DCI Justification
2288.05 - IC SS.docx
Pesticides Data Call In Program (Non-Substantive Change)
DCI Justification
OMB: 2070-0174
Information Collection
Planned Data Call-Ins (DCIs) EPA No. 2288.05; Under OMB Control No. 2070-0174
December 23, 2022
The following information is being provided in accordance with the terms of clearance for the ICR approved under OMB Control Number 2070-0174, which requires EPA to provide OMB with prior notice and opportunity to review the DCI before EPA may issue the DCI under this ICR. Additional information about the collection activities, authority, estimates and related methodologies, is provided in the most recent ICR.
Executive Summary
What Pesticide Active Ingredients will be Subject to the Planned DCI?
EPA’s Office of Pesticide Programs (OPP) is requesting clearance to issue DCI notices to registrants of the following pesticide active ingredient and the pesticide products that are registered for this active ingredient:
No. |
Pesticide Chemical |
Link to Docket |
1. |
Streptomycin sulfate |
Docket number: EPA-HQ-OPP-2016-0067 |
What are the Estimated Total Burdens and Costs for these Planned DCIs?
The total paperwork burden and cost for this specific DCI request is estimated to be 13,664 hours and $1,139,000.00 as summarized in the following table.
Summary of Study Costs, Burden Costs and Hours for these DCIs |
|||
Chemicals |
Study Costs ($) |
Paperwork Burden Costs ($) |
Paperwork Burden Hours |
Streptomycin sulfate |
$3,253,200.00 |
$1,139,000.00 |
|
Totals: |
$3,253,200.00 |
$1,139,000.00 |
13,664 |
These figures reflect an overestimate because the methodology is based on the assumption that all of the registrants will generate all of the data requested in these DCIs. In reality, however, the registrants may not need to generate all of the data requested. The Agency will waive data requirements it ultimately finds any are inappropriate but will ensure that sufficient data are available to make the determinations required by the applicable statutory standards (40 CFR §158.45). Regarding pollinator data, EPA’s risk assessment process for bees proceeds in a tiered manner, with the need for higher tier studies (Tier II and Tier III) conditioned on the results from lower tier studies (Tier I). Since it is not possible to know at this time the exact number of studies that will be needed, all studies that could be potentially needed are listed in this request.
What are the Planned DCIs?
The following pages provide this information for this pesticide: a list of the data that will be included in the planned DCIs, along with the related test cost and burden estimates; a discussion of public comments received (if any) and EPA responses; and a brief justification for any non-codified data requested.
How are Estimated Study Costs and Burden Costs and Hours Depicted in the individual Case Tables below for Data to be Called-In?
The following standard footnotes “a” through “e” are associated with each individual case table and are provided here rather than repeating them for each table:
Guideline Number a |
40 CFR 158 |
Data |
Study Type c |
Average Test Cost |
Total Paperwork Burden Cost d |
Total Paperwork Burden Hours e |
Test Guidelines provide non-binding guidance on how to conduct the test and are available at https://www.epa.gov/test-guidelines-pesticides-and-toxic-substances.
40 CFR Part 158 can be accessed online at http://www.ecfr.gov/cgi-bin/text-idx?c=ecfr&tpl=/ecfrbrowse/Title40/40cfr158_main_02.tpl.
Study types, which are explained in more detail in the ICR, are identified as follows: HH = human health and toxicity; OR = occupational and residential exposure; RC = residue chemistry; EE = ecological effects; EF = environmental fate; PC = product chemistry; NC = non-codified study.
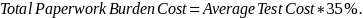 Additional
detail about the methodology is in the ICR.
Additional
detail about the methodology is in the ICR.
Percent staff burden (of the total paperwork burden cost) is estimated as 20% managerial, 65% technical, and 15% clerical. Fully loaded labor rates include the estimated costs of wages, overhead, and benefits paid to an employee. Additional details about the methodology are in the ICR.
