Form CALC- (W, 1, 2, 3) CALC- (W, 1, 2, 3) STUDY PROTOCOL FOR A COMPASSIONATE AQUACULTURE INVESTIGA
Administration of U.S. Fish and Wildlife Service Investigational New Animal Drug (INAD) Program
Calceinpro revised protocol for website
Calcein (Se–Mark®) INAD #10–987 - Private Sector
OMB:
OMB Control No. 1018-####
Expires ##/##/20##
STUDY PROTOCOL FOR A COMPASSIONATE AQUACULTURE
INVESTIGATIONAL NEW ANIMAL DRUG (INAD)
EXEMPTION FOR CALCEIN (SE-MARK®)
(INAD #10-987)
Sponsor:
U.S. Fish and Wildlife Service, Division of the National Fish Hatchery System
______________________ ___________________
Sponsor Signature Date Approved
Manufacturer:
Western Chemical Inc.
1269 Lattimore Road
Ferndale, WA 98248
Facility for Coordination of Calcein (SE-MARK®) INAD:
Aquatic Animal Drug Approval Partnership Program
4050 Bridger Canyon Road
Bozeman, Mt 59715
Proposed Starting Date June 1, 2008
Proposed Ending Date May 31, 2012
Study Director Mr. Jim Bowker
_________________________ ________________
Study Director Signature Date
Clinical Field Trial Location and Trial Number:
__________________________________________ _________________
Type or Print Facility Name Trial Number
Investigator______________________________________________________
Type or Print Name
__________________________________________ _________________
Investigator Signature Date
I. STUDY IDENTIFICATION AND TITLE 3
III. INVESTIGATORS/FACILITIES 3
IV. PROPOSED STARTING AND COMPLETION DATES: 4
XII. TREATMENT RESPONSE PARAMETERS 15
XIII. FORMS FOR DATA COLLECTION 16
XIV. RECORD KEEPING PROCEDURES 17
XV. DISPOSITION OF INVESTIGATIONAL ANIMALS 17
XVI. DISPOSITION OF INVESTIGATIONAL DRUG 18
XVII. DATA HANDLING, QUALITY CONTROL, MONITORING, ADMINISTRATIVE RESPONSIBILITIES 18
XVIII. PLANS FOR DATA ANALYSIS 20
XIX. PROTOCOL AND PROTOCOL AMENDMENTS 20
Appendix IV. Material Safety Data Sheet (MSDS) for Calcein 23
Form CALC-W: Worksheet 24
FORM CALC-1: Report on Receipt of Drug 26
STUDY PROTOCOL FOR A COMPASSIONATE AQUACULTURE INVESTIGATIONAL NEW ANIMAL DRUG (INAD) EXEMPTION FOR CALCEIN (SE-MARK®) UNDER INAD #10-987
I. STUDY IDENTIFICATION AND TITLE
Clinical field trials to determine the efficacy of calcein (SE-MARK®) for use in the skeletal marking of freshwater and marine finfish, and freshwater mussels. INAD #10-987.
Dr. David Erdahl, U.S. Fish and Wildlife Service, Branch Chief, Aquatic Animal Drug Approval Partnership (AADAP) Program, 4050 Bridger Canyon Road, Bozeman, MT 59715; Phone: 406‑994‑9904; Fax: 406‑582‑0242; Email:
Manufacturer: Western Chemical, Inc.
1269 Lattimore Road
Ferndale, WA 98248
Contact Person at Western Chemical, Inc.:
Ron Malnor
Ph. 1-800-283-5292
Fax: 360-384-0270
Study Director: Mr. Jim Bowker
U.S. Fish and Wildlife Service ‑ AADAP
4050 Bridger Canyon Road
Bozeman, MT 59715
Phone: 406‑994‑9910
Fax: 406‑582‑0242
Email: [email protected]
Clinical Field Trial Coordinator: Ms. Bonnie Johnson, USFWS - AADAP
INAD Study Monitors: See Appendix II for names and addresses.
See Appendix IIIa for names and addresses.
IV. PROPOSED STARTING AND COMPLETION DATES:
Proposed Starting Date: June 1, 2008
Proposed Completion Date: May 31, 2012
A. Background
Fisheries management programs throughout the United States are dependent upon artificial propagation and stock supplementation to insure the maintenance of healthy wildstock populations. Restoration/recovery, mitigation, subsistence, recreational, and commercial fisheries programs are all dependent to some extent on stock supplementation to meet management strategies and maintain viable populations. As the popularity of fishing and associated recreation continues to expand, the pressure on existing fish populations will also continue to increase.
Historically, many fish stocking programs were based on the relatively simple premise that the more fish stocked, the better the odds of enhancing wildstock populations. As a result, many stocking programs were little more than a numbers game, where “more” was always better. While this philosophy was obviously a bit of an oversimplification of resource needs and resulted in the somewhat indiscriminate stocking of large number of fish, it was not without success. The subsequent establishment of viable populations of wildstock fish populations (both native and non-native) throughout the United States is witness to this fact. None-the-less, the evolution of fisheries management over the years has recognized the need to evaluate all stock supplementation programs on a case-by-case basis. This evaluation should include not only a pre-plant justification of stocking need and potential basin-wide impacts, but also a post-plant evaluation to determine the overall impact of stock supplementation, including the fate of stocked fish.
Over the years, a multitude of methods have been developed to mark or tag individual fish prior to stocking. Commonly used external tags have included floy tags, spaghetti tags, wire tags, jaw tags, freeze brands, and visual implant tags. A number of internal tags have also been used including coded wire tags and passive induction transponders. While these methodologies have, and continue to be, widely used by fisheries managers to evaluate stock supplementation programs, they are labor intensive as they require the handling and marking of individual fish. As fish must be individually handled and virtually all tags result in some degree of perturbation, these techniques may result in a significant degree of stress to pre-release fish. Although somewhat dependent upon tag type, these methodologies can also result in a significant cost.
More recently, fisheries managers have become increasingly interested in methodologies for the mass marking of larval or juvenile fishes. Fish culturists have found that the immersion of young fish in oxytetracycline hydrochloride will mark otoliths with single or multiple marks (Hettler, 1984; Secor et. al, 1991; Brooks et. al, 1994). Immersion marking allows fish to be mass marked with minimal handling, low cost, and minimal labor. Oxytetracycline marking has been relatively widely used in a variety of fish species with varying degrees of success. Primary factors that affect the usefulness of oxytetracycline marking include mark retention and mark detection. Although it has been reported that oxytetracycline mark retention is generally “good”, (Secor et. al, 1991; Brooks et. al, 1994), mark retention is somewhat variable dependent upon species and life stage at the time of treatment. Oxytetracycline mark detection is a quite laborious process. Mark detection requires that fish are sacrificed, otoliths removed and mounted, and otolith slides then viewed with a fluorescent microscope. Otolith processing and mounting alone requires approximately 30 min per fish.
