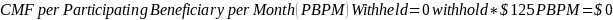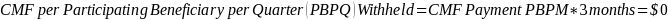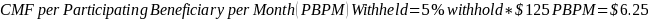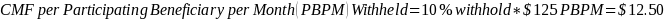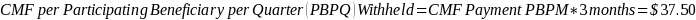Form CMS-10728 Patient Agreement
Value in Opioid Use Disorder Treatment Demonstration (CMS-10728)
ViT PA Final clean 10.15.2020
Participation Agreement
OMB: 0938-1388


Centers for Medicare & Medicaid Services Center for Medicare & Medicaid Innovation Prevention and Population Health Group 7500 Security Blvd.
Mail Stop WB-06-05 Baltimore, MD 21244
Value in Treatment
Participation Agreement
Last Updated: August 24, 2020
Table of Contents
I. Agreement Term; Model Performance Period; Performance Years 3
II. Definitions 4
III. Participant Requirements 5
IV. OUD Care Team 7
V. Participation of Applicable Beneficiaries and Beneficiary Protections 9
VI. Payments 11
VII. ViT Monitoring and Evaluation 14
VIII. Data Sharing and Reports 16
IX. Overlap Policy 21
X. Other Government Authorities 21
XI. Agreement to Comply with Laws 21
XII. Certification of Data and Information 22
XIII. Audits and Record Retention 22
XIV. Remedial Action 23
XV. Termination 24
XVI. Miscellaneous 26
APPENDIX A: VALUE IN TREATMENT PROGRAMMATIC WAIVERS 31
APPENDIX B: VALUE IN TREATMENT PBIP METHODOLOGY 33
PARTICIPATION AGREEMENT
This Participation Agreement (“Agreement”) is between the Centers for Medicare & Medicaid Services (“CMS”) and (“Participant”) (each a “Party” and collectively the “Parties”).
CMS is the agency within the U.S. Department of Health and Human Services (HHS) that is charged with administering the Medicare, Medicaid, and the Children’s Health Insurance Program (CHIP) programs.
The Participant is an entity or individual that is enrolled in Medicare and that is a physician (as defined in section 1861(r)(1) of the Social Security Act (the “Act”)); a group practice comprising at least one physician; a nurse practitioner; a group practice comprising at least one nurse practitioner; a hospital outpatient department; a federally qualified health center (FQHC) (as defined in section 1861(aa)(4) of the Act); a rural health clinic (RHC) (as defined in section 1861(aa)(2) of the Act); a community mental health center (as defined in section 1861(ff)(3)(B) of the Act); a clinic certified as a certified community behavioral health clinic pursuant to section 223 of the Protecting Access to Medicare Act of 2014, P.L. 113–93; an opioid treatment program (OTP) (as defined in section 1861(jjj)(2) of the Act); or a critical access hospital (as defined in section 1861(mm)(1) of the Act).
CMS is implementing the Value in Opioid Use Disorder Treatment Demonstration Program (ViT), a 4-year demonstration program authorized under section 1866F of the Act, which was added by section 6042 of the Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act (the SUPPORT Act), P.L. 115-271, enacted on October 24, 2018.
The purpose of ViT, as stated in the statute, is to “increase access of applicable beneficiaries to opioid use disorder treatment services, improve physical and mental health outcomes for such beneficiaries, and to the extent possible, reduce [Medicare program expenditures].”
The Participant submitted an application to participate in ViT and CMS selected the Participant for participation in ViT pursuant to the application and selection process established under “Value in Opioid Use Disorder Treatment Demonstration Program, Request for Applications (RFA)”, published Month XX Date XX, 2020.
The Parties, intending to be legally bound, therefore agree as follows:
The effective date of this Agreement (“Effective Date”) is the date this Agreement is signed by the last Party to sign it (as indicated by the date associated with that Party’s signature).
The term of this Agreement (“Agreement Term”) begins on the Effective Date and expires two years after the last Day of the Demonstration Performance Period, unless this Agreement is sooner terminated by either Party in accordance with Section XV of the Agreement, in which case the Agreement Term shall expire on the effective date of termination.
The performance period for this Agreement (“Demonstration Performance Period”) begins on the later of April 1, 2021 or the Effective Date (“Start Date”) and ends on December 31, 2024, unless the Agreement is sooner terminated by either Party in accordance with Section XV, in which case the Demonstration Performance Period ends immediately upon the effective date of such termination. The Demonstration Performance Period includes the following four performance years (each a “Performance Year”):
Performance Year 2021: Start Date through December 31, 2021
Performance Year 2022: January 1, 2022 through December 31, 2022
Performance Year 2023: January 1, 2023 through December 31, 2023
Performance Year 2024: January 1, 2024 through December 31, 2024
In this Agreement, the following definitions apply:
“Applicable Beneficiary” means an individual who: is entitled to, or enrolled for, benefits under Medicare Part A and enrolled for benefits under Medicare Part B; is not enrolled in a Medicare Advantage plan under Medicare Part C; and has a current diagnosis for an OUD. An Applicable Beneficiary may include an individual who is dually eligible for benefits under Medicare and Medicaid if such individual satisfies all of these criteria.
“Beneficiary Agreement Form” means the form used by the Participant to obtain consent from an Applicable Beneficiary: (1) for the Applicable Beneficiary to participate in ViT and to receive OUD Treatment Services from the Participant; and (2) if applicable, for CMS to share the Applicable Beneficiary’s health care information with the Participant.
“C.F.R.” means the Code of Federal Regulations, as may be amended from time to time.
“CMF” refers to the care management fee and means a per-Participating Beneficiary per-month fee paid to the Participant by CMS pursuant to this Agreement. The CMF shall be paid in addition to any other amount otherwise payable to the health care practitioners in the Participant’s OUD Care Team or, if applicable, to the Participant under title XVIII of the Act.
“Day” means calendar day unless otherwise specified.
“Eligible Practitioner” means a physician or other health care practitioner, such as a nurse practitioner, who: (1) is enrolled under section 1866(j)(1) of the Act; (2) is authorized to prescribe or dispense narcotic drugs to individuals for maintenance treatment or detoxification treatment; and (3) has in effect a waiver in accordance with section 303(g) of the Controlled Substances Act (21 U.S.C. § 823(g)) for such purpose and is otherwise in compliance with regulations promulgated by the Substance Abuse and Mental Health Services Administration to carry out such section.
“OUD” stands for opioid use disorder, including opioid abuse, opioid dependence, and opioid use.
“OUD Care Team” means a team of health care practitioners established by the Participant in accordance with Section IV.A.1 that furnishes OUD Treatment Services to Participating Beneficiaries.
“OUD Treatment Services” means, with respect to an Applicable Beneficiary, services that are furnished for the treatment of OUD and that utilize drugs approved under section 505 of the Federal Food, Drug, and Cosmetic Act (21 U.S.C. § 355) for the treatment of OUD in an outpatient setting, and includes: medication-assisted treatment; treatment planning; psychiatric, psychological, or counseling services (or any combination of such services), as appropriate; social support services, as appropriate; and care management and care coordination services, including coordination with other providers of services and suppliers not on an OUD Care Team.
“Participant Survey” means a survey instrument conducted securely online to obtain qualitative information on execution of the Participant’s Implementation Plan (as such term is defined in section III.B) and the Participant’s experience implementing ViT.
“Participating Beneficiary” means an Applicable Beneficiary who has agreed to receive OUD Treatment Services from the Participant by signing the Beneficiary Agreement Form.
“PBIP” refers to the performance based incentive payment and means a payment made to the Participant by CMS based on the Participant’s performance with respect to the quality and cost criteria specified in Appendix B of this Agreement.
“Per-Participating Beneficiary Cap” means the maximum number of Participating Beneficiaries to whom the Participant is permitted to furnish OUD Treatment Services under this Agreement in any given quarter, calculated in accordance with Section VI.C.
“PHI” means protected health information as defined in 45 C.F.R § 160.103.
“Program Integrity Screening” means a review of an individual’s or entity’s program integrity history, which may include a review of the individual’s or entity’s history of exclusion or other sanctions imposed with respect to participation in Medicare, Medicaid, or CHIP; history of failure to pay Medicare debts in a timely manner; current or prior law enforcement investigations or administrative actions; affiliations with individuals or entities that have a history of program integrity issues; and other information pertaining to the trustworthiness of the individual or entity.
“TIN” means a federal Taxpayer Identification Number, which in some cases may be a Social Security Number.
“U.S.C.” means the United States Code, as may be amended from time to time.
Participant Requirements
-
The Participant shall be a separate and unique legal entity identified by a TIN that is formed under applicable federal, state, or tribal law and authorized to conduct business in each state in which it operates.
The Participant shall ensure compliance with the requirements of ViT as set forth in this Agreement, including by establishing mechanisms to report quality measures and other data to CMS.
The Participant shall notify CMS in writing of any noncompliance or deficiencies that would result in the Participant losing eligibility to participate in ViT within 15 Days of discovery, unless a different timeframe for notification is specified in this Agreement.
The Participant shall notify CMS of any administrative or other action that may affect the Participant’s Medicare enrollment status, or the Medicare enrollment status of a member of the Participant’s OUD Care Team, within 30 Days of the Participant’s receipt of notice of such action.
The Participant and the Participant’s OUD Care Team members must continue participation in all applicable CMS quality reporting initiatives for the duration of their participation in ViT.
-
The Parties acknowledge that the Participant submitted a plan for implementing ViT (“Implementation Plan”) together with its application to participate in ViT pursuant to the application and selection process established under the ViT RFA.
OUD Treatment Services
The Participant shall furnish OUD Treatment Services through its OUD Care Team, or arrange for such services to be furnished, to Participating Beneficiaries according to the terms of this Agreement. The Participant shall ensure that all OUD Treatment Services furnished to Participating Beneficiaries under ViT are furnished in an outpatient setting.
