that a successful quality incentive program will promote the delivery of high-quality health care services in the renal dialysis facility setting. Under section 1881(h)(2) of the Act, the Secretary is required to specify quality measures for evaluating the quality of care ESRD patients receive at renal dialysis facilities. While the Act outlines few mandatory measure topics, the Secretary is authorized to adopt measures on specified areas or medical topics determined appropriate by the Secretary (§ 1881(h)(2)). The ESRD QIP began in calendar year (CY) 2011 with an initial set of three quality measures and has increased and refined the measure set over the intervening years through notice and comment rulemaking.
In order to score facility performance on quality measures, CMS must be able to collect data on these measures. CMS collects these data from multiple sources, including Medicare claims and other tools such as the In-Center Hemodialysis Consumer Assessment of Healthcare providers and Systems (ICH CAHPS) and the Centers for Disease Control and Prevention’s (CDC) National Healthcare Safety Network (NHSN) Dialysis Event Protocol. To further expand the measures used to evaluate the quality of care provided to ESRD patients in renal dialysis facilities, CMS also collects data using the ESRD Quality Reporting System (EQRS), formerly known as the Consolidated Renal Operations in a Web-Enabled Network (CROWNWeb) system. Because of the complexity of the existing systems and because of the need to comply with the strong approved protections for private or confidential data, CROWNWeb was implemented in phases starting in February 2009. CROWNWeb went into production nationally on June 14, 2012 and brought together all of CMS’ information systems that collect, maintain, and report on data about ESRD patients and provides electronic reporting tools for use by renal dialysis facilities. On November 9, 2020,1 we launched the EQRS, which contains the functionalities of the three legacy ESRD Systems, including CROWNWeb, in one global application, and aims to provide ongoing support to the ESRD user community to foster accurate and timely monthly data submission. This migration eliminates the need for multiple user accounts, and will in the long-term also improve the overall user experience and reduce burden due to enhanced navigation features.
The ESRD QIP is updating this PRA package to ensure that it remains specific to reporting and validating EQRS data for the payment years addressed in the CY 2023 ESRD PPS final rule (i.e. Payment Year (PY) 2025 and PY 2026).
Data Collection for ESRD QIP Measures
In selecting measures for adoption into the ESRD QIP measure set, CMS strives to achieve several objectives. First, the measures should consider national priorities such as those established by the Department of Health and Human Services’ Meaningful Measures Framework. Second, the measures should be tailored to the needs of improved quality in the renal dialysis facility setting; thus, the measures selected are most relevant to renal dialysis facilities. Finally, the burden of measure compliance on renal dialysis facilities should be weighed against the potential for improvements in patient health and well-being resulting from the measure’s collection.
Many measures currently finalized in the ESRD QIP are extracted from Medicare claims and therefore require no additional effort on the part of dialysis facilities to report.2 However, some quality data relevant to the care received by ESRD patients cannot be derived from Medicare claims or other administrative forms. For these measures, dialysis facilities are required to submit data via a web-based tool such as EQRS or the CDC’s NHSN system. The burden associated with submitting measure data to the NHSN Bloodstream Infection Modules3 and for the In-Center Hemodialysis Consumer Assessment of Healthcare Providers and Systems survey (ICH CAHPS)4 are already captured under previously approved packages. Although we are finalizing the adoption of a new COVID-19 Vaccination Coverage among Healthcare Personnel (HCP) reporting measure beginning in PY 2025 that would require measure data to be submitted through the NHSN, the CDC does not currently estimate burden for COVID-19 vaccination reporting under the CDC PRA package approved under OMB control number 0920-1317 because the agency has been granted a waiver under section 321 of the National Childhood Vaccine Injury Act (NCVIA).5 Therefore, this package is specific to the burdens associated with ESRD QIP measure data submitted via EQRS.
The CY 2023/PY 2025 ESRD QIP
The CY 2023 ESRD Prospective Payment System (PPS) final rule finalizes updates to program requirements for the CY 2023/PY 2025 ESRD QIP. During CY 2023/PY 2025, we will continue collecting data for the follow measures using EQRS:
Hemodialysis Vascular Access: Standardized Fistula Rate Clinical Measure (82 FR 50776 through 50777): Measures the use of an AV fistula as the sole means of vascular access as of the last hemodialysis treatment session of the month. Facilities report in EQRS the vascular access type.