Note: all values for “Average Test Cost” and “Paperwork Burden Costs” have been rounded to the nearest $100, while all values for “Total Paperwork Burden Hours” have been rounded to the nearest whole number of hours.
Commonly Appearing Acronyms.
Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), Endangered Species Act (ESA), FIFRA Endangered Species Task Force (FESTF), Organization for Economic Cooperation and Development (OECD).
Studies to be Called-In:
Guideline Number a |
40 CFR 158 Citation b |
Data Requirement |
Study Type c |
Average Test Cost |
Total Paperwork Burden Cost d |
Total Paperwork Burden Hours e |
835.1230 |
158.1300 |
Sediment and soil absorption/desorption for parent and degradates |
EF |
$52,000.00 |
$18,200.00 |
218 |
835.2130 |
158.75 |
Hydrolysis as a function of pH and temperature (40 CFR 721.10619) |
EF |
$58,100.00 |
$20,300.00 |
244 |
835.2240 |
158.1300 |
Direct photolysis rate of parent and degradates in water |
EF |
$62,300.00 |
$21,800.00 |
262 |
835.2410 |
158.1300 |
Photodegradation of parent and degradates in soil |
EF |
$89,900.00 |
$31,500.00 |
378 |
835.4100 |
158.1300 |
Aerobic soil metabolism |
EF |
$131,300.00 |
$46,000.00 |
551 |
835.4200 |
158.1300 |
Anaerobic soil metabolism |
EF |
$182,600.00 |
$63,900.00 |
767 |
835.4300 |
158.1300 |
Aerobic aquatic metabolism |
EF |
$124,300.00 |
$43,500.00 |
522 |
835.4400 |
158.1300 |
Anaerobic Aquatic Metabolism (two-sediment study) |
EF |
$250,000.00 |
$87,500.00 |
1050 |
835.6100 |
158.1300 |
Terrestrial Field Dissipation (incl. storage stability) |
EF |
$442,000.00 |
$154,700.00 |
1856 |
850.1035 |
158.630 |
Mysid acute toxicity test |
EE |
$28,000.00 |
$9,800.00 |
118 |
850.1055 |
158.630 |
Bivalve acute tox larval (embryo/larval) |
EE |
$28,000.00 |
$9,800.00 |
118 |
850.1075 |
158.630 |
Fish acute toxicity test (1 species estuarine/marine) |
EE |
$15,100.00 |
$5,300.00 |
63 |
850.1350 |
158.630 |
Mysid chronic tox - aquatic invertebrate life-cycle (saltwater) |
EE |
$50,500.00 |
$17,700.00 |
212 |
850.1400 |
158.630 |
Fish early-life stage toxicity test |
EE |
$51,900.00 |
$18,200.00 |
218 |
850.1735 |
158.630 |
Whole sediment: acute freshwater invertebrates1 |
EE |
$64,000.00 |
$22,400.00 |
269 |
850.1740 |
158.630 |
Whole sediment: acute marine invertebrates |
EE |
$30,000.00 |
$10,500.00 |
126 |
850.3030 |
158.630 |
Honey Bee Toxicity of Residues on Foliage |
EE |
$21,000.00 |
$7,400.00 |
88 |
850.3040 |
158.630 |
Field testing for Pollinators |
EE |
$302,000.00 |
$105,700.00 |
1,268 |
850.4100 |
158.660 |
Seedling emergence and seedling growth (Tier I) |
EE |
$22,200.00 |
$7,800.00 |
93 |
850.4100 |
158.660 |
Seedling emergency and seedling growth (Tier II) |
EE |
$28,300.00 |
$9,900.00 |
119 |
850.4150 |
158.660 |
Vegetative Vigor (Tier I) |
EE |
$22,600.00 |
$7,900.00 |
95 |
850.4150 |
158.660 |
Vegetative Vigor (Tier II) |
EE |
$44,900.00 |
$15,700.00 |
189 |
850.4500 |
158.660 |
Algal Toxicity |
EE |
$35,200.00 |
$12,300.00 |
148 |
Non GDLN |
158.75 |
Honey bee adult acute oral toxicity |
NC |
$11,000.00 |
$3,900.0 |
46 |
Non GDLN |
158.75 |
Honey bee larvae acute oral toxicity |
NC |
$25,000.00 |
$8,800.00 |
105 |