The Participant shall furnish OUD Treatment Services to each Participating Beneficiary, or arrange for such services to be furnished, based on the Participating Beneficiary’s individualized OUD treatment plan, in alignment with other services furnished to the Participating Beneficiary for purposes of treating his or her OUD, and with a reasonable expectation that such services will improve the overall health and function of the Participating Beneficiary.
The Participant shall ensure that OUD Treatment Services are furnished to Participating Beneficiaries on a face-to-face basis, with the exception of the following services, which may be furnished on a non-face-to-face basis using telecommunications technology:
Offering Participating Beneficiaries, with the Participant serving as the originating site, telehealth visits with an OUD treatment center of excellence or “hub”;
Assisting Participating Beneficiaries to arrange for transportation or legal assistance;
Crisis intervention;
Psychotherapy
Naloxone training;
Contingency management, defined as a behavior modification intervention that establishes a connection between new, targeted behavior and the opportunity to obtain a desired reward; and
Other care management activities that do not include direct beneficiary interaction.
Participant Changes
The Participant shall provide written notice to CMS at least 60 Days before any change in the Participant’s legal name. The notice of legal name change must include a copy of any legal document effecting the name change, authenticated by the appropriate state official (if applicable), and the Parties must execute an agreement reflecting the change of the Participant’s legal name.
The Participant shall provide written notice to CMS as soon as practicable, but no later than 30 Days after any change in TIN, National Provider Identifier (NPI), or other identifier specified by CMS with respect to the Participant or a member of the Participant’s OUD Care Team. After review of such notice, CMS may terminate this Agreement, demand immediate payment of any amount owed by the Participant to CMS under this Agreement, or may take any other actions consistent with the terms of this Agreement.
OUD Care Team
-
The Participant shall establish an OUD Care Team by employing or contracting with heath care practitioners to include, at a minimum:
at least one physician (as defined in section 1861(r)(1) of the Act) who will furnish primary care services or addiction treatment services to one or more Participating Beneficiaries;
at least one Eligible Practitioner who will furnish primary care services or addiction treatment services to one or more Participating Beneficiaries; and
at least one Merit-based Incentive Payment System (MIPS) eligible clinician.
The Participant may include in its OUD Care Team other practitioners licensed under state law to furnish psychiatric, psychological, counseling, and social services to Applicable Beneficiaries.
The Participant shall have a collaborative, integrated relationship with each OUD Care Team member.
OUD Care Team List
The Participant shall maintain and update a list of OUD Care Team members in accordance with this Section IV.B.
The Parties acknowledge that the Participant submitted with its application to participate in ViT, pursuant to the application and selection process established under the ViT RFA, an initial list of OUD Care Team members, identified by name, address, and NPI.
The Participant shall report to CMS, in a form and manner and by a deadline specified by CMS, any changes to its list of OUD Care Team members at least biannually.
Notwithstanding the prohibition in Section IX.A, the Participant may include physicians and non-physician practitioners who participate in Comprehensive Primary Care Plus (CPC+), Primary Care First (PCF), or the Maryland Primary Care Program (MDPCP) on its list of OUD Care Team members.
-
OUD Care Team Arrangements
The Participant shall have a written arrangement with each OUD Care Team member who is not employed by the Participant (each an “OUD Care Team Member Arrangement”).
Each OUD Care Team Member Arrangement must be in writing and the only parties to the arrangement must be the Participant and the OUD Care Team member.
Each OUD Care Team Member Arrangement must require that the OUD Care Team member:
Be available to provide OUD Treatment Services to Participating Beneficiaries on a face-to-face basis except as permitted under Section III.C.3;
Comply with all applicable laws and regulations, including those specified in Section XI.A;
Comply with the applicable provisions of this Agreement;
Update his or her Medicare enrollment information on a timely basis in accordance with Medicare program requirements, and notify the Participant of any changes to its Medicare enrollment information, name, address, or NPI; and
Notify the Participant within 7 Days of becoming aware that he or she is under investigation or has been sanctioned (including, without limitation, the imposition of program exclusion, debarment, civil monetary penalties, loss of medical license or equivalent, corrective action plans, and revocation of Medicare billing privileges) by the federal, state or local government, or any licensing authority.
Each OUD Care Team Member Arrangement must permit the Participant to take remedial action against the OUD Care Team member, including termination of the OUD Care Team Member Arrangement, to address noncompliance with the terms of ViT as set forth in this Agreement or any program integrity issues identified by CMS or the Participant.
CMS provides no opinion on the legality of any contractual or other arrangement that the Participant, any of its OUD Care Team members, or any other individual or entity involved in the Participant’s implementation of ViT has proposed, implemented, or documented. The receipt by CMS of any such documentation over the course of the application process or otherwise shall not be construed as a waiver or modification of any applicable laws, rules or regulations, and will not preclude CMS, HHS, or its Office of Inspector General, a law enforcement agency, or any other federal or state agency from enforcing any and all applicable laws, rules, and regulations.
Participation of Applicable Beneficiaries and Beneficiary Protections
Voluntary Participation by Applicable Beneficiaries
Participation in ViT is voluntary for Applicable Beneficiaries. The Participant and its OUD Care Team members are prohibited from providing gifts or other remuneration to, and from withholding anything from Applicable Beneficiaries to induce them to agree to participate in ViT and receive OUD Treatment Services from the Participant and its OUD Care Team, or to agree for CMS to share their data with the Participant. The Participant must permit an Applicable Beneficiary to terminate his or her participation in ViT at any time.
The Participant must use the Beneficiary Agreement Form to obtain consent from an Applicable Beneficiary: (1) for the Applicable Beneficiary to participate in ViT and receive OUD Treatment Services from the Participant; and (2) for CMS to share the Applicable Beneficiary’s data with the Participant in accordance with Section VIII. A signature by the Applicable Beneficiary or his or her authorized representative on the Beneficiary Agreement Form is required to confirm the Applicable Beneficiary’s agreement to participate in ViT and receive OUD treatment services from the Participant and, if applicable, to confirm the Applicable Beneficiary’s agreement to share his or her data with the Participant. The Participant shall not complete, and shall prohibit its OUD Care Team members from completing the Beneficiary Agreement Form on behalf of an Applicable Beneficiary.
The Participant may distribute the Beneficiary Agreement Form to an Applicable Beneficiary only together with the CMS-provided beneficiary notice and patient FAQs, and only as permitted by this Section V.A.
CMS will provide the Participant with templates for the Beneficiary Agreement Form and for the accompanying beneficiary notice and patient FAQs. The Participant shall make no changes to these CMS-provided templates, except as expressly permitted by CMS in writing. The Participant shall submit the completed templates to CMS, in a form and manner specified by CMS, for review and approval. Completed templates are deemed approved 10 business days following their submission to CMS unless CMS disapproves the completed templates. CMS may issue a written notice of disapproval of at any time, including after the expiration of the 10-buinsess day review period.
The Participant shall not use, and shall prohibit its OUD Care Team members from using, the Beneficiary Agreement Form, beneficiary notice, and patient FAQs until completed by the Participant and approved by CMS. The Participant shall, and shall require its OUD Care Team members to, immediately discontinue use of any Beneficiary Agreement Form, beneficiary notice, or patient FAQs disapproved by CMS.
The Participant shall retain copies of all Beneficiary Agreement Forms, beneficiary notices, and patient FAQs distributed to Applicable Beneficiaries in accordance with Section XIII.B.
Access to Services and Beneficiary Cost Sharing
The Participant shall make, and shall require its OUD Care Team members to make, medically necessary covered services available to an Applicable Beneficiary, regardless of whether the Applicable Beneficiary agrees to participate in ViT or to receive OUD Treatment Services from the Participant.
The Participant shall not require an Applicable Beneficiary to relinquish access to any Medicare benefit as a condition of receiving services from the Participant.
Participation in ViT by a Participating Beneficiary shall not affect coverage of or payment under Medicare for any other item or service furnished to the Participating Beneficiary, except that Medicare will pay 100 percent of the CMF and PBIP payments made for services furnished under ViT, and 100 percent of the bundled payments under 42 C.F.R. § 410.67 for opioid use disorder treatment services furnished by opioid treatment programs, physicians and non-physician practitioners to Participating Beneficiaries while such beneficiaries are participating in ViT. Accordingly, the Participant shall not collect any monies, such as coinsurance, copayments, or deductibles, from a Participating Beneficiary for such services, and shall return any such monies erroneously collected.
Prescription Drug Monitoring Program (PDMP)
If a PDMP exists in the state in which the Participant is furnishing OUD Treatment Services, to the extent permitted under applicable state law, the Participant shall query the state’s PDMP for each new Participating Beneficiary prior to initiating treatment under ViT, and at least quarterly thereafter through the course of the Participating Beneficiary’s participation in ViT.
HIPAA Requirements
The Participant acknowledges that it is a covered entity or a business associate, as those terms are defined in 45 C.F.R. § 160.103, of OUD Care Team members who are covered entities.
The Participant shall have all appropriate administrative, technical, and physical safeguards in place before the Start Date to protect the privacy and security of PHI in accordance with 45 C.F.R. § 164.530(c).
The Participant shall maintain the privacy and security of all ViT-related information that identifies individual beneficiaries in accordance with the Health Insurance Portability and Accountability Act (HIPAA) Privacy and Security Rules and all relevant HIPAA Privacy and Security guidance applicable to the use and disclosure of PHI of covered entities, as well as applicable state laws and regulations.
Payments
General
On a quarterly basis, CMS shall pay the Participant a CMF for each month during the quarter, subject to the limitations in Sections VI.B and VI.E.