Calcein is a fluorochrome compound that chemically binds with alkaline earth metals such as calcium, and upon binding, shows a marked increase in fluorescence when excited with blue light of about 500 nm wavelength. Calcein has been used an indicator to determine calcium content in limestone and gypsum, as a flourescent marker for elasmobranch vertebral cartilage, as a stain for photography and angiography of the eye, and to assess tear exchange in the fitting of soft contact lenses (Diehl and Ellinboe, 1956; Gelsleichter et. al, 1997; Oncel et. al, 1990; and Refojo et. al, 1972). Calcein has also been evaluated as a method of marking fish otoliths (Wilson et al. 1987; Beckman et al. 1990; Brooks et al. 1994; Bumguardner and King 1996) as well as fin rays, scales, and other calcified tissues (Alcobendas et al. 1991; Gelsleichter et al. 1997; Mohler 1997, Leips et al. 2001; Mohler et al. 2002). In addition, calcein has been used experimentally on freshwater mussels (Eads and Layzer In Press) marine mussels (Kaehler and McQuaid 1999) gastropods (Day et al. 1995) as well as brachiopods and other marine organisms (Rowley and Mackinnon 1995).
These studies indicated that immersion marking of fish using calcein resulted in a bright green fluorescent mark that was similar in width, intensity, and duration to the mark produced by oxytetracycline. General conclusions from these studies also indicated that immersion marking in calcein was as effective, if not more effective, than similar immersion in oxytetracycline.
In 1995, the U.S. Fish and Wildlife Service’s Northeast Fishery Center (NEFC), Lamar, PA initiated a series of studies investigating the use of calcein immersion to mass mark the otoliths of larval Atlantic salmon. Results of these studies have shown that not only does calcein treatment mark otoliths, it also produces a brilliant green fluorescence in fin rays and scales. Marks on fin rays and scales were easily visible using a hand-held or bench-top fluorescent detection device on live fish. Using refined procedures, calcein marks at the base of the pelvic and pectoral fins have been found to be readily visible on 100% of 2½ yr-old fish maintained at the NEFC. To date, no deleterious effects have been observed in fish treated with calcein. Larval fish marked with calcein at the NEFC in 1995 have been reared to maturity and successfully spawned. The ability to mass mark fin rays and observe marks on live fish would be of obvious benefit to fisheries managers.
Important parameters for an effective and practical procedure for the mass-marking of larval fish include: 1) the mark must be easy to apply simultaneously to large numbers of fish; 2) the mark must be easy to detect under both laboratory and field conditions; 3) the mark must be non-lethally detectable; and 4) the mark must be detectable for years after application to very young fish. Prior to studies conducted on calcein by the NEFC, no mass marking technique was available that met all of these criteria. Obviously, a new animal drug approval for the use of calcein to mass-mark larval fish would be of tremendous benefit to fish restoration and aquaculture programs throughout the United States.
B. Purpose of INAD:
The purpose of this compassionate INAD for calcein (SE-MARK®) is to develop clinical efficacy field trial data that will be used to determine the most appropriate treatment regime for the use of calcein (SE-MARK®) to mark otoliths, fin rays, scales, or other calcified tissues in larval and juvenile finfish and mussels. The use of calcein (SE-MARK®) in a variety of fish and mussel species will be evaluated. These data will be used to support a new animal drug application (NADA) for calcein (SE-MARK®).
The U. S. Fish and Wildlife Service (USFWS) anticipates requesting the U.S. Food and Drug Administration (FDA) to grant extensions of this INAD for additional years. The USFWS believes that data from at least 3-4 treatment seasons will be required in order to adequately assess the efficacy of calcein (SE-MARK®) as a marking agent for use in fish and mussels, and to collect sufficient data to support a NADA(s).
The two major objectives of this study protocol are as follows:
1. Collect scientific data necessary to establish the effectiveness of calcein (SE-MARK®) for use in the skeletal marking of freshwater and marine finfish, and freshwater mussels.
2. Provide an opportunity for fish culturists and fisheries managers to legally use calcein (SE-MARK®) as a marking agent to effectively manage finfish and mussel stocks during the period of time necessary for collection of efficacy, safety, and residue data required for an NADA(s) for calcein (SE-MARK®) use in finfish and mussels.
A. Test and control articles:
1. Drug Identity
a. Active ingredient
Trade Name: SE-MARK®
Common Name: Calcein
Chemical Name: Calcein solution: Bis[N,N-bis(carboxymethyl)aminomethyl]fluorescein
C.A.S. Registry No.: 1461-15-0
Molecular Formula: C30H26N2O13
Formula Weight: 622.5
Form: Liquid
Color: Yellow/green tint
Odor: Slight
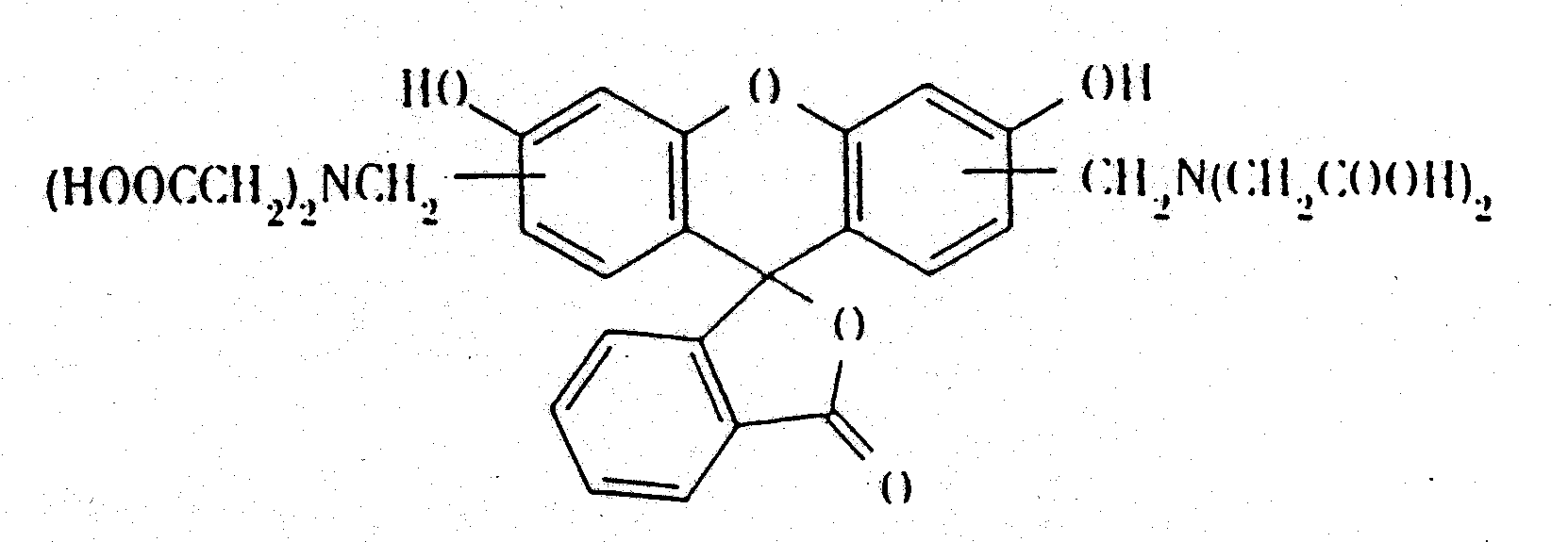
Chemical structure:
b. Strength and dosage form
1.0% solution
c. Manufacturer, source of supply
Western Chemical, Inc.