Non GDLN |
158.75 |
Honey bee larvae chronic oral toxicity |
NC |
$40,000.00 |
$14,000.00 |
168 |
Non GDLN |
158.75 |
Honey bee adult chronic oral toxicity |
NC |
$29,000.00 |
$10,200.00 |
122 |
Non GDLN |
158.75 |
Semi-field testing for pollinators – tunnel study only (Tier II) |
NC |
$108,000.00 |
$37,800.00 |
454 |
Non GDLN |
158.75 |
Semi-field testing for pollinators – colony feeding study only (Tier II) |
NC |
$572,000.00 |
$200,200.00 |
2,402 |
Non GDLN |
158.75 |
Residues in pollen and nectar/field residue analysis (Tier II) |
NC |
$143,000.00 |
$50,100.00 |
601 |
Non GDLN |
158.630 |
Whole sediment – chronic toxicity test, freshwater invertebrates (with TGAI): 28-day study2 |
EE |
$126,000.00 |
$44,100.00 |
529 |
Non GDLN |
158.630 |
Whole sediment – chronic toxicity test, saltwater invertebrate Leptocheirus plumulosus (with TGAI): 28-day study |
EE |
$63,000.00 |
$22,100.00 |
265 |
Totals: |
$3,253,200.00 |
$1,139,000.00 |
13,664 |
|||
Pesticide Overview
Streptomycin is an antibiotic of the aminoglycoside class and is derived from the bacterium Streptomyces griseus. The pesticidal mode of action of streptomycin is classified as a member of glucopyranosyl antibiotic group. Streptomycin is also used to treat bacterial infections in humans and animals. The first pesticide product containing streptomycin was registered in 1955. Streptomycin may be used as a foliar spray on fruit trees, seed treatment for bean, potatoes, and tomatoes, seedling treatment for celery pepper, tobacco, and tomato, and foliar spray/cutting dip for ornamentals. Streptomycin is also registered for use on apple and pear trees in residential gardens. In recent years, emergency exemptions under FIFRA Section 18 have been granted for streptomycin use on citrus in Florida and California to manage Huanglongbing (HLB), also known as citrus greening. Most recently, EPA amended two existing streptomycin pesticide products to add time-limited uses on Citrus Crop Group 10-10 for management of HLB. EPA concluded that the registration of this new use would not cause unreasonable effects on the environment.
However, EPA did not make effects determinations for the new use of streptomycin on Citrus Crop Group 10-10, and the registration were ultimately challenged on both FIFRA and ESA grounds (Migration Clinicians Network et al. v. EPA, No. 21-70719, Ninth Circuit). EPA has asked the Court to remand EPA’s decision without vacating the registration amendments to allow EPA to complete its effects determinations for streptomycin. EPA committed to conducting effects determinations for streptomycin no sooner than Fall 2026, in part based on the need to obtain additional data from the registrants to conduct the effects determinations. All data listed in the table in Section I, above, is required to complete effects determinations for currently registered uses of streptomycin, unless noted otherwise. See Background for Planned Streptomycin Data Call-In (DCI) Under EPA No. 2288.04, OMB Control No. 2070-0174 for more information.
Justification for Non-Codified Study
See rationales for required non-guideline studies in the Appendix, at the end of this document.