In order to incentivize the Participant to achieve quality and cost outcomes under ViT, subject to the limitation in Section VI.D.1 and the limitations in Section VI.E, CMS shall pay Participant a PBIP based on the Participant’s performance on the quality and cost measures specified in Appendix B during a Performance Year. CMS shall calculate the amount of the PBIP in accordance with Section VI.D and Appendix B.
-
CMS will pay the Participant the CMF on quarterly basis, as detailed in Appendix B.
The amount of the CMF is set at a flat rate of $125 per Participating Beneficiary per month, minus the applicable PBIP quality withhold and any applicable Medicare sequestration adjustments; the adjusted monthly amount is multiplied by 3 to determine the quarterly payment amount, as described in Appendix B.
Claims for the CMF under ViT must be submitted to the Medicare Administrative Contractor (MAC) appropriate to the Participant using the ViT-specific G-code specified by CMS (ViT Code). No other billing or procedure codes may be included on the claim.
Only the Participant may submit a claim with the ViT Code.
An ICD-10-CM diagnosis code for an OUD diagnosis specified by CMS must be present on the claim in order for the claim to be eligible for a CMF payment under ViT.
CMS shall pay the CMF to the Participant in addition to any amount that may otherwise be made under Medicare, including:
payment for existing care management codes in the Medicare Physician Fee Schedule, unless the Participant is an FQHC or RHC, in which case the Participant is prohibited from billing HCPCS codes G0511 (general care management) or G0512 (psychiatric collaborative care model) within a calendar quarter period of having billed the ViT Code for the same beneficiary; and
bundled payments for opioid use disorder treatment services furnished by opioid treatment programs made pursuant to 42 C.F.R. § 410.67.
Per Participating Beneficiary Cap
In order to ensure compliance with the requirements of section 1866F(h)(2) of the Act, in a given calendar quarter of a Performance Year, the Participant shall not furnish services to Participating Beneficiaries under this Agreement in excess of the number of Participating Beneficiaries included in the Participant’s Per Participating Beneficiary Cap.
The Parties acknowledge that CMS notified the Participant of the Participant’s Per Participating Beneficiary Cap prior to the Effective Date, which was calculated based on the Participant’s historical claims and a projection of beneficiary cost sharing amounts.
The Participant may submit a request, in a form and manner specified by CMS, that CMS increase the number of Participating Beneficiaries included in the Participant’s Per Participating Beneficiary Cap.
In response to the Participant’s request or for such other reasons determined by CMS, CMS may, at CMS’s sole discretion, adjust the number of Participating Beneficiaries included in the Participant’s Per Participating Beneficiary Cap in accordance with this Section VI.C.4 to ensure ViT is serving the maximum number of Participating Beneficiaries without exceeding the limitations set forth in sections 1866F(d)(2) and (h)(2) of the Act.
CMS may reduce the number of Participating Beneficiaries included in the Participant’s Per Participating Beneficiary Cap for a subsequent calendar quarter if CMS determines that the Participant has furnished OUD Treatment Services, or arranged for such services to be furnished, to at least 5% fewer Participating Beneficiaries than the number of Participating Beneficiaries included in the Participant’s Per Participating Beneficiary Cap for two or more consecutive quarters.
CMS may increase the number of Participating Beneficiaries included in the Participant’s Per Participating Beneficiary Cap for a subsequent calendar quarter if: (1) the Participant submits a request pursuant to Section VI.C.3; (2) the Participant has consistently furnished OUD Treatment Services, or arranged for such services to be furnished, to the number of Participating Beneficiaries already included in the Participant’s Per Participating Beneficiary Cap; and (3) CMS determines, at CMS’s sole discretion, that there are sufficient funds under section 1866F(h)(2) of the Act to make CMF and PBIP payments to the Participant for furnishing OUD Treatment Services to the additional number of Participating Beneficiaries requested by the Participant for inclusion in the Participant’s Per Participating Beneficiary Cap.
CMS shall provide the Participant with written notice of any adjustments to the Participant’s Per Participating Beneficiary Cap at least 30 Days prior to the start of the calendar quarter in which such adjustments will take effect.
The Per Participating Beneficiary Cap is specific to ViT, and in no way restricts a beneficiary’s freedom of choice or ability to access other items and services from the Participant.
Performance-Based Incentive Payment (PBIP)
CMS shall pay the Participant a PBIP for a Performance Year only if the Participant has complied with the data reporting requirements set forth in Section VII.B.
CMS will calculate the amount of the Participant’s PBIP, if any, for the Performance Year based on the total amount withheld from the CMF payments made to the Participant for the Performance Year in accordance with Section VI.B.2 and Appendix B.
CMS will pay the Participant a PBIP for a Performance Year only if the Participant’s performance on the cost and quality measures specified in Appendix B during the Performance Year meets or exceeds the applicable performance threshold as determined by CMS in accordance with the methodology specified in Appendix B.
CMS will assess the Participant’s performance on the cost and quality measures specified in Appendix B during the Performance Year after one quarter claims run out following the Performance Year.
If CMS determines that the Participant is entitled to a PBIP for a Performance Year, CMS shall make the PBIP to the Participant no later than the third quarter following the Performance Year.
Denial of Payment
CMS shall make a CMF payment to no more than one individual or entity participating in ViT for a given Participating Beneficiary under ViT during a calendar quarter. If two or more individuals or entities participating in ViT submit a claim for the CMF under ViT to a single Participating Beneficiary during a single calendar quarter, CMS shall determine which individual or entity will receive a CMF for that Participating Beneficiary based on the date of claim submission. That is, CMS will pay a CMF to the individual or entity that submits the first valid CMF claim for that Participating Beneficiary during the calendar quarter, irrespective of whether that claim had the earliest date of service.
CMS shall not pay the Participant a CMF or PBIP for an individual who does not meet the definition of a Participating Beneficiary.
CMS shall not pay the Participant a CMF or PBIP for any Participating Beneficiaries in excess of the number of Participating Beneficiaries included in the Participant’s Per Participating Beneficiary Cap.
Payment to CMS
If CMS determines that a payment made by CMS pursuant to this Section VI was made in error or was otherwise inconsistent with the terms of this Agreement, CMS shall send the Participant a demand letter for the amount of such payment, and may take a remedial action as described in Section XIV. The Participant shall pay any such amount within 30 Days of the date of the demand letter.
If CMS does not receive payment of the full amount owed by the date specified in the demand letter, CMS may assess interest at the rate applicable to other Medicare debts pursuant to 42 C.F.R. §405.378 on any outstanding unpaid amounts. Interest will be calculated in 30-Day periods and assessed for each 30-Day period that payment is not made in full.
If the Participant fails to pay CMS the full amount owed by the date specified in the demand letter, CMS will recoup monies owed from present and future Medicare payments otherwise owed to the Participant. If CMS is unable to recoup the full amount owed via Medicare payments, CMS will invoke all legal means to collect the debt, including referral of the remaining debt to the United States Department of Treasury, pursuant to 31 U.S.C. 3711(g).
ViT Monitoring and Evaluation
CMS Monitoring and Evaluation
CMS will monitor the Participant’s maintenance of additional services specified in the Participant’s Implementation plan, the Participant’s performance under ViT, the Participant’s experience implementing ViT, and qualitative information on the functional, physical, mental, and overall health status of Participating Beneficiaries in order to detect non-compliance with ViT requirements as set forth in this Agreement, inappropriate care furnished to Participating Beneficiaries, overutilization, inappropriate reductions in care, cost-shifting to other payers or populations, and any other program integrity risks.
CMS will contract with an independent evaluator to study the design and implementation of the Demonstration. The Participant agrees to support the independent evaluation by providing requested data to assess the impact of the Demonstration and by participating in check-in calls and/or phone interviews with the independent evaluation contractor during the Demonstration and at least a year after the Demonstration officially ends. CMS will conduct an independent evaluation of the ViT to assess the extent that the demonstration program:
Reduces hospitalizations and emergency department visits;
Increases use of medication-assisted treatment for OUD;
Improves health outcomes of individuals with OUD, including reducing the incidence of infectious diseases (such as Hepatitis C and HIV);
Does not increase the total Medicare spending on items and services;
Reduces deaths from opioid overdose; and
Reduces the utilization of inpatient residential treatment.
In addition, the evaluation findings will include the extent to which the PBIP:
Increased retention in treatment;
Increased use of pharmacotherapy for OUD;
Increased follow-ups after an emergency department visit for alcohol and other drug abuse or dependence; and
Initiated as well as increased engagement in alcohol and other drug dependence treatment.
Participant Cooperation
As a condition of payment under Section VI, the Participant shall submit to CMS or its designees, in such form, manner, and frequency specified by CMS, with respect to each Participating Beneficiary to whom the Participant furnishes OUD Treatment Services through its OUD Care team, or for whom the Participant arranges for such services to be furnished, data and such other information as CMS determines appropriate to monitor and evaluate ViT.
The Participant shall prepare and submit a financial report for each Performance Year to CMS in a form and manner and by a date specified by CMS.
CMS shall provide the Participant with a template for the financial report in which CMS will pre-fill the CMF and PBIP payments received by the Participant from CMS under ViT during that Performance Year;
The Participant shall confirm whether the CMF and PBIP amounts pre-filled by CMS are correct and shall report any discrepancies in the payment amounts or eligibility for such payments to CMS.
Each financial report must include a summary of the Participant’s expenditures of such CMF and PBIP payments, including the approximate clinical labor, non-clinical labor, non-labor expenses, service types as specified in the financial report template provided by CMS.
The Participant shall respond, and shall require the members of its OUD Care Team to respond to any survey or interview conducted by CMS or its designees to monitor and evaluate ViT to include, without limitation, the Participant Survey.