1269 Lattimore Road
Ferndale, WA 98248
Contact Person for Calcein (SE-MARK®) at Western Chemical, Inc. is:
Ron Malnor
Ph: 1-800-283-5292
Fax: (360) 384-0207
The shipment procedure for calcein (SE-MARK®) is as follows: Western Chemical, Inc. (Ron Malnor) to Investigators (See Section VII.A.6 Accountability [page 7] for details and Appendix IIIa for names and addresses of Investigators).
2. Verification of drug integrity/strength:
The manufacturer (Western Chemical, Inc.) will provide the analytical data necessary to establish the purity of each lot/batch of calcein (SE-MARK®) used under INAD 10-987. Western Chemical, Inc. will also provide analytical support in the event any questions arise regarding product quality. The lot number and date of manufacture for each batch of calcein (SE-MARK®) will be placed on the label of each container by the manufacturer. The form Report on Receipt of Drug - Guide for Reporting Investigational New Animal Drug Shipments for Poikilothermic Food Animals (Form CALC-1) will clearly identify the lot number and date of manufacture of calcein (SE-MARK®) shipments. If the integrity of the calcein (SE-MARK®) is compromised (i.e., by spilling or contamination of the stock container) the event will be carefully recorded, dated, and signed in the Chemical Use Log (Form CALC-2). The Study Monitor assigned to the Investigator involved will be immediately notified and the remaining material will be returned to the Study Monitor along with the properly recorded Form CALC-1.
3. Storage Conditions
Calcein (SE-MARKTM) will be stored in the original container supplied by the Manufacturer with the appropriate investigational label attached. Calcein (SE-MARK®) has high stability and should be stored at room temperature in a dry location away from direct sunlight, or in a refrigerator. Calcein (SE-MARK®) should be stored in a secure location.
4. Handling Procedures
Each Study Monitor and Investigator will be required to have a current copy of the Material Safety Data Sheet (MSDS) for calcein (SE-MARK®; Appendix IV). Each person involved with the study and each person who may be present during the use of calcein (SE-MARK®) shall be required to read the MSDS. Safety precautions as outlined in the MSDS will be followed at all times when working with calcein (SE-MARK®). Standard laboratory equipment such as gloves, lab coats or aprons, eye protection, etc., will be worn at all times.
5. Investigational labeling
A copy of the label to be attached to each container of calcein (SE-MARK®) is provided in Appendix V. If a shipment of calcein (SE-MARK®) is received from the manufacturer that does not contain the investigational label, it is the responsibility of the Investigator to ensure a proper label is affixed to all containers.
6. Accountability
Western Chemical, Inc. will be the sole supplier of calcein (SE-MARK®) to all Investigators under INAD 10-987.
1. USFWS and Non-USFWS Facilities
Immediately upon receiving an order/shipment of calcein (SE-MARK®), the Investigator will complete Form CALC-1 “Report on Receipt of Drug - Guide for Reporting Investigational New Animal Drug Shipments for Poikilothermic Food Animals". The investigator will archive the original in the facilities INAD file, and send a copy to his/her Study Monitor. Both the Investigator and the Study Monitor are required to sign Form CALC-1. The Study Monitor will then forward a copy to the Clinical Field Trial Coordinator at the Aquatic Animal Drug Approval Partnership Program. The Clinical Field Trial Coordinator will archive one copy, and send two copies of Form CALC-1 to FDA. Arrangements should be made between Investigators and Study Monitors to insure completed Form CALC-1s are received by the Clinical Field Trial Coordinator in a timely manner.
All Investigators are also responsible for maintaining an accurate inventory of calcein (SE-MARK®) on-hand. A Chemical Use Log (Form CALC-2) will be supplied to each Investigator. Each time calcein (SE-MARK®) is used, it must be recorded by the Investigator on Form CALC-2.
At the conclusion of the study, all remaining calcein (SE-MARK®) will be shipped to Emerald Services, Inc., 1825 Alexander Avenue, Tacoma, WA 98451 according to procedures detailed in general Waste-stream Profile #216200B. Procedures and forms for Waste-stream Profile #216200B are provided in Appendix VII. Form CALC-2 should be updated by the Investigator, signed, and forwarded to the Study Monitor.
7. Preparation Procedures
Calcein (SE-MARK®) should be prepared according to directions for normal use. This should include accurately measuring out the calculated amount of calcein (SE-MARK®) to obtain the desired dose, adding hatchery water to establish a calcein (SE-MARK®) stock solution, vigorously stirring the solution to ensure thorough mixing, and then uniformly distributing and mixing measured aliquots of stock solution to treatment tank water. To facilitate the preparation of a calcein (SE-MARK®) stock solution pH should be maintained at approximately 7.0 (i.e. near ambient hatchery water pH). Dependent upon pH and buffering capacity of hatchery water, it may be beneficial to add a small amount of sodium hydroxide or acetic acid to adjust the pH (+/-) of the stock solution prior to use.
B. Items Needed for Treatment, Sample Collection, Observations, Etc.:
Treatment and diagnostic equipment should include a graduated cylinder, flask, treatment tank, recovery tank, thermometer, stop watch, dissolved oxygen meter, pH meter, SE-MARK® detection apparatus, and supplemental aeration equipment.
The experimental unit in this clinical field trial will consist of a contained or isolated group of fish. This will generally be a group of fish contained in a tank, raceway, or pond. In some cases, the experimental unit may be individual animals.
A. Facilities/Investigators
The proposed facility and the Investigator must be listed in Appendix IIIa of this Study Protocol before calcein (SE-MARK®) can be ordered and dispensed under this INAD. Last minute deviations can be requested by the Sponsor, Study Director, or by an Investigator in case emergency use-pattern needs should arise (See Section XX). However, it is important to note that poor planning and/or lack of preparation will not be considered an emergency situation.
B. The characteristics of the study animals is presented in Appendices VIa and VIb. Treatment is restricted to fish having a body weight of 2 grams or less and juvenile life-stage mussels.
C. Environmental conditions
Environmental conditions will be variable and include a broad spectrum of temperatures a water quality parameters. Environmental conditions will be reported on Form CALC-3.
D. Ability of investigator to fulfill all the requirements of the Study Protocol
See Appendix IIIb for example of knowledge required of hatchery managers (i.e., Investigators).
Prior to initiating each treatment event, the Investigator must first complete Form CALC-W. “Worksheet for Designing Individual Field Trials” that pertains to each specific treatment event. The worksheet should be filled out, signed, and sent by Fax to the Study Monitor. The Study Monitor will review the planned treatment (worksheet), sign it, and forward (Fax) the paperwork to the Aquatic Animal Drug Approval Partnership (AADAP) Office. The AADAP Office will then review the worksheet, assign the approved treatment a Study Number, and then notify both the Investigator and the Study Monitor of the assigned number and approval to proceed. In most cases, this entire process should be able to be accomplished within a single working day. The Investigator should record the assigned study number on Form CALC-3, as well as on any additional correspondence regarding that specific treatment event. If for some reason the Investigator is unable to reach his/her Study Monitor with regards to worksheet approval, and infection/disease/treatment need is rapidly escalating, the Investigator should contact the AADAP Office for a study number and permission to proceed.
A. A treatment group or experimental unit may be an entire tank, pond, raceway, or group of fish, or it may be individual animals.