Hemodialysis Vascular Access: Long-Term Catheter Rate Clinical Measure (82 FR 50777 through 50778): Measures the use of a catheter continuously for 3 months or longer as of the last hemodialysis treatment session of the month. Facilities report in EQRS the vascular access type.
Hypercalcemia Clinical Measure (76 FR 72203): Proportion of patient-months with 3-month rolling average of total uncorrected serum calcium greater than 10.2 mg/dL.
Kt/V Dialysis Adequacy Comprehensive Clinical Measure (80 FR 69053): Percentage of all patient-months for patients whose delivered dose of dialysis (either hemodialysis or peritoneal dialysis) met the specified threshold during the reporting period
Clinical Depression Screening and Follow-Up Reporting Measure (79 FR 66203): Facility reports in EQRS one of the six conditions listed for each qualifying patient once before February 1 of the year following the Performance Period.
Ultrafiltration Rate Reporting Measure (81 FR 77915): Facilities must report the following data to EQRS for all hemodialysis sessions during the week of the monthly Kt/V draw submitted to EQRS for that patient-month, for each qualifying patient: (1) HD Kt/V Date; (2) Post-Dialysis Weight; (3) Pre-Dialysis Weight; (4) Delivered Minutes of BUN Hemodialysis; (5) Number of sessions of dialysis delivered by the dialysis unit to the patient in the reporting month.
Medication Reconciliation for Patients Receiving Care at Dialysis Facilities Reporting Measure (83 FR 57008 through 57010): Percentage of patient-months for which medication reconciliation was performance and documented by an eligible professional.
Table A. Measures Collected via EQRS in CY 2023
NQS Goal |
NQF Endorsement Number |
Measure Title |
Data Collected |
Clinical Care |
NQF #2977 |
Hemodialysis Vascular Access: Standardized Fistula Rate Clinical Measure |
Vascular Access Type |
Clinical Care |
NQF #2978 |
Hemodialysis Vascular Access: Long-Term Catheter Rate Clinical Measure |
Vascular Access Type |
Clinical Care |
NQF #1454 |
Hypercalcemia |
Uncorrected serum calcium |
Clinical Care |
N/A |
Dialysis Adequacy Comprehensive |
Kt/V Value |
Clinical Care |
N/A |
Clinical Depression Screening and Follow-Up |
One of six clinical depression screening and follow up conditions |
Clinical Care |
Based upon NQF #2701 |
Ultrafiltration Rate Reporting Measure |
|
Safety |
NQF #2988 |
Medication Reconciliation for Patients Receiving Care at Dialysis Facilities Reporting Measure |
|
The CY 2024/PY 2026 ESRD QIP
For the CY 2024/PY 2026 ESRD QIP, we will continue to collect data using EQRS for the measures referenced earlier in the section for the CY 2023/PY 2025 ESRD QIP. We will also continue to collect these measures in subsequent years unless we deem their removal appropriate based on the measure removal criteria outlined in the CY 2013 ESRD PPS final rule (77 FR 67475)—further clarified in the CY 2015 ESRD PPS final rule (79 FR 66171 through 66173) and the CY 2019 ESRD PPS final rule (83 FR 56983 through 56985).
EQRS Data Validation for the ESRD QIP
One of the critical elements of the ESRD QIP’s success is ensuring that the data submitted to calculate measure scores and facility Total Performance Scores (TPS) are accurate. We began a pilot validation study program for the ESRD QIP in CY 2013. That validation study has continued in subsequent years. In the CY 2019 ESRD PPS final rule, we finalized a policy to make the CROWNWeb validation study a permanent element of the Program rather than a continued pilot study (83 FR 57001 through 57003). Making the CROWNWeb validation study permanent did not alter the methodology that we employ to validate CROWNWeb data and signals the importance that we place on accurate and complete quality data to participating ESRD facilities. Although we have transitioned from CROWNWeb to EQRS, we continue the validation using the data that was previously submitted to CROWNWeb and is now submitted to EQRS. Specifically, we will continue sampling the same number of records (approximately 10 per facility) from the same number of facilities (300 facilities). If a facility is randomly selected to participate in the validation but does not provide us with the requisite medical records within 60 calendar days of receiving a request, then we will deduct 10 points from the facility’s TPS.