The Participant shall cooperate, and shall require its OUD Care Team members to cooperate, with audits conducted by CMS and its designees to determine compliance with the requirements of ViT as set forth in this Agreement, to include without limitation the submission of medical charts, medical records, Beneficiary Agreement Forms, and other data pertaining to Participating Beneficiaries.
The Participant shall cooperate, and shall require its OUD Care Team members to cooperate, with CMS efforts to conduct the independent evaluation of ViT, to include:
Engagement and cooperation with site visits and focus groups; and
Other activities that CMS determines appropriate to conduct a comprehensive evaluation of ViT.
If the Participant submits any PHI to CMS or its designees pursuant to this Section VII, the Participant shall submit such PHI via a secure method specified by CMS and in accordance with the requirements of Section V.D. The Participant shall not disclose PHI to CMS or its designees in response to a survey or interview conducted by CMS or its designees described in Section VII.C.3.
Program Integrity Screening
CMS may, at its sole discretion, subject the Participant and its OUD Care Team members to periodic Program Integrity Screening throughout the Demonstration Performance Period.
Data Sharing and Reports
General
Subject to the limitations discussed in this Agreement, and in accordance with applicable law, in advance of each Performance Year and at any other time deemed necessary by CMS, CMS will offer the Participant an opportunity to request certain data and reports, which are described in Section VIII.B and Appendix C of this Agreement.
The data and reports provided to the Participant under the preceding paragraph will omit individually identifiable data for each Participating Beneficiary who has not agreed for CMS to share his or her data with the Participant as described in Section V.A, and for each Participating Beneficiary who has elected to terminate his or her consent for CMS to share his or her data with the Participant as described in Section VIII.D.
The Participant shall not require an Applicable Beneficiary to agree to share his or her data with the Participant in order to participate in ViT and receive OUD Treatment Services from the Participant.
Provision of Certain Claims Data
CMS believes that the care coordination and quality improvement work of the Participant (acting on its own behalf as a HIPAA covered entity (“CE”) or as a business associate (“BA”) acting on behalf of its OUD Care Team members who are HIPAA CEs) would benefit from the receipt of certain beneficiary-identifiable claims data on Participating Beneficiaries. CMS will therefore offer to the Participant an opportunity to request specific beneficiary-identifiable claims data by completing the HIPAA-Covered Disclosure Request Attestation and Data Specification Worksheet (Appendix C). All requests for beneficiary-identifiable claims data will be granted or denied at CMS’ sole discretion based on CMS’ available resources, the limitations in this Agreement, and applicable law.
In offering this beneficiary-identifiable claims data which omits individually identifiable data for each Participating Beneficiary who has not agreed for CMS to share his or her data with the Participant as described in Section V.A, CMS does not represent that the Participant has met all applicable HIPAA requirements for requesting data under 45 CFR § 164.506(c)(4). The Participant and its OUD Care Team members should consult with their own counsel to make those determinations prior to requesting this data from CMS.
The beneficiary-identifiable claims data available is the data described in Appendix C.
The Parties mutually agree that, except for data covered by Section VIII.B.15 below, CMS retains all ownership rights to the data files referred to in Appendix C, and the Participant does not obtain any right, title, or interest in any of the data furnished by CMS.
The Participant represents, and in furnishing the data files specified in Appendix C, CMS relies upon such representation, that such data files will be used solely for the purposes described in this Agreement. The Participant agrees not to disclose, use or reuse the data except as specified in this Agreement or except as CMS shall authorize in writing or as otherwise required by law. The Participant further agrees not to sell, rent, lease, loan, or otherwise grant access to the data covered by this Agreement.
The Participant intends to use the requested information as a tool to conduct quality improvement activities and deliver seamless, coordinated care for Participating Beneficiaries. Information derived from the CMS files specified in Appendix C may be shared and used within the legal confines of the Participant and its OUD Care Team members in a manner consistent with Section VIII.B.7 to enable the Participant to improve access to OUD Treatment Services and care integration, and be a patient-centered organization.
The Participant may reuse original or derivative data without prior written authorization from CMS for clinical treatment, care management and coordination, and quality improvement activities but shall not disseminate individually identifiable original or derived information from the files specified in Appendix C to anyone who is not a HIPAA CE OUD Care Team member in a treatment relationship with the subject Participating Beneficiaries; a HIPAA BA of such an OUD Care Team member; the Participant’s BA, where the Participant is itself a HIPAA CE; the Participant’s sub-BA, which is hired by the Participant to carry out work on behalf of the CE OUD Care Team members; or a non-participant HIPAA CE in a treatment relationship with the subject Participating Beneficiary(ies). When using or disclosing PHI or personally identifiable information (“PII”), obtained from files specified in Appendix C, the Participant must make “reasonable efforts to limit” the information to the “minimum necessary” to accomplish the intended purpose of the use, disclosure, or request. The Participant shall further limit its disclosure of such information to the types of disclosures that CMS itself would be permitted make under the “routine uses” in the applicable systems of records listed in Appendix C.
Subject to the limits specified above and elsewhere in this Agreement and applicable law, the Participant may link individually identifiable information specified in Appendix C (including directly or indirectly identifiable data) or derivative data to other sources of individually-identifiable health information, such as other medical records available to the Participant and its OUD Care Team members. The Participant may disseminate such data that has been linked to other sources of individually identifiable health information provided such data has been de-identified in accordance with HIPAA requirements in 45 CFR § 164.514(b).
The Participant agrees to establish appropriate administrative, technical, and physical safeguards to protect the confidentiality of the data and to prevent unauthorized use or access to it. The safeguards shall provide a level and scope of security that is not less than the level and scope of security requirements established for federal agencies by the Office of Management and Budget (OMB) in OMB Circular No. A-130, Appendix I--Responsibilities for Protecting and Managing Federal Information Resources (https://www.whitehouse.gov/sites/whitehouse.gov/files/omb/circulars/A130/a130revised.pdf) as well as Federal Information Processing Standard 200 entitled “Minimum Security Requirements for Federal Information and Information Systems” (https://nvlpubs.nist.gov/nistpubs/FIPS/NIST.FIPS.200.pdf); and, NIST Special Publication 800-53 “Recommended Security Controls for Federal Information Systems” (https://nvlpubs.nist.gov/nistpubs/SpecialPublications/NIST.SP.800-53r4.pdf).
The Participant acknowledges that the use of unsecured telecommunications, including the Internet, to transmit directly or indirectly individually identifiable information from the files specified in Appendix C, or any such derivative data files is strictly prohibited. Further, the Participant agrees that the data specified in Appendix C, must not be physically moved, transmitted, or disclosed in any way from or by the site of the Custodian indicated in Appendix C, other than as provided in this Agreement without written approval from CMS, unless such movement, transmission, or disclosure is required by a law.
The Participant agrees to grant access to the data and/or the facility(ies) in which the data is maintained to the authorized representatives of CMS or HHS Office of Inspector General, including at the site of the custodian indicated in Appendix C, for the purpose of inspecting to confirm compliance with the terms of this Agreement.
The Participant agrees that any use of CMS data in the creation of any document concerning the purpose specified in this section and Appendix C, of the Agreement must adhere to CMS’ current cell size suppression policy. This policy stipulates that no cell (e.g., admittances, discharges, patients, services) representing 10 or fewer beneficiaries may be displayed. Also, no use of percentages or other mathematical formulas may be used if they result in the display of a cell representing 10 or fewer beneficiaries.
The Participant agrees to report any breach of PHI or PII from or derived from the CMS data files, loss of these data or improper use or disclosure of such data to the CMS Action Desk by telephone at (410) 786-2850 or by email notification at [email protected] within one hour. Furthermore, the Participant agrees to cooperate fully in any federal incident security process that results from such improper use or disclosure.
The Parties mutually agree that the individual named in Appendix C is designated as Custodian of the CMS data files on behalf of the Participant and will be responsible for the observance of all conditions of use and disclosure of such data and any derivative data files, and for the establishment and maintenance of security arrangements as specified in this Agreement to prevent unauthorized use or disclosure. Furthermore, such Custodian is responsible for contractually binding any downstream recipients of such data to the terms and conditions in this Agreement as a condition of receiving such data. The Participant agrees to notify CMS within fifteen (15) Days of any change of custodianship. The parties mutually agree that CMS may disapprove the appointment of a custodian or may require the appointment of a new custodian at any time.
Data disclosed to the Participant pursuant to Appendix C may be retained by the Participant until the conclusion or termination of this Agreement. The Participant is permitted to retain any individually identifiable health information from such data files or derivative data files after the conclusion or termination of the Agreement if the Participant is a HIPAA CE, and the data has been incorporated into the subject Participating Beneficiaries’ medical records that are part of a designated record set under HIPAA. Furthermore, any HIPAA CE to whom the Participant provides such data in the course of carrying out ViT may also retain such data if the recipient entity is a HIPAA CE or BA and the data is incorporated into the subject Participating Beneficiaries’ medical records that are part of a designated record set under HIPAA. The Participant shall destroy all other data and send written certification of the destruction of the data files and/or any derivative data files to CMS within 30 Days following the expiration or termination of the Agreement or except as CMS shall authorize in writing or as otherwise required by law. Except for disclosures for treatment purposes, the Participant shall bind any downstream recipients to these terms and conditions as a condition of disclosing such data to downstream entities and permitting them to retain such records under this paragraph. These retention provisions survive the expiration or termination of the Agreement.
De-Identified Reports
CMS will provide the following reports to the Participant, which will be de-identified in accordance with HIPAA requirements in 45 CFR § 164.514(b):
Annual Financial Reports -These reports will include year-to-date information on total ViT Medicare payments to individuals and entities participating in ViT. This aggregate information will not include individually identifiable health information and will incorporate de-identified data from Participating Beneficiaries who have not opted in for data sharing.