Non-guideline study rationales:
Study Title: Honey bee adult acute oral toxicity, Tier I |
Rationale for Requiring the Data |
Terrestrial invertebrates are likely to be impacted if exposed to pesticides in various use settings. Pesticide residues may be transferred to pollen and/or nectar of treated plants and subsequently brought back to the hive. Therefore, potential acute effects to adult honey bees and other insect pollinators from oral exposure to some pesticides could exist. Currently available toxicity studies do not address possible effects of oral exposure on adult terrestrial insect survival. Because of the potential for pollen and nectar to be contaminated with pesticide residues, and subsequently brought back to the hive, it is important to determine the acute oral toxicity of this compound to adult honey bees and other insect pollinators.
The Office of Pesticide Programs has made guidance available regarding ecological testing for bees using the honey bee as a surrogate test species. The guidance discusses Tier I laboratory-based acute oral toxicity studies of individual adult bees as a critical component of the screening-level risk assessment process for examining potential adverse effects from specific routes of exposure. The guidance can be found at: http://www2.epa.gov/pollinator-protection/pollinator-risk-assessment-guidance. Additional guidance on the honey bee acute oral toxicity test design can be found in OECD Test Guideline 213: http://www.oecd-ilibrary.org/environment/test-no-213-honeybees-acute-oral-toxicity-test_9789264070165-en. |
Practical Utility of the Data |
How will the data be used? The Tier I acute oral toxicity data on adult honey bees serve as a foundation for the screening-level assessment of potential risk to non-target organisms, such as federally listed threatened or endangered and non-listed terrestrial invertebrate insects, including pollinators, from acute oral exposures to pesticides. The data will be used to reduce uncertainties associated with the risk assessment for terrestrial invertebrates and will improve EPA’s understanding of the potential direct and indirect effects on a broad range of taxa. This study will also provide information with which to compare whether oral toxicity estimates differ from contact toxicity estimates obtained from other Tier I studies. If acute oral effects data for adult honey bees are not available, risks to terrestrial insects from acute oral exposure will be assumed.
How could the data impact the Agency’s future decision-making? The data will inform the determination required under FIFRA or the ESA as to whether continued registration of a pesticide is likely to result in unreasonable adverse effects to non-target species or is likely to adversely affect federally listed threatened or endangered species and/or modify their designated critical habitat. Without these data, EPA may need to presume risk, which will limit the flexibility of pesticide products to comply with FIFRA and the ESA and could result in use restrictions. |
Study Title: Honey bee larvae acute oral toxicity |
Rationale for Requiring the Data |
Terrestrial invertebrates are likely to be impacted if exposed to pesticides in various use settings. Pesticide residues may be transferred to pollen and/or nectar of treated plants and subsequently brought back to the hive where developing larvae and pupae may be exposed. Therefore, potential adverse effects to developing bees and other insect pollinators could result from exposure to pesticide residues. Available toxicity studies do not address possible effects on brood (larvae and pupae) survival/development. Because of the potential for pollen and nectar to be contaminated with pesticide residues, and subsequently brought back to the hive, it is important to determine the acute toxicity of this compound to bee brood.
The Office of Pesticide Programs has made guidance available regarding ecological testing for bees using the honey bee as a surrogate test species. The guidance discusses Tier I laboratory-based acute toxicity studies of individual honey bee larvae as a critical component of the screening-level risk assessment process for examining potential risks from specific routes of exposure. The guidance can be found at: http://www2.epa.gov/pollinator-protection/pollinator-risk-assessment-guidance. Additional guidance on larval honey bee acute oral toxicity test design can be found in OECD Test Guideline 237: http://www.oecd-ilibrary.org/environment/test-no-237-honey-bee-apis-mellifera-larval-toxicity-test-single-exposure_9789264203723-en. |
Practical Utility of the Data |
How will the data be used? The Tier I acute toxicity data on honey bee larvae serve as a foundation for the screening-level assessment of potential risk to non-target organisms, including federally listed threatened or endangered and non-listed terrestrial invertebrates, including pollinators, and/or modify their designated critical habitat from acute oral exposures to pesticides. The data will be used to reduce uncertainties associated with the risk assessment for terrestrial invertebrates and will improve EPA’s understanding of the potential effects on terrestrial species and whether there is a differential sensitivity of larval bees relative to adult bees. If acute effects data for larvae are not available, risks to terrestrial insects from acute exposure will be assumed.