Justification
Need and Legal Basis
Section 1881(h)(2) of the Act requires that the Secretary specify measures for each year of the program and with each successive year of the ESRD QIP, CMS has increased the sophistication and scope of the Program’s measure set. While Medicare claims can be an appropriate data source for some measures, claims do not represent the entirety of the ESRD population and are also limited in the depth of information available. For these reasons, in furtherance of our obligations under section 1881(h)(2) of the Act, we have specified several measures utilizing data reported by renal dialysis facilities using the EQRS system described below. These collections are authorized under section 494.180(h) of the Conditions for Coverage of End-Stage Renal Disease Facilities, which requires renal dialysis facilities to furnish data and information (both clinical and administrative) electronically to CMS at intervals specified by the Secretary. CMS proposes and finalizes data reporting requirements for the ESRD QIP through notice and comment rulemaking.
Trend summaries included below depict the progression of measure results over the past several years to determine the impact of the ESRD QIP on improved quality and outcomes in ESRD populations. However, those trends cannot be attributed directly to the ESRD QIP. Several other national initiatives such as Fistula First, Catheter Last (a national vascular access improvement initiative), Dialysis Facility Compare (DFC), quality improvement activities by dialysis organizations, the changes to the PPS ESRD Payment Bundle, and technical support provided by the ESRD networks have all collectively contributed to improvements in ESRD care and services. The implementation of the Medicare ESRD PPS in 2011 and the ESA labeling change later that year are likely to have contributed to improvements in care for this population.
Rates of hypercalcemia have declined, meaning improved patient calcium rates over time starting in CY 2013 when the measure was introduced in the ESRD QIP final rule. Hypercalcemia rates improved from 3.7% in CY 2012 to 1.1% in CY 2018.
Facility performance on the vascular access type (VAT) measures (i.e. fistula and catheter) improved in the first few years that the measures were included in the program and have remained stable over the past four years. Fistula rates have increased from 62.1% in CY 2012 to 66.3% in CY 2018.
Kt/V Comprehensive rates have improved since the measure was introduced in the ESRD QIP in PY 2019. Rates improved from 94.6% in CY 2016 to 95.9% in CY 2019.
Performance on risk adjusted measures including readmissions, hospitalizations, and transfusions has remained stable since the measures were introduced in the ESRD QIP, with the exception of the NHSN Bloodstream Infection (BSI) ratio, where facility performance is improving slightly each year. Average BSI ratios have decreased from 1.05 in CY 2014 to 0.75 in CY 2018.
Mortality rates have steadily declined from 2010 to 2017.
The data show a substantial decrease in readmission rates from 30.3 in 2011 to 25.2 in 2016.
While the ESRD QIP was not solely intended as a cost saving program, below we show the Program’s estimated payment reductions in recent years. We note that the estimated payment reductions for PY 2023, PY 2025, and PY 2026 have been updated from the estimates in the CY 2023 ESRD PPS proposed rule, due to updated information about the total number of facilities expected to receive a payment reduction and the estimated impact of policies in the CY 2023 ESRD PPS final rule on facilities.
PY 2026; $32,457,692.52 (CY 2023 ESRD PPS final rule)
PY 2025; $32,457,692.52 (CY 2023 ESRD PPS final rule)
PY 2024; $17,104,030.59 (86 FR 62011)
PY 2023; $5,548,652.69 (CY 2023 ESRD PPS final rule)
PY 2022; $0 (86 FR 62011)6
PY 2021; $32,196,724 (83 FR 57061)
PY 2020; $31,581,441 (81 FR 77960)
PY 2019; $15,470,309 (80 FR 69074)
PY 2018; $11,576,214 (79 FR 66257)
PY 2017; $11,954,631 (79 FR 66255)
Information Users
Section 1881(h) of the Act requires the Secretary, generally, to adopt a set of quality measures and to assess the quality of care provided by renal dialysis facilities using those measures. CMS and others use these data to monitor and assess the quality and type of care provided to ESRD patients. Specifically, CMS uses these data to calculate performance scores on certain measures included in the ESRD QIP measure set (described in detail below) and conducts a validation each year to ensure that those data are accurate.