B. Non-treated control groups will not be a requirement for clinical field trials evaluating the efficacy of calcein (SE-MARK®) as a marking agent. Fish that are not treated, will obviously not be “marked”. However, Investigators are encouraged to record observations with respect to the behavior and physiological state of fish prior to calcein (SE-MARK®) treatment. This information will provide a “pseudo-control” as to fish condition without, or prior to, calcein (SE-MARK®) treatment.
Although untreated control groups are not a required element of treatment under this INAD exemption and are at the discretion of the Investigator, they are strongly encouraged whenever circumstances permit. Control groups are extremely important to not only document response to treatment, but also to validate potential adverse reactions in treated animals. Assignment to control and treatment groups should be random and designed to avoid bias. It is important that all fish are treated in a similar fashion. If fish are physically moved into separate test groups or different rearing units, caution should be used so that handling and rearing conditions are as similar as possible. Control fish should be kept under conditions as similar as possible to treated fish for valid comparison. Use of control groups will ensure that results of efficacy studies provide useful information that will support a NADA.
Blinded studies can reduce bias in data collection. Whenever possible, investigators should consider methods by which treatment response observations are recorded by individuals who are unaware which fish have been treated, at what dosage levels fish have been treated, and/or which fish are controls.
A. Route of administration
Calcein (SE-MARK®) will be administered as a static immersion bath treatment. Calcein (SE-MARK®) will be prepared according to directions for normal use. Dependant upon the desired dosage, the calculated amount of calcein (SE-MARK®) should be accurately measured out and uniformly distributed and mixed with treatment tank/container water. In some cases, it may be desirable to make a calcein (SE-MARK®) stock solution to facilitate uniform distribution in the treatment tank. In some cases, it may also be desirable to place fish in a screened container within the treatment tank so they can easily be moved into and out of the marking solution.
B. Dose to be administered
Calcein (SE-MARKTM) should be applied as a static immersion bath. Calcein (SE-MARK®) treatment will be restricted to two separate use patterns with respect to treatment dosage that include Option A (125 -250 mg/L); and Option B (2.5 - 5.0 g/L). Within these ranges, the actual concentration applied will be at the discretion of the Investigator. Dosage will likely vary within these ranges with respect to species, life stage, water temperature, and type of mark desired (e.g. otolith vs fin ray/scale mark). Specific restrictions regarding these treatment dosages are provided below.
C. Dosing interval and repetition (note: same for both Option A and Option B)
Calcein (SE-MARK®) will be applied as a single treatment event, or as repeated treatments. Repeated treatments may be conducted to establish multiple marks. If a multiple treatment regimen is used an interval of at least 2 days should be observed between treatment events.
D. Duration of treatment
Option A: Calcein (SE-MARK®) treatment at 125 - 250 mg/L - Finfish and Mussels
Treatment duration will be variable, and dependant on species, life stage, water temperature, and type of mark desired (e.g. otolith or fin ray/scale mark). Duration of calcein (SE-MARK®) treatment will range from 1-6 hr. After completion of calcein (SE-MARK®) treatment, fish or mussels should immediately be moved to fresh water.
Option B: Calcein (SE-MARK®) treatment at 2.5 - 5.0 g/L - Finfish only
Treatment duration will be variable, and dependant on species, life stage, water temperature, and type of mark desired (e.g. otolith or fin ray/scale mark). Duration of calcein (SE-MARK®) treatment will range from 1-7 min (note: it is anticipated that most fish treated under Option B will be pre-treated with a 1-5% solution of non-iodized salt for ~3.5 min to facilitate calcein (SE-MARK®) uptake via osmotic induction). After completion of calcein (SE-MARK®) treatment, fish should immediately be moved to fresh water.
Disposition of marking solution
No discharge of marking solution will be allowed under INAD 10-987. Although calcein (SE-MARK®) solution is a non-hazardous, non-Resource Conservation and Recovery Act (RCRA) regulated liquid, it does require solidification and disposal in a landfill, or incineration. All calcein (SE-MARK®) solution remaining in static baths following completion of treatment should be collected in a secure, leak-proof container that clearly identifies container contents. Based on the non-hazardous, non-regulated status of calcein (SE-MARK®) solution, these containers may be retained on-station for a period of time before disposal. However, all calcein (SE-MARK®) solution must ultimately be disposed of by shipment to Emerald Services, Inc., 1825 Alexander Avenue, Tacoma, WA 98451 according to procedures detailed in general Waste-stream Profile #216200B. Procedures and forms for Waste-stream Profile #216200B are provided in Appendix VII.
Note: Although calcein (SE-MARK®) solution may be retained on station as described above, Investigators are encouraged to properly dispose of calcein (SE-MARK®) solution immediately following completion of treatment.
F. Detailed procedures for drug administration
Standard laboratory equipment such as gloves, lab coats or aprons, eye protection, etc. should be worn at all times when working with calcein (SE-MARK®). The chemical should be accurately measured for each treatment immediately prior to treatment. To aid in the uniform distribution of chemical, calcein (SE-MARK®) may be diluted with fresh water to establish a stock solution prior to addition to the treatment tank.
G. Permissible concomitant therapy
Since efficacy data are being collected during the INAD process, there should be little or no concomitant therapy. Preferably, there should be no other therapy during a period extending from 2 weeks prior to treatment to 2 weeks after treatment. Investigators must be prepared to minimize changes in fish cultural procedures or environmental conditions, and apply no other treatments following treatment with calcein (SE-MARK®). However, if concomitant therapy is required in order to protect valuable fish stocks, it should be fully documented and the efficacy data from the calcein (SE-MARK®) treatment involved should be appropriately labeled.
Procedures for mark detection and determination of mark quality (finfish and mussels)
When exposed to ultraviolet light, calcein exhibits a bright green fluorescence. Optimal fluorescence is typically observed when calcein is exposed to blue light of ~500 nm wavelength. The only commercially available field calcein detection device for use on fish is the SE-MARK® Fluorescent Detector, which is available from Western Chemical, Co., Ferndale, Washington, phone: 1-800-283-5292. Hence, it is anticipated that in all field evaluations and hatchery evaluations mark detection and determination of mark quality will be evaluated using the SE-MARK® detector. To facilitate mark detection, the AADAP Office has 2 SE-MARK® detectors that are available to investigators as “loaners”. The detectors will be distributed on a first-come-first-serve basis, so investigators are encouraged to plan ahead.
As calcein will potentially bind to all calcified structures, it is important that investigators examine a variety of bony structures when evaluating mark detection and quality including: fin rays of all fins (especially pectoral and pelvic); operculum areas; jaw bones (dorsal, ventral, and lateral); and scales. Fish should be evaluated for mark detection and mark quality immediately following treatment. Fish that are retained on station for any period of time after marking (i.e. > 30 days) should be evaluated a second time for mark detection and mark quality as close as possible to the time of release (i.e. within 1 week of stocking). Mark detection and quality should be determined using an ordinal scale (0, 1, 2, and 3) as described on the top of Form CALC-3 Results Report Form. A minimum of 15 fish should be individually examined during each evaluation. As one of primary benefits of calcein marking is the ability to mark fish with an externally visible mark, evaluation of calcified tissues that require sacrifice and dissection of fish (e.g. otoliths) will not be evaluated under INAD 10-987. Although it will be at the discretion of individual investigators to determine which bony structures they choose to evaluate for mark detection and determination of mark quality, the selected structure(s) must be clearly identified on Form CALC -3 Results Report Form.