Other Reports - CMS will periodically provide reports to the Participant regarding the Participant’s financial performance throughout the Performance Year. The reports will not contain individually identifiable health information and will incorporate de-identified data from Participating Beneficiaries who have opted out of data sharing.
Beneficiary Rights to Opt in to or Terminate Data Sharing
The Participant shall provide Participating Beneficiaries who inquire about or wish to modify their preferences regarding claims data sharing for care coordination and quality improvement purposes with information about how to modify their data sharing preferences. Such communications shall note that, even if a Participating Beneficiary has elected to decline claims data sharing, CMS may still engage in certain limited data sharing for quality improvement purposes.
The Participant shall allow Participating Beneficiaries to reverse a data sharing preference at any time by calling 1-800-MEDICARE.
CMS will maintain the data sharing preferences of Participating Beneficiaries.
The Participant may affirmatively contact a Participating Beneficiary who has elected to decline claims data sharing no more than once in a given Performance Year to provide information regarding data sharing. Such contact includes mailings, phone calls, electronic communications, or other methods of communicating with beneficiaries outside of a clinical setting.
Overlap Policy
Prohibition
The Participant shall not concurrently participate in ViT and any of the following Innovation Center initiatives: PCF, CPC+, or MDPCP.
CMS reserves the right to potentially include additional requirements, revise initiative parameters, or ultimately prohibit simultaneous participation in other CMS initiatives, based on a number of factors, including CMS’s capacity to avoid counting savings twice in interacting initiatives and to conduct a robust evaluation of each such initiative.
Permitted Overlap and Notification
The Participant may concurrently participate in ViT and certain other CMS initiatives, including shared savings, total cost of care, and medical home initiatives.
The Participant must give written notice to CMS if the Participant is selected to participate in any other CMS initiative after the Effective Date. Such notice must be given within 30 Days of receiving notification that the Participant has been selected to participate in the initiative.
Other Government Authorities
None of the provisions of this Agreement limit or restrict any other federal government agency that is permitted by law to audit, evaluate, investigate, or inspect the Participant or its OUD Care Team members.
Agreement to Comply with Laws
The Participant shall comply with, and shall require all OUD Care Team members to comply with the applicable terms of this Agreement and all applicable statutes and regulations, including without limitation: (a) federal criminal laws; (b) the False Claims Act (31 U.S.C. § 3729 et seq.); (c) the anti-kickback statute (42 U.S.C. § 1320a-7b(b)); (d) the civil monetary penalties law (42 U.S.C. § 1320a-7a); and (e) the physician self-referral law (42 U.S.C. § 1395nn).
This Agreement does not waive any obligation of the Participant or its OUD Care Team members to comply with the terms of any other CMS contract, agreement, model, or demonstration.
The failure by CMS to require performance of any provision of this Agreement does not affect CMS’s right to require performance at any time thereafter, nor does a waiver of any breach or default of this Agreement constitute a waiver of any subsequent breach or default or a waiver of the provision itself.
Certification of Data and Information
With respect to data and information that CMS requires to be certified when submitted to CMS by the Participant, the Participant shall ensure that an individual with the authority to legally bind the individual or entity submitting such data or information certifies the accuracy, completeness, and truthfulness of that data and information to the best of his or her knowledge information, and belief.
Audits and Record Retention
-
The Participant agrees, and must require all OUD Care Team members to agree, that the government, including CMS, HHS, and the Comptroller General or their designees, has the right to audit, inspect, investigate, and evaluate any books, contracts, records, documents and other evidence of the Participant and OUD Care Team members that pertain to the following:
The Participant’s compliance with the terms of this Agreement, including provisions that require the Participant to impose duties or requirements on its OUD Care Team members;
Whether the OUD Care Team members complied with the duties and requirements imposed on them by the Participant pursuant to the terms of this Agreement;
The quality of the OUD Treatment Services performed under this Agreement; and
The Participant’s right to CMF and PBIP.
Maintenance of Records
-
The Participant agrees, and must require all OUD Care Team members to agree, to the following:
To maintain and give the government, including CMS, HHS, and the Comptroller General or their designees, access to all books, contracts, records, documents, and other evidence (including data related to Medicare utilization and costs, quality performance measures, and other financial arrangements) sufficient to enable the audit, evaluation, inspection, or investigation of the following:
the Participant’s compliance with the terms of this Agreement, including provisions that require the Participant to impose duties or requirements on OUD Care Team members;
whether the OUD Care Team members complied with the duties and requirements imposed on them by the Participant pursuant to the terms of this Agreement;
the quality of services furnished under this Agreement; and
the Participant’s right to CMF and PBIP.
To maintain such books, contracts, records, documents, and other evidence for a period of 10 years from the expiration or termination of this Agreement or from the date of completion of any audit, evaluation, inspection, or investigation, whichever is later, unless:
CMS determines there is a special need to retain a particular record or group of records for a longer period and notifies the Participant at least 30 Days before the normal disposition date; or
There has been a termination, dispute, or allegation of fraud or similar fault against the Participant, or its OUD Care Team members in which case the records shall be maintained for an additional six years from the date of any resulting final resolution of the termination, dispute, or allegation of fraud or similar fault.
Remedial Action
Remedial Action
If CMS determines that the Participant has failed to meet one or more ViT requirements as set forth in this Agreement, or that the Participant or a member of its OUD Care Team has past or present program integrity issues, CMS may take one or more of the following actions:
Notify the Participant and, if appropriate, the OUD Care Team member of the violation;
Require the Participant to provide additional information to CMS or its designees;
Place the Participant on a monitoring and/or auditing plan developed by CMS;
Require the Participant to remove an OUD Care Team member from the Participant’s list of OUD Care Team members and to terminate its OUD Care Team Member Arrangement, immediately or within a timeframe specified by CMS, with that OUD Care Team member;
Request that the Participant submit a proposed Corrective Action Plan (“CAP”), subject to CMS review and approval, which the Participant must implement once approved by CMS;
Amend this Agreement without the consent of the Participant to provide that any and all waivers of existing law made pursuant to Section 1866F(i) of the Act will be inapplicable;
Discontinue the provision of data sharing and reports to the Participant under Section VIII;
Require the repayment of any CMF or PBIP.
CMS may impose additional remedial actions or terminate this Agreement pursuant to Section XV if CMS determines that remedial actions were insufficient to correct noncompliance with the terms of this Agreement or other program integrity issues.
Further action
CMS and its contractors will work with CMS’ Center for Program Integrity and the HHS Office of Inspector General to report and refer any suspected non-compliance, fraud, or abuse for further investigative or administrative action as appropriate under existing law.
CMS reserves the right to terminate this Agreement, or to require the Participant to terminate its OUD Care Team Member Arrangement with one or more OUD Care Team Members, for reasons including but not limited to:
CMS determines that the Agency no longer has funds to support ViT.
CMS determines that the Participant or, if applicable, a member of the Participant’s OUD Care Team has:
Failed to comply with any term of this Agreement or any other Medicare program requirement, rule, or regulation;
Failed to comply with a monitoring plan and/or auditing plan imposed pursuant to Section XIV.A;
Failed to implement a CMS-approved CAP imposed pursuant to Section XIV.A;
Failed to demonstrate improved performance following any remedial action;
Taken any action that threatens the health or safety of a Participating Beneficiary or other patient;
Failed to repay money owed to the Medicare program as required under the Agreement or any audit issued pursuant thereto;
Consistently failed to meet quality performance thresholds or benchmarks required under the Agreement;
Failed to meet reporting requirements specified in the Agreement, including failure to report to CMS quality measures or information CMS has determined appropriate to monitor and evaluate ViT;
Unreasonably interfered with or impeded CMS’s and its designees’ monitoring and evaluation activities;
Submitted false data or made false representations, warranties, or certifications in connection with any aspect of ViT.
CMS determines that the Participant or any member of its OUD Care Team is subject to sanctions or other actions of an accrediting organization or a federal state, or local governmental agency, to include action by the Department of Health and Human Services (HHS) or the Department of Justice involving violations of applicable laws, statutes, and regulations, including but not limited to: federal criminal laws, the federal False Claims Act, antitrust laws, the federal anti-kickback statute, the federal civil monetary penalties law, the federal physician self-referral law or any other applicable Medicare laws, rules or regulations that are relevant to ViT.
CMS determines that the Participant is unable to implement its participation in ViT due to federal, state, or local laws or scope of practice barriers.
Termination by Participant
The Participant may terminate this Agreement upon advance written notice to CMS. Such notice must specify the effective date of the termination; which date may be no sooner than 30 Days following the date of that notice.
Notification Requirements
Upon termination of the Agreement by the Participant or CMS, the Participant shall provide written notice of such termination to all members of its OUD Care Team. The Participant shall deliver such written notice in a time and manner determined by CMS. The Participant shall include in such notices any content specified by CMS, including information regarding data destruction and record keeping, as applicable.
Upon termination of the Agreement by the Participant or CMS, the Participant shall provide written notice of such termination to each Participating Beneficiary. The Participant shall deliver such written notice in a time and manner determined by CMS. The Participant shall include in such notices any content specified by CMS. Any notice to Participating Beneficiaries is subject to review and approval by CMS.
Miscellaneous
Unless otherwise stated in writing after the Effective Date, all notifications and reports required under this Agreement shall be submitted to the Parties at the addresses set forth below:
CMS:
Value In Treatment
Centers for Medicare & Medicaid Services Center for Medicare and Medicaid Innovation
7500 Security Boulevard
Mailstop: WB-06-05XX
Baltimore, MD 21244
Email: [email protected]
Participant:
Organization Name:
Address:
Email:
Phone Number:
In the event the Participant enters into proceedings relating to bankruptcy, whether voluntary or involuntary, the Participant agrees to furnish, by certified mail, written notification of the bankruptcy to CMS. This notification shall be furnished within 5 Days of the initiation of the proceedings relating to bankruptcy filing. This notification shall include the date on which the bankruptcy petition was filed, the identity of the court in which the bankruptcy petition was filed, and a listing of all federal government contracts, project agreements, contract officers, and project officers for all government contracts and project agreements against which final payment has not been made. This obligation remains in effect until the expiration or termination of this Agreement and final payment under this Agreement has been made.