CMS will make available to renal dialysis facilities their scores on individual measures and their total performance score for their use in internal quality improvement initiatives. CMS will also make available to facilities information on the performance of other facilities on individual measures and their total performance score. Most importantly, facility performance on individual measures and their TPS is available to beneficiaries, as well as to the public, to assist them in making decisions about their health care. Facilities, beneficiaries, and the public do not have access to validation results. CMS intends to use information on facility performance on measures and their TPS as well as validation results to direct its contractors to focus on areas of improvement and to develop quality improvement initiatives. This includes targeted training if underreporting or inaccurate reporting is identified and user error is suspected as the cause. CMS uses the validation to independently sample and test the reliability and validity of the clinical data submitted electronically in EQRS against providers’ source medical records, and to encourage facilities to accurately report data to EQRS.
Use of Information Technology
As noted previously, CMS developed EQRS to reduce the burden to renal dialysis facilities of submitting data to CMS. This system brings together all of CMS’ information systems that collect, maintain, and report on data about ESRD patients and provides electronic reporting tools for use by renal dialysis facilities. Renal dialysis facility users are required to open an account under their CMS Certification Number and are then able to complete the necessary data submission.
Duplication of Efforts/Similar Information
The information to be collected is not duplicative of similar information collected by the Centers for Medicare and Medicaid Services.
Small Businesses
Information collection requirements were designed to impose minimal burdens on small renal dialysis facilities subject to the ESRD QIP, and to facilitate the collection and reporting of required data. Specifically, the EQRS was created to allow small renal dialysis facilities to enter data via a web-based application rather than using paper-based data submissions or employing a full electronic health record, which can be prohibitively expensive for these facilities.
Less Frequent Collection
Measures developers employ clinical and statistical knowledge during the measure development process to determine the optimal schedule for collecting measure data. These data are then collected on the schedules provided in Table B to best evaluate the care provided to ESRD patients. Without this frequency of information collection, CMS would be unable to assess the correlations between the endpoints collected and the health and well-being of ESRD patients treated by the renal dialysis facilities participating in the ESRD QIP.
Table B. Measure Collection Schedule/Frequency
Measure Title |
Measure Collection Schedule/Frequency |
Hypercalcemia |
Monthly |
Dialysis Adequacy Comprehensive |
Monthly |
Clinical Depression Screening and Follow-Up |
Annually |
Ultrafiltration Rate Reporting Measure |
4 data elements are reported for every HD Kt/V session during the week of the monthly Kt/V draw, and Kt/V date is reported monthly |
Hemodialysis Vascular Access Type: Standardized Fistula Rate Clinical Measure |
Monthly |
Hemodialysis Vascular Access Type: Long-Term Catheter Rate Clinical Measure |
Monthly |
Medication Reconciliation for Patients Receiving Care at Dialysis Facilities (MedRec) Measure |
Monthly |
Special Circumstances
There are no special circumstances.
Federal Register Notice/Outside Consultation
The CY 2023 ESRD PPS proposed rule’s publication, serving as the 60-day Federal Register notice, was published on June 28, 2022 (87 FR 38586). The final rule published on November 07, 2022 (87 FR 67136).
Payment or Gift to Respondent
Dialysis facilities are required to submit measure data to CMS as part of the Conditions for Coverage of End-Stage Renal Disease Facilities (see 42 CFR 494.180(h)). No additional payments or gifts will be given to respondents for compliance with the reporting requirements of the ESRD QIP measures submitted via EQRS.