A number of general factors should be considered during procedures for mark detection and determination of mark quality including: a) quality of the original mark (i.e “time zero” post-treatment); b) size and life stage of fish marked; c) ability of the investigator to see the color green; and d) condition of the fish. These factors are briefly described below.
quality of the original mark .- To obtain highest quality calcein marks always follow established protocols for marking the particular species and life stage of fish being marked and use only SE-MARK® calcein solution. To accurately determine mark retention over time, it is essential to always determine the quality of the original mark.
elapsed time since fish were marked.- Calcein marks are retained at their point of origin in calcified fish tissues. Since growth of fin rays in fish is terminal, the calcein mark will be most intense at the base of fins, particularly if much growth has occurred since fish were first marked. Growth of scales is also terminal, and is similar in pattern to growth rings of trees. Hence, calcein marks will be found on scales at the same location at which they were first induced. The presence of calcein-marked scales on a fish can be observed with the detection device, but accurate verification of multiple marks resulting in a banding pattern may require use of fluorescence microscopy techniques. For inexperienced investigators, it is helpful to have an unmarked fish of the same age/size available for comparison with marked fish.
color recognition of investigator.- Since the calcein mark is manifested as a green fluorescence, the ability of the investigator to recognize this color is obviously essential to mark recognition.
condition of the fish.- Fish are generally held in the investigator’s hand or laid on some other surface during calcein mark detection. Therefore, it is strongly recommended that fish be anesthetized prior to examination to not only facilitate close examination of fin rays, scales, and other calcified structures, but also to minimize trauma to fish. If fish are evaluated within 6 months of marking, a quick glance through the activated detection unit will immediately reveal bright fluorescent marks and the need for anesthesia may not be as great. None-the-less, light anesthesia of all fish that are being evaluated for calcein mark detection is highly recommended to ensure accurate determination of mark presence and quality.
Mark detection procedures are summarized in Appendix VIII.
XII. TREATMENT RESPONSE PARAMETERS
The collection and reporting of source data begins with the decision to treat valuable fish based on hatchery records or field management practices that indicate treatment is warranted. Daily morbidity and mortality records, case history records, as well as any extenuating or mitigating
circumstances that may affect treatment response need to be documented. All pertinent treatment response parameters should be reported on Form CALC-3. Treatment response parameters that should be addressed include the following:
1. Primary Parameters
Primary parameters include the efficacy of the marking procedure, mark retention data (if possible), and morbidity and mortality data related to the marking procedure. Whenever possible, control fish should be included in the clinical field trial. These control fish should be part of the normal population and be held under the same conditions as the treated fish.
2. Secondary Parameters
Secondary parameters include general observations on the effect of treatment on fish behavior and response to routine culture/management activities. Secondary parameters would include such responses as feeding activity, feed consumption, apparent level of stress, negative fish behavior, post-release behavior, etc.
3. Adverse Reactions
Any adverse reaction to treatment should be reported immediately to the Study Monitor, who will in turn notify the Study Director. Such responses might include changes in water quality, extremely negative responses/behavior by the fish, or hazards to the applicator. There is little information on the sensitivity of various fish species to calcein (SE-MARK®). It is possible adverse reactions may occur under certain environmental conditions or with respect to specific species/strains of fish. Careful observation of all treated fish for signs of any adverse reaction to treatment is extremely important, and all observations of adverse reactions should be documented. If any signs of drug toxicity are detected, they should also be documented and immediately reported to the Study Monitor, who will in turn notify the Study Director.
Note: Investigators are strongly encouraged to record observations/comments with respect to all phases of treatment. This may include a description of events before, during, and post-treatment. All extenuating or mitigating treatment circumstances need to be described in detail. Such information is imperative so that accurate study/data analysis can be performed.
4. Procedures for the determination of mark quality
As a result of the potential diversity of species, specific life stages, and environmental conditions involved in these studies, Investigators are encouraged to provide detailed descriptions of all study variables. Investigators may also choose to create their own forms for purposes of recording source data under this INAD. Supplementary data forms should be attached to Form CALC-3.
XIII. FORMS FOR DATA COLLECTION
When the Study Protocol has been approved and treatments are scheduled, the Investigator at each facility covered by Calcein (SE-MARK®) INAD 10-987 will need to complete the following forms:
Form CALC-W. Worksheet for Designing Individual Field Trials
Form CALC-1. Report on Receipt of Drug - Guide for Reporting Investigational New Animal Drug Shipments for Poikilothermic Food Animals
Form CALC-2. Chemical Use Log for Clinical Field Trials Using Calcein (SE-MARK®) under INAD #10-987
Form CALC-3. Results Report Form for use of Calcein (SE-MARK®) under INAD #10-987
Copies of these forms are attached to this Study Protocol.
XIV. RECORD KEEPING PROCEDURES
The data should be recorded in permanent ink (preferably black). The data should be recorded on the official data record forms at the time the observations are made. The raw data should be original, i.e., they should be the first recording of the observations, rather than a transcription of original observations to another data sheet. Each original data sheet should be legibly signed and dated by the person making the observation and recording the entry. If more than one person makes and records the observations, entries should be properly attributed to each person. The data should be accurate and legible. If a mistake is made, it should be crossed out using a single strike-through and the correct data should be recorded next to it; each change to the raw data should be initialed and dated by the person making the change, and a statement should be provided explaining why the change was made. If the data sheet needs to be copied, all data should be transferred, including the properly noted changes; the original record should be retained and submitted with the revised copy, along with a memo explaining the reason for the copying.
XV. DISPOSITION OF INVESTIGATIONAL ANIMALS
Animals that die during treatment should be disposed of by burial or incineration. No investigational withdrawal time is required for fish weighing 2 grams because the body weight restriction ensures a long inherent withdrawal time. No investigational withdrawal time is necessary for fish (of any size) that are classified as endangered. No investigational withdrawal is necessary for mussels due to their treatment at an early life-stage (i.e., juveniles) and the limited human consumption of freshwater mussels.
No slaughter authorization is provided for fish weighing greater than 2 grams.
No withdrawal period shall be required for dead fish that will be buried or rendered into non-edible products. The Investigator must record the disposition of all treated fish on Form CALC-3.
XVI. DISPOSITION OF INVESTIGATIONAL DRUG
Calcein (SE-MARK®) will be used only in the manner and by the individuals specified in the Study Protocol. At the conclusion of the study, all remaining calcein (SE-MARK®) will be shipped to the Study Monitor along with the properly recorded Chemical Use Log (Form CALC-2). The Study Monitor will then verify the Drug Inventory Form against the quantity of calcein (SE-MARK®) remaining. All remaining calcein (SE-MARK®) will then be returned to the Manufacturer. The investigational drug may not be redistributed to others not specified by the protocol and may not be retained by the Investigator after completion of the study.
XVII. DATA HANDLING, QUALITY CONTROL, MONITORING, ADMINISTRATIVE RESPONSIBILITIES
A. Drug distribution
See Section VII.A.6. Accountability (page 6) for information and details.
B. Study Monitors
The Study Monitors are generally fish health professionals with experience in diagnosing and treating fish diseases. There is one Study Monitor assigned to each facility within the USFWS that is covered by the calcein (SE-MARK®) INAD. Non-Service facilities must have a similar Study Monitor - Investigator relationship in place. A list of Study Monitors, along with addresses and phone numbers, can be found in Appendix II. The Study Monitors are responsible for supervision of the trials, adherence of the Investigator to the Study Protocol, and inspection of the site.