How could the data impact the Agency’s future decision-making? The data will inform the determination required under FIFRA or the ESA as to whether continued registration of a pesticide is likely to result in unreasonable adverse effects to non-target species or is likely to adversely affect federally listed threatened or endangered species and/or modify their designated critical habitat. Without these data, EPA may need to presume risk, which will limit the flexibility of pesticide products to comply with FIFRA and the ESA and could result in use restrictions. |
Study Title: Honey bee larvae chronic oral toxicity |
Rationale for Requiring the Data |
Terrestrial invertebrates are likely to be impacted if exposed to pesticides in various use settings. Pesticide residues may be transferred to pollen and/or nectar of treated plants and subsequently brought back to the hive where larvae and pupae may be exposed. Therefore, potential effects to developing bees could result from chronic oral exposure to pesticide residues. Available toxicity studies do not address possible chronic effects on brood (larvae and pupae) survival. Because of the potential for pollen and nectar to be contaminated with pesticide residues, and subsequently brought back to the hive, it is important to determine chronic larval/pupal toxicity and whether adult emergence is adversely affected. This study will provide information on whether honey bee larvae differ in sensitivity from adult bees following chronic exposure.
The Office of Pesticide Programs has made guidance available regarding ecological testing for bees using the honey bee as a surrogate test species. The guidance discusses Tier 1 laboratory-based chronic toxicity studies of individual honey bee larvae as a critical component of the screening-level risk assessment process for examining potential risks from specific routes of exposure. The guidance can be found at: http://www2.epa.gov/pollinator-protection/pollinator-risk-assessment-guidance. Additional information on the larval honey bee repeated exposure (chronic) toxicity test design can be found in the OECD guidance document: https://one.oecd.org/document/ENV/JM/MONO(2016)34/en/pdf. |
Practical Utility of the Data |
How will the data be used? The Tier I chronic oral toxicity data on bee larvae serve as a foundation for the screening-level assessment of potential risk to non-target organisms including federally listed threatened or endangered and non-listed terrestrial invertebrate insects, including pollinators, from chronic oral exposures to pesticides. The data will be used to reduce uncertainties associated with the risk assessment for terrestrial invertebrates and will improve EPA’s understanding of the potential direct and indirect lethal and sublethal effects on a broad range of terrestrial species, particularly insect pollinators. These data will also assist in determining whether early life stages of the bee differ in their sensitivity to pesticides relative to adults. If chronic oral effects data for larvae are not available, risks to terrestrial insects from chronic oral exposure will be assumed.
How could the data impact the Agency’s future decision-making? The data will inform the determination required under FIFRA or the ESA as to whether continued registration of a pesticide is likely to result in unreasonable adverse effects to non-target species or is likely to adversely affect federally listed threatened or endangered species and/or modify their designated critical habitat. Without these data, EPA may need to presume risk, which will limit the flexibility of pesticide products to comply with FIFRA and the ESA and could result in use restrictions. |
Study Title: Honey bee adult chronic oral toxicity |
Rationale for Requiring the Data |
Terrestrial invertebrates are likely to be impacted if exposed to pesticides in various use settings. Pesticide residues may be transferred to pollen and/or nectar of treated plants and subsequently brought back to the hive. Therefore, potential chronic effects to adult honeybees and other pollinators from oral exposure to some pesticides could exist. Currently available toxicity studies do not address possible lethal and sublethal effects of chronic oral exposure on adult terrestrial invertebrates and will assist in determining whether the sensitivity of adult bees differs from that of earlier life stages. Because of the potential for pollen and nectar to be contaminated with pesticide residues, and subsequently brought back to the hive, it is important to determine the chronic oral toxicity of this compound to adult honeybees and other pollinators.