Confidentiality
CMS adheres to all confidentiality-related statutes, regulations, and agency policies. All information collected under ESRD QIP will conform to all applicable Federal laws and regulations and Federal, HHS, and CMS policies and standards as they relate to information security and data privacy. The laws and regulations that may apply include, but are not limited to: The Privacy Act of 1974; the Federal Information Security Management Act of 2002; the Computer Fraud and Abuse Act of 1986; the Health Insurance Portability and Accountability Act of 1996; the EGovernment Act of 2002, the Clinger Cohen Act of 1996; the Medicare Modernization Act of 2003, and the corresponding implementing regulations. OMB Circular A–130, Management of Federal Resources, Appendix III, Security of Federal Automated Information Resources also applies. Federal, HHS, and CMS policies and standards include but are not limited to: All pertinent National Institute of Standards and Technology publications; the HHS Information Systems Program Handbook and the CMS Information Security Handbook.
SORN #: 09-70-0520 – ESRD Program Management and Medical Information System (PMMIS) published 6/17/2002 (67 FR 41244), updated 5/8/2007 (72 FR 26126), and revised 6/26/2009 (74 FR 30606).
Sensitive Questions
There are no questions of a sensitive nature being collected as part of this quality assessment.
Burden Estimates
This burden estimate includes measures which CMS is continuing to collect as part of the ESRD QIP and the ongoing EQRS (formerly CROWNWeb) data validation. As noted in section A.1. of this supporting statement, this estimate excludes burden associated the NHSN Bloodstream Infection clinical measure and the ICH-CAHPS measure because the burden associated with these measures is captured under OMB numbers 0920-0666 (The National Healthcare Safety Network) and 0938-0926 (ICH-CAHPS Survey), respectively. This estimate also excludes burden associated with the finalized COVID-19 Vaccination Coverage among Healthcare Personnel (HCP) reporting measure because the CDC has been granted a waiver under section 321 of the National Childhood Vaccine Injury Act (NCVIA). This burden estimate also excludes the burden associated with training facilities to use EQRS, which will continue to be accounted for in OMB Control Number 0938-0386. The burden associated with the NHSN BSI Data Validation is captured under OMB Control Number 0938-1340.
The assumptions used to compute the estimated burdens associated with submitting ESRD QIP measure data via EQRS and the ongoing EQRS data validation are described here.
We estimate the burden hours for reporting measure data using the EQRS system for CY 2023/PY 2025 to be 4,908,291 hours; for CY 2024/PY 2026 this figure is also 4,908,291. We estimate that the total burden hours associated with the PY 2025 EQRS validation is 750. The total burden hours for these two activities per year is 4,909,041 (4,908,291 + 750). Accordingly, we estimate the annual burden for the 3-year OMB approval period to be 3,272,444 hours (9,817,332 / 3 years).
a. Data Collection for ESRD QIP Measures Using EQRS
We have used the following equation to estimate the burden associated with these data collection and submission efforts.

Table C. EQRS Data Collection Burden Estimate Elements
Burden Estimate Elements |
CY 2023/ PY 2025 |
CY 2024/ PY 2026 |
Number of facilities7 |
7,847 |
7,847 |
Number of ESRD patients, nationally8 |
514,406 |
514,406 |
The time spent for data entry and submission per element9 |
2.5 minutes |
2.5 minutes |
Annual Hour Burden Nationally |
4,908,291 hours |
4,908,291 hours |
Median hourly wage of a Medical Records and Health Information Technician (Fringe benefit is calculated at 100%). |
$44.86 |
$44.86 |
We estimate the number of patients per facility by calculating the mean number of patients per ESRD PPS-eligible facility nationwide, based on CY 2021 data, even though we recognize that the number of patients per renal dialysis facility is also highly variable, and may vary from month to month within a given facility. To estimate the total burden per facility, the mean number of patients per facility is then multiplied by the number of required elements per patient-year for each measure and the estimated time per element entry, as shown in Table D1. The estimated time per element entry for the EQRS measure is based on historical estimates previously finalized in the CY 2016 ESRD PPS final rule regarding the amount of time required to enter one data element for one patient (i.e. we assumed that it takes 2.5 minutes to report a data element, even though the time required is highly variable) (80 FR 69070).