C. Special equipment and materials
Most of the equipment and materials required for this study, with the exception of the calcein (SE-MARK®) itself and the SE-MARK® detection apparatus, are already available at each participating facility. The treatment of fish with various chemicals is a relatively common occurrence at most fish hatcheries and in many fisheries management programs.
Fish hatchery managers and fisheries managers (i.e., Investigators) are well trained and well equipped to supervise these procedures (see Appendix IIIb). If any additional equipment or materials are required, they will be provided by the Study Monitors (See Section VII.B. Items needed for sample collection, observations, etc., page 7).
D. Administrator of the drug
Calcein (SE-MARK®) will be administered directly by the assigned Investigator (fish hatchery manager or fisheries manager) or under the Investigator's direct supervision (see Appendix IIIa for names). Calcein (SE-MARK®) will be maintained in a secure location, and only the Investigator or a person under his/her direct supervision will have access.
E. Drug accountability records
See Section VII.A.6. Accountability (page 6) for details and Form CALC-W, Form CALC-1, Form CALC-2, and Form CALC-3 for actual forms to be used in the study.
F. Recording observations
The Investigator or a person under his/her direct supervision will be responsible for implementing the Study Protocol, making observations, collecting samples, and recording data during the clinical field trials. After the data have been collected and recorded on the forms, the Investigator will send the data to the Study Monitors who will ensure that all required information is provided. The Study Monitors will in turn send the data to the Study Director. The Study Director will analyze and summarize the data and prepare an annual report that will be submitted to the FDA. Note: If the Study Monitor does not think all required information has been provided, or forms have not been satisfactorily completed, he/she should contact the Investigator and rectify the situation before forwarding the package to the Study Director.
G. Data storage
The Investigator is responsible for complete and accurate data collection. The Investigator is also responsible for archiving a complete set of all original data. A copy of Form CALC-1 should be sent immediately to the Study Monitor, who will in turn forward a copy to the Study Director. Original raw data on Form CALC-2 should be retained by the Investigator until completion of the calendar year, at which time copies should be sent to the Study Monitor. Original raw data on Form CALC-3 should be retained by the Investigator until completion of the study, at which time copies should be sent to the Study Monitor. Study Monitors should carefully check each set of data for accuracy and completeness. If there are any discrepancies in the data, the Study Monitor should contact the Investigator immediately to rectify the problem. After review, Study Monitors should forward all data to the Study Director. As stated above, a complete set of raw data should be archived by the Investigator. All data should be stored in a secure place. Another complete data set (copies) will be archived by the Study Director.
Form CALC-3 Results Report Form is to be completed no later than 30 days after a course of therapy is completed. The purpose of this form and supplementary data is to document the results of the treatment. In addition to the data solicited by the form, attach original source data that may have been collected to document any treatment effect.
XVIII. PLANS FOR DATA ANALYSIS
Data analysis will be completed by the Study Director located at the AADAP Office. Data from the treatment year will be summarized through tabulation and appropriate statistical analysis. An annual INAD report will be prepared and submitted to the FDA. This submission may include a request for an extension of the INAD based on the data collected during that year. When sufficient data are collected, the entire INAD data set will be summarized in a final report for submission to support a full NADA.
XIX. PROTOCOL AND PROTOCOL AMENDMENTS
A signed copy of the Study Protocol must be retained by each Investigator. At any time before the study begins, desired changes in the Study Protocol should be brought to the attention of the Study Director. The desired changes will be fully described in the form of an amendment along with the reason for the change. The amendment will be signed by the Sponsor (or its representative). Copies of the signed amendment will be attached to each copy of the Study Protocol. Investigators will be liable for non-compliance violation if drugs are used without a Study Protocol or differently than specified in the Study Protocol, if forms are not filed on time, or if the study data are not properly collected, maintained, and reported. The Study Monitor is responsible for ensuring that all INAD procedures are being followed as defined by the Study Protocol.
Deviations from the established Study Protocol occasionally cannot be avoided. If deviations occur, the Study Monitor should be contacted immediately for advice. Protocol deviations should be fully documented and should be accompanied by a written explanation of what happened, why, and what steps were taken to mitigate the deviation. Deviation statements should be signed and dated. These statements should be forwarded to the Study Monitor along with the quarterly data summaries, and ultimately be submitted to the Study Director.
Alcobendas, M., and five coauthors. 1991. Mass labeling of elvers with fast balneation in fluorochromes. Application to tetracycline labeling of 500kg of elvers. Bull. Fr. Peche Piscic. 321:43-54.
Beckman, D.W., C.A. Wilson, F. Lorica, and J.M. Dean. 1990. Variability in incorporation of calcein as a fluorescent marker in fish otoliths. Pages 547-549 in N.C. Parker and five coeditors, Fish-marking techniques American Fisheries Society, Symposium 7, Bethesda, Maryland.
Brooks, R.C., R.C. Heidinger, and C.C. Kohler. 1994. Mass-marking otoliths of larval and juvenile walleyes by immersion in oxytetracycline, calcein, or calcein blue. North American Journal of Fisheries Management 14:143-150.
Bumguardner, B.W, and T.L. King. 1996. Toxicity of oxytetracycline and calcein to juvenile striped bass. Transactions of the American Fisheries Society 125:1443-145.
Day, R.W., M.C. Williams, and G.P. Hawkes. 1995. A comparison of fluorochromes for marking abalone shells. Marine Freshwater Research 46(3):599-605.
Diehl, H., and J.L. Ellingboe. 1956. Indicator for titration of calcium in presence of magnesium using disodium dihydrogen ethylenediamine tetraacetate. Analytical Chemistry 28:882-884.
Du, S.J., V. Frenkel, G. Kindschi, and Y. Zohar. 2001. Visualizing normal and defective bone development in zebrafish embryos using the fluorescent chromophore calcein. Developmental Biology. Academic Press.
Eads, C.B. and J.B. Layzer. In Press. How to pick your mussels out in a crowd: using fluorescence to mark juvenile freshwater mussels. Journal of the North American Benthological Society
Gelsleichter, J., E. Cort^es, C.A. Manire,, R.E. Hueter, and J.A. Musick. 1997. Use of calcein as a fluorescent marker for elasmobranch vertebral cartilage. Transactions of the American Fisheries Society 126:862-865.
Hettler, W.F. 1984. Marking otoliths by immersion of marine fish larvae in tetracycline. Transactions of the American Fisheries Society 113:370-373.
Kaehler, S. and C.D. McQuaid. 1999. Use of the fluorochrome calcein as an in-situ growth marker in the brown mussel Perna perna. Marine Biology 133:455-460.
Leips, J., C.T. Baril, F.H. Rodd, D.N. Reznick, F. Bashey, G.J. Visser, and J. Travis. 2001. The suitability of calcein to mark poeciliid fish and a new method of detection. Transactions of the American Fisheries Society 130:501-507.
Mohler, J.W. 1997. Immersion of larval Atlantic salmon in calcein solutions to induce a non-lethally detectable mark. North American Journal of Fisheries Management 17:751-756.