The Office of Pesticide Programs has made available guidance regarding ecological testing for invertebrates with the honeybee. The guidance discusses Tier I laboratory-based chronic oral toxicity studies of individual adult honeybees as a critical component of the screening-level risk assessment process for examining potential risks from specific routes of exposure. The guidance can be found at: http://www2.epa.gov/pollinator-protection/pollinator-risk-assessment-guidance. Study design elements for the chronic 10-day oral toxicity test with honeybees are similar to the OECD Test Guideline 213 acute oral toxicity test http://www.oecd-ilibrary.org/environment/test-no-213-honeybees-acute-oral-toxicity-test_9789264070165-en. |
Practical Utility of the Data |
How will the data be used? The Tier I chronic oral toxicity data on adult bees serve as a foundation for the screening-level assessment of potential risk to non-target organisms including federally listed threatened or endangered species and non-listed terrestrial invertebrate insects, including pollinators, from chronic oral exposures to pesticides. The data will be used to reduce uncertainties associated with the risk assessment for terrestrial invertebrates and will improve EPA’s understanding of the potential direct and indirect lethal and sublethal effects on a broad range of terrestrial species, particularly insect pollinators and to determine whether adult toxicity differs substantially from other life stages evaluated in other Tier I tests. If chronic oral effects data for adults are not available, risks to terrestrial insects from chronic oral exposure will be assumed.
How could the data impact the Agency’s future decision-making? The data will inform the determination required under FIFRA or the ESA as to whether continued registration of a pesticide is likely to result in unreasonable adverse effects to non-target species or is likely to adversely affect federally listed threatened or endangered species and/or their designated critical habitat. Without these data, EPA may need to presume risk, which will limit the flexibility of pesticide products to comply with FIFRA and the ESA, and could result in use restrictions. |
Study Title: Tier II Semi-field testing for pollinators (tunnel or colony feeding studies) |
Rationale for Requiring the Data |
Tier II studies are conditional on the outcome of the screening-level assessment where acute and/or chronic risk levels of concern have been exceeded for terrestrial invertebrates. Terrestrial invertebrates are likely to be impacted if exposed to pesticides in various use settings. Pesticide residues may be transferred to pollen and/or nectar of treated plants and subsequently brought back to the hive and may adversely affect developing brood (egg, larvae, and pupae) and adult bees. Screening-level (Tier I) studies of individual bees do not address possible effects and/or exposure to pesticide residues at the colony-level. Because of the potential for pollen and nectar to be contaminated with pesticide residues, and subsequently brought back to the hive, it is important to determine whether bee colonies may be negatively affected under relatively controlled exposure conditions of a semi-field study. In addition to providing effects data, these studies can provide data on exposure as pesticide residues in pollen/nectar of treated plants.
The Office of Pesticide Programs has made available guidance regarding ecological testing for invertebrates with the honeybee. The guidance describes the tiered testing process and can be found at: http://www2.epa.gov/pollinator-protection/pollinator-risk-assessment-guidance. Additional information on honeybee colony studies under semi-field conditions can be found in the OECD Guidance 75 http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono%28.
|
Practical Utility of the Data |
How will the data be used? Tier II colony-level data will be used to assess potential risk to non-target organisms including listed and non-listed terrestrial social invertebrate species and to determine whether effects observed in the screening-level (Tier I) laboratory-based studies of individual bees are evident in colony-level studies under semi-field conditions. The Tier II semi-field test of whole colonies is a relatively controlled study, i.e., bees are confined to a specific area, that is designed to represent potential field-level exposure and account for hive dynamics, which are not achievable from other pollinator studies. This study will be used to determine whether adverse effects to insect pollinators at the whole colony level, may result for the use of pesticides and will help to refine risk estimates derived in the screening-level risk assessment for beneficial terrestrial invertebrates. Measured residues in pollen/nectar can also be used to refine risk estimates derived from model-based or default values in the screening-level assessment.