In the event that one or more of the provisions contained herein shall, for any reason, be held to be invalid, illegal or unenforceable in any respect, such invalidity, illegality or unenforceability shall not affect any other provisions of this Agreement, but this Agreement shall be construed as if such invalid, illegal or unenforceable provisions had never been contained herein, unless the deletion of such provision or provisions would result in such a material change so as to cause completion of the transactions contemplated herein to be unreasonable.
This Agreement, including all Appendices, constitutes the entire agreement between the Parties. The Parties may amend this Agreement or any Appendix hereto at any time by mutual written agreement; provided, however, that CMS may unilaterally amend this Agreement or any Appendix hereto as specified in this Agreement including its Appendices, or for good cause or as necessary to comply with applicable federal or state law, regulatory requirements, accreditation standards or licensing guidelines or rules. To the extent practicable, CMS shall provide the Participant with 30 Days advance written notice of any such unilateral amendment, which notice shall specify the amendment’s effective date.
Termination or expiration of this Agreement by any party shall not affect the rights and obligations of the Parties accrued prior to the effective date of the termination or expiration of this Agreement, except as provided in this Agreement. The data privacy and security requirements articulated in this Agreement survive for the duration that CMS data remains in the possession of the Participant. The rights and duties under the following Sections of this Agreement shall also survive termination of this Agreement and apply thereafter:
Section III.D [Participant Changes]
Section V.D [HIPAA Requirements]
Section VI [Payments];
Section VII [ViT Monitoring and Evaluation];
Section VIII [Data Sharing and Reports];
Section XII [Certification of Data and Information];
Section XIII [Audits and Record Retention];
XV.C [Notification Requirements];
Section XVI.B [Notice of Bankruptcy]; Section XVI.E [Survival];
Section XVI.G [Prohibition on Assignment];
Section XVI.H [Change of Control]; and
Appendix B [Value in Treatment CMF and PBIP Payment Methodology].
If any provision of this Agreement conflicts with a provision of any document incorporated herein by reference, the provision of this Agreement shall prevail.
Except with the prior written consent of CMS, the Participant shall not transfer, including by merger (whether the Participant is the surviving or disappearing entity), consolidation, dissolution, or otherwise: (1) any discretion granted it under this Agreement; (2) any right that it has to satisfy a condition under this Agreement; (3) any remedy that it has under this Agreement; or (4) any obligation imposed on it under this Agreement. The Participant shall provide CMS 90 Days advance written notice of any such proposed transfer. This obligation remains in effect until the expiration or termination of this Agreement and final payment by the Participant under this Agreement has been made. CMS may condition its consent to such transfer on full or partial reconciliation of any monies owed to CMS under the terms of this Agreement. Any purported transfer in violation of this Section is voidable at the discretion of CMS.
CMS may terminate this Agreement or require immediate payment of any monies owed under this Agreement if the Participant undergoes a Change of Control. For purposes of this paragraph, a “Change of Control” shall mean: (1) the acquisition by any “person” (as such term is used in Sections 13(d) and 14(d) of the Securities Exchange Act of 1934) of beneficial ownership (within the meaning of Rule 13d-3 promulgated under the Securities Exchange Act of 1934), directly or indirectly, of voting securities of the Participant representing more than 50% of the Participant’s outstanding voting securities or rights to acquire such securities; (2) upon any sale, lease, exchange or other transfer (in one transaction or a series of transactions) of all or substantially all of the assets of the Participant; or (3) a plan of liquidation of the Participant or an agreement for the sale or liquidation of the Participant is approved and completed. The Participant shall provide CMS 90 Days advance written notice of a Change of Control. This obligation remains in effect until the expiration or termination of this Agreement and final payment by the Participant under this Agreement has been made.
The individual signing this Agreement on behalf of the Participant certifies to the best of his or her knowledge, information, and belief that the information contained in this Agreement (inclusive of Appendices), is accurate, complete, and truthful and that he or she is authorized by the Participant to execute this Agreement and to legally bind the Participant on whose behalf he or she is executing this Agreement to its terms and conditions.
The Participant has been represented (or has had the opportunity to be represented) by their attorneys throughout the transactions contemplated by this Agreement in connection with the execution of this Agreement and any agreements and instruments executed in connection herewith. As a consequence, the Parties do not intend that the presumptions of laws or rules relating to the interpretation of contracts against the drafter of any particular clause should be applied to this Agreement or any document or instrument executed in connection herewith, and therefore waive their effects.
This Agreement and any amendments thereto may be executed in counterparts, each of which shall be deemed to be an original, but all of which, taken together, shall constitute one and the same Agreement. This Agreement and any amendments hereto may be signed by autopen or electronic signature (e.g., DocuSign or similar electronic signature technology) and may be transmitted by electronic means. Copies of this Agreement and any amendments hereto that are so executed and delivered have the same force and effect as if executed with handwritten signatures and physically delivered.
[SIGNATURE PAGE FOLLOWS]
Each Party is signing this Agreement on the date stated opposite that Party’s signature. If a Party signs but fails to date a signature, the date that the other Party receives the signing Party’s signature will be deemed to be the date that the signing Party signed this Agreement.
PARTICIPANT:
Date:
By:

Name of authorized signatory

Title
NPI:
Application ID:
CMS:
Date:
By:

Name of authorized signatory

Title
APPENDIX A: VALUE IN TREATMENT PROGRAMMATIC WAIVERS
Under the authority of 1866F(i) of the Act, CMS finds it necessary solely for purposes of carrying out ViT to waive the following requirements:
Waivers of Beneficiary Cost Sharing:
As necessary to carry out ViT, CMS waives the requirements of sections 1833(a)(1)(F), 1833(a)(1)(L), 1833(a)(1)(N), 1833(a)(1)(U), 1833(a)(1)(CC), and 1833(b) of the Act to the extent otherwise applicable for Medicare Part B payment systems and for OTPs permitted to submit claims on CMS form 835A, such that Medicare will pay 100 percent of the cost of services related to highly coordinated and integrated OUD Treatment Services furnished through ViT to Participating Beneficiaries. This waiver applies on a uniform basis without regard to patient-specific factors and shall be applied to ViT Code claims.
As necessary to carry out ViT, CMS waives the requirements of sections 1833(a)(1)(F), 1833(a)(1)(L), 1833(a)(1)(N), 1833(a)(1)(U), 1833(a)(1)(CC), and 1833(b) of the Act to the extent otherwise applicable for Medicare Part B payment systems, such that Medicare will pay 100 percent of the bundled payment for opioid use disorder treatment services furnished to Participating Beneficiaries by OTPs under 42 C.F.R. 410.67(d), and for OTPs permitted to submit claims on CMS form 835A. This waiver applies on a uniform basis without regard to patient-specific factors and shall be applied to claims for HCPCS codes G2067 through G2080, and any other HCPCS codes specified by CMS for use by OTPs in billing for opioid use disorder treatment services.
As necessary to carry out ViT, CMS waives the requirements of sections 1833(a)(1)(F), 1833(a)(1)(L), 1833(a)(1)(N), 1833(a)(1)(U), 1833(a)(1)(CC), and 1833(b) of the Act to the extent otherwise applicable for Medicare Part B payment systems, such that Medicare will pay 100 percent of the bundled payment for an episode of OUD treatment furnished to Participating Beneficiaries by physicians and other practitioners in the office setting. This waiver applies on a uniform basis without regard to patient-specific factors and shall be applied to claims for HCPCS codes G2086 through G2088 submitted for services furnished to Participating Beneficiaries.
As necessary to carry out ViT, CMS waives the requirements of section 1834(m) of the act, as necessary to allow payment to be made for services furnished to a patient remotely using telecommunications technology in accordance with the terms of this Agreement.
APPENDIX B: VALUE IN TREATMENT CMF AND PBIP PAYMENT METHODOLOGY
Payments
During the 4 Performance Years of the ViT, CMS will pay the Participant a CMF on a quarterly basis. CMS will also pay the Participant a PBIP annually. The payment timeline and methodology for each of these two payments are detailed in the following subsections of this Appendix B.
Timeline
CMS will pay the CMF and PBIP in accordance with the timeline outlined in Table 1. The CMF will be paid as a per Participating Beneficiary per quarter payment for the calendar quarter in which the Participant furnishes and submits a ViT Code claim and will be paid after the claim is processed. The PBIP for a given Performance Year will be paid annually no later than Q3 of the year following the Performance Year.