Mohler, J.W., M.J. Millard, and J.W. Fletcher. 2002. Predation by captive wild brook trout on calcein-marked versus nonmarked Atlantic salmon fry. North American Journal of Fisheries Management 22:223-228.
National Native Mussel Conservation Committee. 1998. National strategy for the conservation of native freshwater mussels. Journal of Shellfish Research. 17:1419-1428.
Oncel, M., B. Khoobehi, and G.A. Peyman. 1990. Calcein angiography: a preliminary report on an experimental dye. International Ophthalmology 14:245-250.
Refogo, M.F., D. Miller, and A.S. Fiore. 1972. A new stain for soft hydrophilic lens fitting. Archives of Ophthalmology 87:275-277.
Rowley, R.J. and D.I. Mackinnon. 1995. Use of the fluorescent marker calcein in biomineralization studies of brachiopods and other marine organisms. Musee Oceanographique: Monaco. Proceedings of 7th international symposium on biomineralization 14:111-120.
Secor, D.H., M.G. White, and J.M. Dean. 1991. Immersion marking of larval and juvenile hatchery-produced striped bass with oxytetracycline. Transactions of the American Fisheries Society 120:261-266.
Wilson, C.A., D.W. Beckman, and J.M. Dean. 1987. Calcein as a fluorescent marker of otoliths of larval and juvenile fish. Transactions of the American Fisheries Society. 116:668-670.
Appendix IV. Material Safety Data Sheet (MSDS) for Calcein
The MSDS for calcein can be found at the drug sponsors website
http://www.syndel.com/downloads/dl/file/id/54/se_mark_calcein_solution_msds.pdf
Form CALC-W: Worksheet for Designing Individual Field Trials Under
SE- MARK® INAD 10-987
INSTRUCTIONS
1. Investigator must fill out Form CALC-W for each trial conducted under this INAD before actual use of SE-MARK® for marking. The Investigator is responsible that Form CALC-W is completed accurately.
2. Investigator should keep the original on file, and fax a copy to the Study Monitor for review.
3. After review, the Study Monitor will fax a copy to the AADAP Office for assignment of the Study Number.
4. The AADAP Office will review the worksheet, and then fax the assigned trial Study Number to both the Investigator and Study Monitor, at which time the trial may be initiated.
5. Note: Both Investigator and Study Monitor should sign and date Form CALC-W.
SITE INFORMATION
Facility |
|
||
Address |
|
||
|
|
||
Investigator |
|
||
Reporting Individual (if not Investigator) |
|
||
Phone |
|
Fax |
|
FISH CULTURE AND DRUG TREATMENT INFORMATION
Fish species to be treated |
|
Number of fish to be treated |
|
Average fish weight (gm) |
|
Average fish length (in) |
|
Volume of treatment tank/ container (gal) |
|
Number of fish per treatment tank/container |
|
Planned duration of drug treatment (e.g. min or hr) |
|
Intended drug dosage (mg/L) |
|
Salt pre-treatment (yes or no) |
|
% salt solution and duration of treatment (min) |
|
Anticipated date treatment will be initiated |
|
||
Estimated total weight of fish treated (lbs) |
|
Estimated total amount of drug needed for proposed treatment (ml) |
|
Drug manufacturer |
Western Chemical |
Drug lot number |
|
STUDY DESIGN: Describe in detail the purpose of the clinical trial. Study design must be carefully focused and lend itself to rigorous evaluation. If more space is required to describe study details, title additional page(s) "Study Design" and attach them to this Worksheet.
Study designed by |
|
DISPOSITION OF TREATED FISH (Human Food Safety Considerations):
|
|
Investigator should initial here to indicate awareness that fish disposition must be in compliance with FDA-mandated withdrawal times as described in the Study Protocol. |
|
|
|
|
|
DISPOSITION OF MARKING SOLUTION (Environmental Safety Considerations):
-
Marking solution will be stored on-site in a secure, leak-proof container that clearly identifies container contents (Investigator should initial).
-
Marking solution will be disposed of by shipment to Emerald Services, Inc., 1825 Alexander Avenue, Tacoma, WA 98451 according to procedures detailed in general Waste-stream Profile #216200B (Investigator should initial).
WORKER SAFETY CONSIDERATIONS:
|
|
Investigator should initial here to indicate that all personnel handling drug have read Material Safety Data Sheet for calcein and have been provided protective equipment, in good working condition, as described in the MSDS. |
|
|
Date Prepared: |
|
Investigator: |
|
|
|
|
|
Date Reviewed: |
|
Study Monitor: |
|
FORM CALC-1: Report on Receipt of Drug - Guide for Reporting Investigational New Animal Drug Shipments for Poikilothermic Food Animals
INSTRUCTIONS
1. Investigator must fill out Form CALC-1 immediately upon receipt of SE-MARK®.
2. Investigator should keep the original on file, and send one copy to the Study Monitor for review.
Within 10 days of receipt, the Study Monitor should send a copy to the AADAP Office.
Note: Both Investigator and Study Monitor should sign and date Form CALC-1.
The sponsor, U.S. Fish and Wildlife Service, submits a notice of claimed investigational exemption for the shipment or delivery of a new animal drug under the provisions of Section 512 of the Federal Food, Drug, and Cosmetics Act.
Name of Drug |
SE-MARK® |
INAD Number |
10-987 |
Proposed Use of Drug |
Marking of calcified structures including otoliths, fin rays, and scales |
||
Date of CVM Authorization Letter |
TBD |
||
Date of Drug Receipt |
|
Amount of Drug Received |
|
Drug Lot Number |
|
Study Worksheet Number |
|
Name of Investigator |
|
||
Address of Investigator |
|
||
Location of Trial |
|
||
Pivotal Study (yes/no) |
|
Non-pivotal Study (yes/no) |
|
Approximate Number of Treated Animals |
|
Approximate Number of Control Animals |
|
Number of Animals Used Previously1 |
|
||
Study Protocol Number |
10-987 |
||
Approximate dates of trial (start/end) |
|
||
Species, Size, and Type of Animals |
|
||
Maximum daily dose and duration |
125-250 mg/L for 1-6 hr 2.5-5.0 g/L for 1-7 min |
||
Methods(s) of Administration |
Immersion (static bath) |
||
Withdrawal Period |
- No investigational withdrawal time for finfish weighing ≤ 2 g - No investigational withdrawal time for mussels - No slaughter authorization for finfish weighing > 2 g |
||
1 To be filled out by the AADAP Office
Date Prepared: |
|
Investigator: |
|
Date Reviewed: |
|
Study Monitor: |
|
Date Reviewed: |
|
Sponsor: |
|
Form CALC-2: Chemical Use Log for Clinical Field Trials Using SE-MARK®
Under INAD #10-987
INSTRUCTIONS
1. Investigator must initiate a new Form CALC-2 immediately upon receipt of each shipment of SE-MARK®.
2. Form CALC-2 should be updated whenever drug is used, transferred, or discarded.
3. Investigator should save all copies of this form until the end of the calendar year, at which time they should maintain all originals on file and send one copy of the completed form(s) to their Study Monitor. Within 10 days of receipt, the Study Monitor will ensure accuracy and send a copy to the AADAP Office for inclusion in the permanent file.