How could the data impact the Agency’s future decision-making? The data will inform the determination required under FIFRA or the ESA as to whether continued registration of a pesticide is likely to result in unreasonable adverse effects to non-target species or is likely to adversely affect federally listed threatened or endangered species and/or their designated critical habitat. Without these data, EPA may need to presume risk, which will limit the flexibility of pesticide products to comply with FIFRA and the ESA, and could result in use restrictions. |
Study Title: Residues in pollen and nectar/ field residue analysis Tier II |
Rationale for Requiring the Data |
Terrestrial invertebrates are likely to be impacted if exposed to pesticide residues in various use settings. Pesticide residues may be transferred to pollen and/or nectar of treated plants and subsequently brought back to hive where all life stages may be exposed. For some pesticides, the quantification of pollinator-relevant residues in treated flowering plants is needed, since pollinators will be exposed to residues from either current or prior season applications (due to the potential for residues to accumulate in plants and trees). Residues in edible/transportable-to-hive parts of treated trees and plants, including (where appropriate), but not limited to, guttation water, sap/resins, whole plant tissue (e.g., leaves, stems), as well as blooming, pollen-shedding, and nectar producing parts (i.e., flowers and, if present, extra-floral nectaries) of plants may inform the potential for risk. Studies should be designed to provide residue data for crops and application methods of concern.
The Office of Pesticide Programs has made available guidance regarding ecological testing for invertebrates with the honeybee. The guidance can be found at: http://www2.epa.gov/pollinator-protection/pollinator-risk-assessment-guidance.
|
Practical Utility of the Data |
How will the data be used? Measured residue data will be used to refine conservative estimates of pesticide exposure and reduce uncertainties associated with the Tier I exposure assessment by providing direct measurements of pesticide concentrations resulting from actual use settings. Measured residues may provide a more realistic understanding of exposure through contact or ingestion with which to calculate risk quotients for individual bees as well as to characterize exposure to the colony. If measured residue data are not available, risk estimates for terrestrial insects will be based on model-generated or default values used to support the screening-level assessment.
How could the data impact the Agency’s future decision-making? The data will inform the determination required under FIFRA or the ESA as to whether continued registration of a pesticide is likely to result in unreasonable adverse effects to non-target species or is likely to adversely affect federally listed threatened or endangered species and/or their designated critical habitat. Without these data, EPA may need to presume risk, which will limit the flexibility of pesticide products to comply with FIFRA and the ESA, and could result in use restrictions. |
Study Title: Chronic sediment toxicity test with Chironomus riparius (with TGAI ) |
Rationale for Requiring the Data |
The submitted daphnid life cycle study was classified as supplemental and is useful for risk assessment purposes. However, the midge (Chironomus riparius) may be more sensitive to streptomycin than the daphnid. There is uncertainty regarding the sensitivity of midges to streptomycin on a chronic exposure basis and EPA is requiring a chronic toxicity study of streptomycin using the midge in a spiked water system. The 28-day study (OCSPP 850.1735) should measure the chronic and reproductive effects of streptomycin on the midge, including survival, growth, and emergence. |
Practical Utility of the Data |
How will the data be used? The data will be used to assess risk to non-target listed and non-listed aquatic invertebrate species. These data would allow the Agency to refine the screening level risk assessment for beneficial terrestrial invertebrates to determine whether streptomycin use causes unreasonable adverse effects to these taxa. Exposure data is an integral part of determining the potential for risk to beneficial terrestrial invertebrates through direct exposure from their food sources.