Table 1: Care Management Fee (CMF) Payments and Performance-Based Incentive Payments (PBIP) Timeline
Calendar Year |
CMF Payments |
PBIP Payments |
|||
Calendar Quarter |
Payment Date |
Performance Year1 |
Claims Runout Period |
Payment Date |
|
2021 |
|
CMF will be paid after the claim is processed
|
04/01/20212 – 12/31/2021 |
01/01/2022 – 03/31/2022 |
None
|
Q2 (04/01/2021 – 06/30/2021) |
|||||
Q3 (07/01/2021 – 09/30/2021) |
|||||
Q4 (10/01/2021 – 12/31/2021) |
|||||
2022 |
Q1 (01/01/2022 – 03/31/2022) |
CMF will be paid after the claim is processed
|
01/01/2022 – 12/31/2022 |
01/01/2023 – 03/31/2023 |
No later than Q3 2023
|
Q2 (04/01/2022 – 06/30/2022) |
|||||
Q3 (07/01/2022 – 09/30/2022) |
|||||
Q4 (10/01/2022 – 12/31/2022) |
|||||
2023 |
Q1 (01/01/2023 – 03/31/2023) |
CMF will be paid after the claim is processed
|
01/01/2023 – 12/31/2023 |
01/01/2024 – 03/31/2024 |
No later than Q3 2024
|
Q2 (04/01/2023 – 06/30/2023) |
|||||
Q3 (07/01/2023 – 09/30/2023) |
|||||
Q4 (10/01/2023 – 12/31/2023) |
|||||
2024 |
Q1 (01/01/2024 – 03/31/2024) |
CMF will be paid after the claim is processed
|
01/01/2024 – 12/31/2024 |
01/01/2025 – 03/31/2025 |
No later than Q3 2025
|
Q2 (04/01/2024 – 06/30/2024) |
|||||
Q3 (07/01/2024 – 09/30/2024) |
|||||
Q4 (10/01/2024 – 12/31/2024) |
|||||
Care Management Fee (CMF)
The amount of the CMF is calculated as $125 per Participating Beneficiary per month (PBPM), adjusted by subtracting the applicable PBIP quality withhold percentage and then applying Medicare sequestration. The adjusted amount is multiplied by three to determine the quarterly payment amount. Claims for the CMF must be billed to the Medicare Administrative Contractor (MAC) appropriate to the Participant.
The MAC will verify that all CMF payment policies outlined in Section VI of the Agreement, and further described below, are met with respect to each CMF claim submitted by the Participant.
Payment Policy
CMS will make a CMF payment to the Participant only if the CMF payment policies outlined in Section VI of this Agreement, and further described below, are met. CMS will monitor CMF payments for compliance with these policies. In addition, the Participant may not simultaneously participate in ViT and the MDPCP, CPC+, or PCF and is therefore ineligible for CMF payments under ViT while also participating in these initiatives. The MAC will verify the absence of such participation overlaps to process CMF claims.
The MAC will deny claims that do not meet the applicable payment policies. CMS will seek repayment of CMF payments that have been made in error or inconsistent with applicable payment policies in accordance with Section VI.F of this Agreement.
The Participant must submit a claim that includes only the ViT Code.
No other billing or procedure codes may be included on the same claim, including the codes used to bill for the bundled payment for opioid use disorder treatment services furnished by OTPs and the codes used to bill for the bundled payment for an episode of OUD treatment furnished by physicians and other practitioners in the office setting.
The MAC will deny any claim that includes the ViT Code and any other billing or procedure code. In such a case, the ViT Code claim for the services furnished to a Participating Beneficiary under ViT must be resubmitted as a standalone claim for the CMF payment to be processed and paid.
Only the Participant may submit a claim with the ViT Code. This means that the billing information on the claim must match the billing information provided to CMS by the Participant, including the Participant’s unique Taxpayer Identification Number (TIN), for the claim to be processed. Other individuals and entities, including the Participant’s OUD Care Team members, may not bill the ViT Code.
A ViT Code claim for services furnished under ViT must be for services furnished to a Participating Beneficiary, as defined in Section II of this Agreement. That is, the Participating Beneficiary must be an Applicable Beneficiary who has agreed to receive OUD Treatment Services from the Participant by signing the Beneficiary Agreement Form.
The MAC will verify that the Participating Beneficiary included on each billed claim satisfies the definition of an Applicable Beneficiary during their claims processing. This means that, on the date of service included on the claim, the Participating Beneficiary must be entitled to, or enrolled for, benefits under Medicare Part A and enrolled for benefits under Medicare Part B (which may include a beneficiary dually-eligible for Medicare and Medicaid); not be enrolled in a Medicare Advantage Plan under Medicare Part C; and have an OUD diagnosis. For purposes of verifying that the Participating Beneficiary had an OUD diagnosis, the submitted claim must contain at least one of the CMS specified ICD-10-CM diagnosis codes for an OUD diagnosis.
To satisfy the definition of a Participating Beneficiary, the beneficiary also must have agreed to receive OUD Treatment Services from the Participant on or before the date of service on the claim. The MAC will not collect proof of the signed Beneficiary Agreement Form for claims processing. Proof that the Participating Beneficiary has signed a Beneficiary Agreement Form agreeing to receive OUD Treatment Services from the Participant will be collected and verified during audits conducted by CMS and its designees described in Sections VII.B.4 and XIII.A of this Agreement.
The ViT Code claim must not be duplicative of another ViT Code claim for services furnished to the same Participating Beneficiary under ViT during the same calendar quarter. If two or more individuals or entities submit a ViT Code claim for services furnished to the same Participating Beneficiary under ViT during the same calendar quarter, only one such claim will be processed. CMS will determine which individual or entity will receive a CMF for that Participating Beneficiary based on the date of claim submission, not based on date of service. That is, CMS will pay a CMF to the individual or entity that submits the first valid ViT Code claim for services furnished to that Participating Beneficiary under ViT during the calendar quarter, irrespective of whether that claim had the earliest date of service.
The submitted claim must not exceed the Per-Participating Beneficiary Cap set for the Participant in accordance with Section VI.C of this Agreement. The Participant is expected to keep track of its billing to monitor its claims volume against the Per-Participating Beneficiary Cap. ViT Code claims submitted for services furnished to any Participating Beneficiary in excess of the number of Participating Beneficiaries included in the Participant’s Per-Participating Beneficiary Cap will be denied by the MAC.
Calculation of CMF Payments with Quality Withhold & Medicare Sequestration Adjustment
The CMF payment made to the Participant is subject to a PBIP quality withhold (No withhold in PY1; 5% in PY2; 10% in each Performance Year thereafter) and, if a sequestration order is in effect, an adjustment to account for sequestration (2%).
Specifically, the amount of the CMF for each ViT Code claim submitted for the calendar quarter in which OUD Treatment Services were furnished to a Participating Beneficiary under ViT is calculated as $125 per Participating Beneficiary per month, minus the applicable PBIP quality withhold and any applicable Medicare sequestration adjustments. This adjusted amount is then multiplied by three to reflect the three-month duration of the calendar quarter. The calculation formulas applicable to each Performance Year are reflected below.
Performance Year 1 (2021)
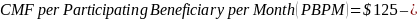 PBPM
PBPM

*Note that the 2% sequestration adjustment is applied only if sequestration is in effect for the period in which the payment is made.
Performance Year 2 (2022)
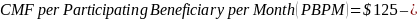 PBPM
PBPM
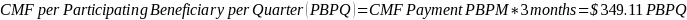
*Note that the 2% sequestration adjustment is applied only if sequestration is in effect for the period in which the payment is made.
Performance Year 3 to 4 (2023 to 2024)
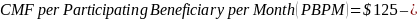 PBPM
PBPM
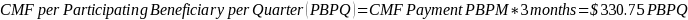
*Note that the 2% sequestration adjustment is applied only if sequestration is in effect for the period in which the payment is made.
Performance-Based Incentive Payments (PBIP)
To encourage the Participant to improve quality of care and reduce healthcare cost, CMS will apply a PBIP quality withhold in calculating the amount of the quarterly CMF payments, as specified in the previous section of this Appendix B. The Participant may earn back the PBIP quality withhold in the form of a PBIP if the Participant meets or exceeds the applicable performance threshold. The PBIP, if any, will be paid to the Participant on an annual basis in accordance to the timeline specified in Table 1 of this Appendix B.
ViT employs the following PBIP design principles and features:
Quality withhold phased in during the Demonstration Performance Period, increasing from none in the first Performance Year, to 5% during the second Performance Year, and to 10% for each of the subsequent Performance Years.
Claim-based performance measures tied to payment are specific to OUD.
The Participant must meet or exceed the performance threshold in order to earn back PBIP quality withhold in the form of a PBIP.
The following policies specify the PBIP quality withhold amount that Participants may earn back based on performance, the quality measures tied to payment, and the quality performance calculation methodology. CMS will notify the Participant of the performance threshold for each Performance Year prior to the start of the Performance Year to which the performance threshold will apply. CMS will also provide the Participant with more detailed information regarding the performance calculation methodology.
PBIP Quality Withhold Calculation
The PBIP quality withhold applied to the $125 PBPM amount used to calculate the CMF payment rate will be 5% in Performance Year 2 (2022) and 10% in each of Performance Years 3 to 4 (2023 to 2024). The formulas and specific amount withheld per quarterly CMF payment are reflected in Table 2.
Table 2: Performance-Based Incentive Payments (PBIP) Quality Withhold Amount
Performance-Based Incentive Payments (PBIP) |
||
Performance Year |
Quality Withhold (%) |
Formula and Specific Amount Withheld |
PY 1 (2021) |
Zero |
|
PY 2 (2022) |
5% |
|
PY 3 (2023) |
10% |
|
PY 4 (2024) |
10% |
|
Quality Measures tied to Payment
For purposes of the PBIP, CMS will compare the Participant’s performance on the quality measures specified in Table 3 to the performance of a national comparison group described below to determine whether the Participant met or exceeded the PBIP performance threshold. These measures are claim-based and require no reporting on the part of the Participant. In addition to the measures modifications described in Table 3, CMS may in CMS’s sole discretion make minor changes to the measures methodologies, including to adjust the numerator and denominator, and to apply risk-adjustments, to ensure each measure is appropriate for use in ViT. CMS will obtain licensing approval to do so, if required. Prior to the start of each Performance Year CMS may also in CMS’s sole discretion opt to not use one or more of the quality measures specified in Table 3. CMS will notify Participant of any changes to the quality measures or measure specifications prior the start of the Performance Year in which such changes will take effect.