4. Note: Both Investigator and Study Monitor should sign and date Form CALC-2.
Qty of SE-MARK®
from previous page (ml): Facility: Reporting individual:______________
Date |
SE-MARK® received (ml) |
Lot number of SE-MARK® received |
Study Number |
SE-MARK® used in treatment (ml) |
SE-MARK® transferred1 (ml) |
SE-MARK® discarded (ml) |
SE-MARK® remaining on hand (ml) |
Inventory by (initials) |
|
|
|
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
|
xxxx |
xxxx |
|
|
|
|
|
|
1 Unused SE-MARK® that is transferred to another facility participating in SE-MARK® INAD #10-987 (Note: SE--MARK® can only be transferred to another facility with prior authorization by the AADAP Office).
Date Prepared: |
|
Investigator: |
|
|
|
|
|
Date Reviewed: |
|
Study Monitor: |
|
STUDY NUMBER |
|
Page 1 of 3 |
Form CALC-3: Results Report Form for Use of SE-MARK® Under INAD 10-987
INSTRUCTIONS
1. Investigator must fill out Form CALC-3 no later than 30 days after completion of treatment. Study Number must be recorded on all pages of Form CALC-3. Attach lab reports and other information.
2. If SE-MARK® was not used under the assigned Study Number, fill out only the Site Information portion on this page, and skip to the end of page 3 and fill out only the "Negative Report" section.
3. Investigator should keep the original on file, and send a copy to the Study Monitor. Within 10 days of receipt, the Study Monitor should send a copy to the AADAP Office for inclusion in the permanent file.
Note: Both Investigator and Study Monitor should sign and date Form CALC-3.
SITE INFORMATION
Facility |
|
Reporting Individual |
|
FISH CULTURE AND DRUG TREATMENT INFORMATION
SE-MARK® lot number |
|
Amount SE-MARK® used (ml) |
|
Treatment option used (see study circle one) |
Option A Option B |
||
Treatment dosage |
|
Treatment duration |
|
Pre-treatment with salt solution (circle one) |
Yes or No |
If yes, salt solution conc. (%) and treatment duration (min) |
|
Fish species treated |
|
Total number of fish treated |
|
Ave fish weight (gm or number/pound); circle one used and enter data) |
|
Average fish length (in) |
|
Treatment bath vol (gal) |
|
Number fish per treatment |
|
Number of rearing units treated |
|
Treatment date |
|
WATER QUALITY PARAMETERS
Ave pre-treatment temp (oF) |
|
Dissolved Oxygen (mg/L) |
|
Ave treatment temp (oF) |
|
pH |
|
Ave post-treatment temp (oF) |
|
Hardness - CaCO3 (mg/L) |
|
Marking Record - Version 1
INSTRUCTIONS
Investigator should fill out the Marking Record as completely as possible.
Enter the AMarking Grade@ for each unit in the proper column to indicate the quality of the mark:
3 = readily visible bright green mark; 2 = clearly visible green mark; 1 = dimly visible dull green mark;
and 0 = no mark.
Use additional copies of this form if more than 1 rearing unit/lot is involved in the trial.
If more that 15 fish are evaluated, append another copy of this form labeled Acontinuation sheet@
|
Facility: |
|
|
||||||
Rearing Unit ID |
|
||||||||
Number of Fish |
|
||||||||
Fish Number |
Date |
Days Post Treatment |
Pectoral Fin Ray Mark |
Pelvic Fin Ray Mark |
Opercle Mark |
Jaw Mark |
Scale Mark |
Other Mark (identify)
|
Observer Initials |
1 |
|
|
|
|
|
|
|
|
|
2 |
|
|
|
|
|
|
|
|
|
3 |
|
|
|
|
|
|
|
|
|
4 |
|
|
|
|
|
|
|
|
|
5 |
|
|
|
|
|
|
|
|
|
6 |
|
|
|
|
|
|
|
|
|
7 |
|
|
|
|
|
|
|
|
|
8 |
|
|
|
|
|
|
|
|
|
9 |
|
|
|
|
|
|
|
|
|
10 |
|
|
|
|
|
|
|
|
|
11 |
|
|
|
|
|
|
|
|
|
12 |
|
|
|
|
|
|
|
|
|
13 |
|
|
|
|
|
|
|
|
|
14 |
|
|
|
|
|
|
|
|
|
15 |
|
|
|
|
|
|
|
|
|
RESULTS: Describe in detail treatment results. Was treatment successful? If treatment did not appear to be successful, explain why not? Were there any mitigating environmental conditions that may have impacted treatment results? Were there any deviations from the Study Protocol?
TOXICITY OBSERVATIONS: Report any apparent drug toxicity including a description of unusual fish behavior.
OBSERVED WITHDRAWAL PERIOD OF TREATED FISH:
|
Investigator should initial here to indicated awareness that fish disposition must be in compliance with FDA-mandated withdrawal times as described in Study Protocol Section XV |
|
Estimated number of days between last treatment and first availability of fish for human consumption (ensure this time period meets the withdrawal period) |
|
|
DISPOSITION OF MARKING SOLUTION
-
SE-MARK® solution has been stored on-site in a secure, leak-proof container that clearly identifies container contents (Investigator should initial)
-
SE-MARK® solution disposed of by shipment to Emerald Services, Inc., 1825 Alexander Avenue, Tacoma, WA 98451 according to procedures detailed in general Waste-stream Profile #216200B (Investigator should initial)
NEGATIVE REPORT SE-MARK® immersion marking was not used at this facility under this Study Number during the reporting period. (Investigator should initial for negative reports as soon as the Study Number is known to be no longer needed or valid.)
Date Prepared: |
|
Investigator: |
|
|
|
|
|
Date Reviewed: |
|
Study Monitor: |
|
NOTICES
Paperwork Reduction Act
In accordance with the Paperwork Reduction Act (44 U.S.C. 3501 et seq.), the U.S. Fish and Wildlife Service collects information necessary to permit the use of an investigational new animal drug to generate data to support a new animal drug approval (NADA) as part of the Fish and Aquatic Conservation fish health network. Your response is voluntary, but is required to obtain or retain a benefit. According to the Paperwork Reduction Act of 1995, an agency may not conduct or sponsor and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. OMB has approved this collection of information and assigned Control No. 1018-####.
ESTIMATED BURDEN STATEMENT
We estimate public reporting for this collection of information to average 4 hours, including time for reviewing instructions, gathering and maintaining data, and completing and reviewing the form. Direct comments regarding the burden estimate or any other aspect of the form to the Service Information Clearance Officer, Fish and Wildlife Service, U.S. Department of the Interior, 5275 Leesburg Pike, MS: PRB (JAO/3W), Falls Church, VA 22041-3803, or via email at [email protected]. Please do not send your completed form to this address.
FREEDOM OF INFORMATION ACT STATEMENT
Information provided to the Service is generally subject to release to the public under the Freedom of Information Act (FOIA). Certain information, however, may be subject to withholding if the Service determines that the information is a trade secret and/or commercial or financial information that is privileged or confidential. To the extent you are submitting business information that falls into one of these categories, you must clearly mark this information as "Business Confidential" in order for the Service to assess the applicability of FOIA Exemption 4. Any information provided by you that is not marked as “Business Confidential” will be considered releasable to the public under the FOIA [43 CFR 2.26 – 2.33].
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Johnson, Bonnie |
| File Modified | 0000-00-00 |
| File Created | 2023-09-28 |
© 2026 OMB.report | Privacy Policy