To derive wage estimates, we used data from the U.S. Bureau of Labor Statistics’ (BLS) May 2021 National Occupational Employment and Wage Estimates.10 We anticipate that the labor required to collect and submit these data will be completed by either Medical Records Specialists or similar administrative staff. The median hourly wage of a Medical Records Specialist is $22.43. Fringe benefits and overhead are calculated at 100% using current HHS department-wide guidance on estimating the cost of fringe benefits and overhead. These are necessarily rough adjustments both because fringe benefits and overhead costs vary significantly from employer to employer and because methods of estimating these costs vary widely from study to study. Nonetheless, there is no practical alternative and we believe that these are reasonable estimation methods.
Using the assumptions described above, we estimate an hourly labor cost of $44.86 as the basis of the wage estimates for all collection of information calculations in the ESRD QIP. We also estimate the total annual burden for reporting measure data using the EQRS for CY 2023/PY 2025 to be $220,185,916 and the total annual burden for reporting measure data using the EQRS for CY 2024/PY 2026 is $220,185,916.
Table D1. CY 2023/PY 2025 EQRS Data Collection Burden Per Measure
Note: Numbers may not add up due to rounding
MEASURE REPORTING Renal Dialysis Facilities CY 2019 Measure Set |
Number of Facilities |
Number of Patients Nationally |
Average number of patients per facility |
Number of Elements per Patient-Year |
Estimated Time for Data Entry per Element (hours) |
Estimated Wage plus Benefits per Hour for Data Entry |
Annual Hour Burden per Facility |
Annual Burden per Facility |
Hemodialysis Vascular Access: Standardized Fistula Rate Clinical Measure |
7,847 |
514,406 |
65 |
12 |
0.042 |
$44.86 |
32.7 |
$1,470.39 |
Hemodialysis Vascular Access: Long-Term Catheter Rate Clinical Measure |
7,847 |
514,406 |
65 |
12 |
0.042 |
$44.86 |
32.7 |
$1,470.39 |
Hypercalcemia |
7,847 |
514,406 |
65 |
12 |
0.042 |
$44.86 |
32.7 |
$1,470.39 |
Comprehensive Dialysis Adequacy |
7,847 |
514,406 |
65 |
12 |
0.042 |
$44.86 |
32.7 |
$1,470.39 |
Clinical Depression Screening and Follow-Up |
7,847 |
514,406 |
65 |
1 |
0.042 |
$44.86 |
2.7 |
$122.53 |
Ultrafiltration Rate Reporting Measure |
7,847 |
514,406 |
65 |
156 |
0.042 |
$44.86 |
426.1 |
$19,115.03 |
Medication Reconciliation for Patients Receiving Care at Dialysis Facilities Reporting Measure |
7,847 |
514,406 |
65 |
24 |
0.042 |
$44.86 |
65.5 |
$2,940.77 |
Table E1. CY 2023/PY 2025 EQRS Total Data Collection Burden
Basis |
Number of Elements |
Annual Hour Burden |
Annual Burden |
Each Facility |
15,011 |
625.49 |
$28,059.88 |
National |
117,798,974 |
4,908,291 |
$220,185,916 |
Table D2. CY 2024/PY 2026 EQRS Data Collection Burden Per Measure
Note: Numbers may not add up due to rounding
MEASURE REPORTING Renal Dialysis Facilities CY 2019 Measure Set |
Number of Facilities |
Number of Patients Nationally |
Average number of patients per facility |
Number of Elements per Patient-Year |
Estimated Time for Data Entry per Element (hours) |
Estimated Wage plus Benefits per Hour for Data Entry |
Annual Hour Burden per Facility |
Annual Burden per Facility |
Hemodialysis Vascular Access: Standardized Fistula Rate Clinical Measure |
7,847 |
514,406 |
65 |
12 |
0.042 |
$44.86 |
32.7 |
$1,470.39 |
Hemodialysis Vascular Access: Long-Term Catheter Rate Clinical Measure |
7,847 |
514,406 |
65 |
12 |
0.042 |
$44.86 |
32.7 |
$1,470.39 |
Hypercalcemia |
7,847 |
514,406 |
65 |
12 |
0.042 |
$44.86 |
32.7 |
$1,470.39 |
Comprehensive Dialysis Adequacy |
7,847 |
514,406 |
65 |
12 |
0.042 |
$44.86 |
32.7 |
$1,470.39 |
Clinical Depression Screening and Follow-Up |
7,847 |
514,406 |
65 |
1 |
0.042 |
$44.86 |
2.7 |
$122.53 |
Ultrafiltration Rate Reporting Measure |
7,847 |
514,406 |
65 |
156 |
0.042 |
$44.86 |
426.1 |
$19,115.03 |
Medication Reconciliation for Patients Receiving Care at Dialysis Facilities Reporting Measure |
7,847 |
514,406 |
65 |
24 |
0.042 |
$44.86 |
65.5 |
$2,940.77 |
Table E2. CY 2024/PY 2026 EQRS Total Data Collection Burden
Note: Numbers may not add up due to rounding.