How could the data impact the Agency’s future decision-making? The data EPA intends to call in are necessary to inform the determination required under FIFRA or the ESA as to whether continued registration of a pesticide is likely to result in unreasonable adverse effects to non-target species or is not likely to jeopardize threatened or endangered species or its critical habitat. The lack of these data will require EPA to make conservative assumptions in its effects determinations and could result in EPA determining that more severe use restrictions are necessary. In addition, the lack of these data may result in an uncertain assumed risk and potential mitigation of streptomycin formulations under FIFRA. |
Study Title: Chronic sediment toxicity test with the freshwater amphipod Hyalella azteca (with TGAI ) |
Rationale for Requiring the Data |
The submitted daphnid life cycle study was classified as supplemental and is useful for risk assessment purposes. However, the amphipod (Hyalella azteca) may be more sensitive to streptomycin than the daphnid. There is uncertainty regarding the sensitivity of amphipods to streptomycin on a chronic exposure basis and EPA is requiring a chronic toxicity study of streptomycin using the amphipod in a spiked water system. The 10-day study (OCSPP 850.1735) should measure the chronic and reproductive effects of streptomycin on the amphipod, including survival, growth, and emergence.
|
Practical Utility of the Data |
How will the data be used? The data will be used to assess risk to non-target listed and non-listed aquatic invertebrate species. These data would allow the Agency to refine the screening level risk assessment for beneficial terrestrial invertebrates to determine whether streptomycin use causes unreasonable adverse effects to these taxa. Exposure data is an integral part of determining the potential for risk to beneficial terrestrial invertebrates through direct exposure from their food sources.
How could the data impact the Agency’s future decision-making? The data EPA intends to call in are necessary to inform the determination required under FIFRA or the ESA as to whether continued registration of a pesticide is likely to result in unreasonable adverse effects to non-target species or is not likely to jeopardize threatened or endangered species or its critical habitat. The lack of these data will require EPA to make conservative assumptions in its effects determinations and could result in EPA determining that more severe use restrictions are necessary. In addition, the lack of these data may result in an uncertain assumed risk and potential mitigation of streptomycin formulations under FIFRA. |
Study Title: Chronic sediment toxicity test with the saltwater invertebrate Leptocheirus plumulosus (with TGAI ) |
Rationale for Requiring the Data |
The submitted daphnid life cycle study was classified as supplemental and is useful for risk assessment purposes. However, the saltwater invertebrates may be more sensitive to streptomycin than the daphnid. There is uncertainty regarding the sensitivity of saltwater amphipods (Leptocheirus plumulosus) to streptomycin on a chronic exposure basis and EPA is requiring a chronic toxicity study of streptomycin using the amphipod in a spiked water system. The 10-day study (OCSPP 850.1740) should measure the chronic and reproductive effects of streptomycin on the amphipod, including survival, growth, and emergence.
|
Practical Utility of the Data |
How will the data be used? The data will be used to assess risk to non-target listed and non-listed aquatic invertebrate species. These data would allow the Agency to refine the screening level risk assessment for beneficial terrestrial invertebrates to determine whether streptomycin use causes unreasonable adverse effects to these taxa. Exposure data is an integral part of determining the potential for risk to beneficial terrestrial invertebrates through direct exposure from their food sources.
How could the data impact the Agency’s future decision-making? The data EPA intends to call in are necessary to inform the determination required under FIFRA or the ESA as to whether continued registration of a pesticide is likely to result in unreasonable adverse effects to non-target species or is not likely to jeopardize threatened or endangered species or its critical habitat. The lack of these data will require EPA to make conservative assumptions in its effects determinations and could result in EPA determining that more severe use restrictions are necessary. In addition, the lack of these data may result in an uncertain assumed risk and potential mitigation of streptomycin formulations under FIFRA. |
1 Doubled because test is for two species.
2 Doubled because test is for two species.
Page
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Gary |
| File Modified | 0000-00-00 |
| File Created | 2023-08-28 |
© 2026 OMB.report | Privacy Policy