Table 3: Performance-Based Incentive Payments (PBIP) Quality Measures
Measure Title |
NQF ID |
Changes to Specifications for ViT? |
Use of pharmacotherapy for opioid use disorder (OUD) |
3400 (NQF endorsed) |
Yes. Measure modified to be specific to Medicare beneficiaries, as the NQF endorsed measure applies to Medicaid beneficiaries ages 18 to 64 with an OUD diagnosis. |
Initiation and Engagement of Alcohol and Other Drug Abuse or Dependence Treatment |
0004 (NQF endorsed) |
No. Will use specifications as defined. ViT will consider this measure two separate measures, as it calculates two rates. |
Continuity of Pharmacotherapy for Opioid Use Disorder (OUD) |
3175 (NQF endorsed) |
No. Will use specifications as defined. |
Emergency Department (ED) Use Due to Opioid Overdose |
N/A (not NQF endorsed) |
CMS will develop specifications to capture the rate of ED visits for opioid overdose events using ICD-9 or ICD-10 diagnosis codes for Medicare beneficiaries aged 18 and older. |
National Comparison Group
CMS will assess the Participant’s performance on the combined quality measures in Table 3 (or such other measures specified by CMS) relative to a national comparison group’s averaged composite score to determine whether the Participant has met or exceeded the performance threshold.
CMS will identify the national comparison group as a group comparable to but excluding those individuals and entities participating in ViT. CMS will assess the performance of this national comparison group on the quality measures in Table 3 (or such other measures specified by CMS) using Medicare Fee-for-Service claims data. CMS will combine the performance scores for each of the quality measures across the national comparison group into an averaged composite score.
Quality Performance Calculation Methodology
The Participant’s performance scores for each of the quality measures in Table 3 will be combined into an averaged composite score. CMS will then compare the Participant’s averaged composite score (rounded to the second decimal) to the averaged composite score for the national comparison group described above (rounded to the second decimal) to establish a percentile score. Finally, CMS will determine whether the Participant’s percentile score meets or exceeds the performance threshold. The performance threshold is an absolute threshold that the Participant must meet or exceed to earn back the PBIP quality withhold amount.
CMS will calculate the Participant’s quality performance on a given quality measure only if a minimum of 125 Participating Beneficiaries are included in the measure. To ensure the Participant has the opportunity to earn back the PBIP quality withhold in the form of a PBIP, CMS will include the Participant in a pool with other ViT Participants to meet the minimum Participating Beneficiary count. The quality performance calculation methodology used to calculate PBIP payments is detailed in Table 4.
Table 4: Performance-Based Incentive Payments (PBIP) Quality Performance Calculation Methodology
ViT Participant with <125 unique Participating Beneficiaries |
ViT Participant with 125+ unique Participating Beneficiaries |
|
|
APPENDIX C: VALUE IN TREATMENT HIPAA-COVERED DISCLOSURE REQUEST ATTESTATION and DATA SPECIFICATION WORKSHEET
Performance Year 2021 HIPAA-Covered Disclosure Request Attestation
The Participant requests the CMS data listed in the Data Specification Worksheet below for Performance Year 2021 and makes the following assertions regarding its ability to meet the HIPAA requirements for receiving such data:
The Participant is (select if applicable):
A HIPAA Covered Entity (CE) as defined in 45 CFR § 160.103.
The Participant is seeking protected health information (PHI), as defined in 45 CFR § 160.103 (select one):
For its own use.
On behalf of a CE for which the Participant is a BA.
Other: Please attach a description of the intended purpose (e.g., for “research” purposes, for “public health” purposes, etc.).
The Participant requests (select all that apply):
For the Participating Beneficiaries who have agreed, pursuant to a Beneficiary Agreement Form, for CMS to share data with the Participant under ViT, for Performance Year 2021: (i) 1 years of historical claims data files for the data elements identified in the Data Specification Worksheet; and (ii) monthly claims data files for the data elements identified in the Data Specification Worksheet, from the following CMS data files:
-
Data Elements
Source
Part A Claims Header
CCW
Part A Claims Revenue Center
CCW
Part B Physicians Header and Lines
CCW
Beneficiary Cross-Reference (XREF)
CCW
Page 1 of 13
Other: Please attach a detailed description of the data requested.
The Participant intends to use the requested data to carry out (select one):
“Health care operations” that fall within the first and second paragraphs of the definition of that phrase under the HIPAA Privacy Rule (45 CFR § 164.501).
Other: Please attach a description of the intended purpose (e.g., for “research” purposes, for “public health” purposes, etc.).
The data requested is (select one):
The "minimum necessary" (as defined at 45 CFR § 164.502) to carry out the health care operations activities described above.
Other: Please attach a description of how (if applicable) the data requested exceeds what is needed to carry out the work described above.
In making this request, the Value in Treatment participant names the following individual as the data custodian. In providing this information, the Value in Treatment participant attests that this individual is either an employee of the Value in Treatment Participant or an employee of a BA of the Value in Treatment Participant that requires access to the requested data for the purpose indicated above. The Participant’s data custodian for the requested data is as follows:
_____________________
(name of individual serving as data custodian)
_____________________
(data custodian organization)
_____________________
(data custodian phone number)
_____________________
(data custodian e-mail)
_____________________
(alternative contact #1 name)
_____________________
(alternative contact #1 organization)
_____________________
(alternative contact #1 phone number)
_____________________
(alternative contact #1 e-mail)
By: Date:
Name of authorized signatory
Title
Data Element Source |
Data Element |
Data Element Description |
Part A Claims |
Claim From Date |
The first day on the billing statement that covers services rendered to the beneficiary. |
Claim Thru Date |
The last day on the billing statement that covers services rendered to the beneficiary. |
|
Claim Payment Amount |
Amount that Medicare paid on the claim. |
|
Facility Provider NPI Number |
Identifies the facility associated with the claim. Each facility is assigned its own unique NPI. |
|
Claim Effective Date |
Date the claim was processed and added to the NCH. Also referred to as the NCH Weekly Processing Date. |
|
Claim Bill Frequency Code |
The third digit of the type of bill (TOB3) code. It indicates the sequence of the claim in the beneficiary's current episode of care (e.g., interim or voided). |
|
Find Claim Frequency Codes here: http://www.resdac.org/cms-data/variables/Claim-Frequency-Code. |
||
Calendar Century Year Month Number |
The year and calendar month number combination in the format 'YYYYMM'. e.g. 200701, 200702, etc. |
|
Part A Claims |
Claim Line From Date |
The date the service associated with the line item began. |
Claim Line Thru Date |
The date the service associated with the line item ended. |
|
Claim Line Institutional Revenue Center Date |
The date that applies to the service associated with the Revenue Center code. |
|
HCPCS Code |
The HCPCS code representing the procedure, supply, product, and/or service provided to the beneficiary. |
|
Claim From Date |
The first day on the billing statement that covers services rendered to the beneficiary. |
|
Claim Thru Date |
The last day on the billing statement that covers services rendered to the beneficiary. |
|
Claim Line Service Unit Quantity |
The number of dosage units of medication that were dispensed in this fill. |
|
Claim Line Covered Paid Amount |
The amount Medicare reimbursed the provider for covered services associated with the claim-line. |
|
Calendar Century Year Month Number |
The year and calendar month number combination in the format 'YYYYMM'. e.g. 200701, 200702, etc. |
|
Part B Physicians |
Claim From Date |
The first day on the billing statement that covers services rendered to the beneficiary. |
Provider Type Code |
Identifies the type of Provider Identifier. |
|
Rendering Provider FIPS State Code |
Identifies the state that the provider providing the service is located in. |
|
Claim Rendering Federal Provider Specialty Code |
Indicates the CMS specialty code associated with the provider of services. CMS used this number to price the service on the line-item. |
|
Claim Line From Date |
The date the service associated with the line item began. |
|
Claim Line Thru Date |
The date the service associated with the line item ended. |
|
HCPCS Code |
The HCPCS code representing the procedure, supply, product, and/or service provided to the beneficiary. |
|
Claim Line Covered Paid Amount |
The amount Medicare reimbursed the provider for covered services associated with the claim-line. |
|
Claim Provider Tax Number |
The SSN or Employee Identification Number (EIN) of the provider of the indicated service. This number identifies who receives payment for the indicated service. |
|
Rendering Provider NPI Number |
A number that identifies the provider rendering the indicated service on the claim line. Each provider is assigned its own unique NPI. |
|
Claim Carrier Payment Denial Code |
Indicates to whom payment was made (e.g., physician, beneficiary), or if the claim was denied. |
|
Claim Line Processing Indicator Code |
Indicates whether the service indicated on the claim line was allowed or the reason it was denied. |
|
Claim Effective Date |
Date the claim was processed and added to the NCH. |
|
Claim Line Allowed Charges Amount |
The amount Medicare approved for payment to the provider. |
|
Claim Line Service Unit Quantity |
The number of dosage units of medication that were dispensed in this fill. |
|
Calendar Century Year Month Number |
The year and calendar month number combination in the format 'YYYYMM'. e.g. 200701, 200702, etc. |
|
Meta Process Date |
The date the CCLF process loaded the historical record in the table |
|
Beneficiary XREF |
Calendar Century Year Month Number |
The year and calendar month number combination in the format 'YYYYMM'. e.g. 200701, 200702, etc. |
Meta Process Date |
The date the CCLF process loaded the historical record in the table |
1 Performance year 1 will be abbreviated and will consist of 9 months.
2 In the event that the Effective Date of this Agreement is a date after April 1, 2021, the start of this Performance Year will be the Effective Date.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | ViT Draft Participation Agreement |
| Author | CMS |
| File Modified | 0000-00-00 |
| File Created | 2023-08-25 |
© 2026 OMB.report | Privacy Policy