Basis |
Number of Elements |
Annual Hour Burden |
Annual Burden |
Each Facility |
15,011 |
625.49 |
$28,059.88 |
National |
117,798,974 |
4,908,291 |
$220,185,916 |
b. EQRS Data Validation
We have used the following equation to estimate the burden associated with the ongoing EQRS data validation:
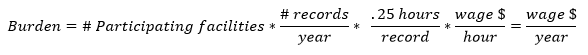
Table F. EQRS Data Validation Burden Estimate Elements
Burden Estimate Element |
CY 2023 (PY 2025) |
Number of facilities participating in the EQRS (formerly, CROWNWeb) data validation, annually |
300 |
Number of medical records per facility per year |
10 |
Time spent for record collection and submission per facility11 |
2.5 hours (approx. 0.25 hours per record) |
Hourly wage per hour engaged in data collection and submission12 |
$44.86 |
Under the EQRS data validation, we will randomly sample records from 300 facilities. Each sampled facility will be required to produce approximately 10 records. The burden associated with these validation requirements is the time and effort necessary to submit the requested records to a CMS contractor. We estimate that it will be take each facility approximately 2.5 hours in total, or 0.25 hours per medical record, to comply with this requirement. We therefore estimate that the total annual hourly burden for the ongoing EQRS data validation for CY 2023 to be 750 hours.
Just as noted above, we anticipate that the labor required to collect and submit these data will be completed by either Medical Records Specialists or similar administrative staff. The median hourly wage of a Medical Records Specialist is $22.43 per hour. Fringe benefits and overhead are calculated at 100 percent. Therefore, using these assumptions, we estimate an hourly labor cost of $44.86 as the basis of the wage estimates for all collection of information calculations in the ESRD QIP. These are necessarily rough adjustments, both because fringe benefits and overhead costs vary significantly from employer to employer and because methods of estimating these costs vary widely from study to study. Accordingly, we estimate the total annual burden for the ongoing EQRS data validation for CY 2023 to be $33,645.
Table G. CY 2023/PY 2025 EQRS Data Validation Burden
DATA VALIDATION Renal Dialysis Facilities CY 2022 |
Number of Facilities |
Number of Records per Year |
Estimated Time per Record |
Estimated Wage plus Benefits per Hour for Record Collection |
Annual Hour Burden per Facility |
Annual Burden per Facility |
EQRS Data Validation |
300 |
10 |
0.25 |
$44.86 |
2.5 |
$112.15 |
Table H. CY 2023/PY 2025 EQRS Total Data Validation Burden
Basis |
Annual Hour Burden |
Annual Burden |
Each Facility |
2.5 |
$112.15 |
National |
750 |
$33,645 |
Capital Cost
There are no capital costs.
Cost to Federal Government
The cost to the Federal Government includes costs associated with the collection and validation of the data. The validation costs are an estimated $535,295 (FY) annually for the validation contract. For the claims-based measures, the cost to the Federal Government is minimal. CMS uses data from the CMS National Claims History system that are already being collected for provider reimbursement; therefore, no additional data will need to be submitted by dialysis facilities for claims-based measures. Additionally, the ESRD QIP program takes three CMS staff at the GS-13 Step 5 level ($117,516 annually per staff member), for an additional cost of $352,548.
Changes to Burden
As discussed above, the ESRD QIP has consistently refined its measure set since the inception of the ESRD QIP in CY 2011. For CY 2023, we are not adding any new measures to be collected using data entered in EQRS. Therefore, we do not estimate any increased burden hours associated with new measures. In addition, the PY 2025 EQRS reporting burden estimate in terms of dollars has increased from the PRA package associated with the CY 2022 ESRD PPS final rule, from $215 million to approximately $220 million, due to the current PRA package using an updated wage estimate for Medical Records Specialists. This estimate is higher the one used in the previous PRA package.
The PY 2025 EQRS reporting burden in terms of hours has decreased from the currently approved PRA package, from approximately 5.08 million hours to approximately 4.9 million hours across all dialysis facilities. This is due to updated data regarding the number of facilities and the number patients nationwide.
The EQRS data validation for CY 2023 is a continuation of the validation process previously finalized for CYs 2015, 2016, 2017, 2018, 2019, 2020, 2021, and 2022. The burden to renal dialysis facilities for CY 2023 EQRS validation will be similar to the burden associated with validation conducted in prior years.
The annual burden hours specified in this PRA package (for the CY 2023 ESRD PPS final rule) for the 3-year OMB approval period have decreased from the currently approved PRA package (associated with the CY 2022 ESRD PPS final rule), from 3,390,283 hours to 3,272,444 hours.
Publication/Tabulation Date
The goal of the data collection is to evaluate facility performance on measures in the ESRD QIP measure set for the given year in order to assess the payment reductions required under section 1881(h)(1) of the Act. This data is also made publicly available pursuant to section 1881(h)(6) of the Act and is used in other programs within the Centers for Medicare and Medicaid Services, such as public reporting of dialysis facility quality data on the CMS Care Compare website (formerly, Dialysis Facility Compare).
Expiration Date
CMS will display the expiration date on the collection instruments.
Explain any exceptions to the certification statement “Certification for Paperwork Reduction Act Submissions” of OMB form 83-I.
There are no exceptions to the certification statement “Certification for Paperwork Reduction Act Submissions” of OMB form 83-I.
2 For example, in the CY 2015 ESRD PPS final rule with comment period, CMS finalized 10 measures using Medicare claims as the primary data source.
3 The NHSN Bloodstream Infection measure is accounted for under OMB Control Number 0920-0666.
4 ICH CAHPS is accounted for under OMB Control Number 0938-0926.
5 Section 321 of the National Childhood Vaccine Injury Act (NCVIA) provides the PRA waiver for activities that come under the NCVIA, including those in the NCVIA at section 2102 of the Public Health Service Act (42 U.S.C. 300aa-2). Section 321 is not codified in the U.S. Code, but can be found in a note at 42 U.S.C. 300aa-1.
6 In the CY 2022 ESRD PPS final rule, we finalized our proposed special scoring methodology and payment policy for PY 2022 (86 FR 61918 through 61919). Under this policy, we will not apply any payment reductions to ESRD facilities for PY 2022.
7 Total number of ESRD PPS facilities in the United States treating ESRD QIP-eligible patients.
8 Total number of patients treated at ESRD PPS facilities in the United States.
9 As stated in the CY 2016 ESRD PPS final rule (80 FR 69070), we estimate the amount of time required to submit measure data to EQRS (formerly CROWNWeb) to be 2.5 minutes.
10 https://www.bls.gov/oes/current/oes292072.htm.
11 As stated in the CY 2020 ESRD PPS final rule (84 FR 60788), we estimate the amount of time required to submit measure data to CROWNWeb (now EQRS) to be 2.5 minutes.
12 https://www.bls.gov/oes/current/oes292072.htm (Estimates are based on national median hourly wage).
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Lobel, Devra |
| File Modified | 0000-00-00 |
| File Created | 2023-08-23 |
© 2026 OMB.report | Privacy Policy