0 - Environ PH Trckg 2023 (REV) SSA
0 - Environ PH Trckg 2023 (REV) SSA.docx
[NCEH] Environmental Public Health Tracking Network (Tracking Network)
OMB: 0920-1175
Environmental Public Health Tracking Network
OMB Control No. 0920-1175 (Expiration Date: 07/31/2023)
Revision of ICR
Supporting Statement Part A –
Justification
Program Official/Project Officer: Patrick Wall
Title: Section Chief (Acting)
Phone: 770-488-3819
Email: [email protected]
Date: 05/26/2023
Table of Contents
A.1. Circumstances Making the Collection of Information Necessary 3
A.2. Purpose and Use of the Information Collection 4
A.3. Use of Improved Information Technology and Burden Reduction 10
A.4. Efforts to Identify Duplication and Use of Similar Information 12
A.5. Impact on Small Businesses or Other Small Entities 12
A.6. Consequences of Collecting the Information Less Frequently 12
A.7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5 13
A.9. Explanation of Any Payment or Gift to Respondents 16
A.10. Protection of the Privacy and Confidentiality of Information Provided by Respondents 16
A.11. Institutional Review Board (IRB) and Justification for Sensitive Questions 17
A.12. Estimates of Annualized Burden Hours and Costs 17
A.13. Estimates of Other Total Annual Cost Burden to Respondents and Record Keepers 20
A.14. Annualized Cost to the Federal Government 21
A.15. Explanation for Program Changes or Adjustments 22
A.16. Plans for Tabulation and Publication and Project Time Schedule 25
A.17. Reason(s) Display of OMB Expiration Date is Inappropriate 26
A.18. Exceptions to Certification for Paperwork Reduction Act Submissions 27
Part A. Justification
THIS IS A REQUEST FOR URGENT REVIEW AS THE EXPIRATION DATE IS 07/31/2023. |
A.1. Circumstances Making the Collection of Information Necessary
The CDC is seeking Paperwork Reduction Act (PRA) clearance to continue to collect state and local information from Cooperative Agreement (CoAg) recipients for three years. This information collection is sponsored by the Environmental Public Health Tracking Section (Tracking Section), Division of Environmental Health Science and Practice (DEHSP), National Center for Environmental Health (NCEH) at CDC. This program is authorized under Sections 311 and 317(k)(2) of the Public Health Service Act, [42 U.S.C. Sections 243 and 247b(k)(2)] as amended (see Attachment 1). The 60-day Federal Register Notice is provided as Attachment 2 and is further discussed in Section A.8. The CDC is requesting to revise the information collection request (ICR) titled Environmental Public Health Tracking Network (Tracking Network) (OMB Control No. 0920-1175, expiration date 07/31/2023) and obtain approval for a 3-year Paperwork Reduction Act (PRA) clearance.
In September 2000, the Pew Environmental Health Commission issued a report entitled America’s Environmental Health Gap: Why the Country Needs a Nationwide Health Tracking Network. The Commission documented a critical gap in “knowledge that hinders our national efforts to reduce or eliminate diseases that might be prevented by better managing environmental factors” due largely to the fact that existing environmental health systems were inadequate and fragmented. They described a lack of data for the leading causes of mortality and morbidity, a lack of data on exposure to hazards, a lack of environmental data with applicability to public health, and barriers to integrating and linking existing data. To address this critical gap, the Commission recommended a “Nationwide Health Tracking Network” for disease and exposures. In response to the report and this critical gap, Congress appropriated funds in the fiscal year 2002 budget for the CDC to establish the National Environmental Public Health Tracking Program (Tracking Program) and Network and has appropriated funds each year thereafter to continue this effort.
The Tracking Program includes state and local health departments (SLHD) which collaborate to (1) build and maintain the Tracking Network, (2) advance the practice and science of environmental public health tracking, (3) communicate information to guide environmental health policies and actions, (4) enhance tracking workforce and infrastructure, and (5) foster collaborations between health and environmental programs. In spring of 2022, under Notice of Funding Opportunity CDC-RFA-EH22-2202 (Attachment 3), the CDC’s Tracking Program funded 33 state and local public health departments (SLHD). The 33 recipients were selected through a competitive objective review process and are managed as CDC cooperative agreements. Awards are for five [5] years and renewed through an Annual Performance Report (APR)/Continuation Application. The Tracking Program collects data from recipients about their activities and progress for the purposes of program evaluation and monitoring (hereinafter referenced as program data).
Environmental public health tracking is the ongoing collection, integration, analysis, and dissemination of health, exposure, and hazard data (hereinafter referenced as Tracking Network data) to inform public health actions that protect the population from harm resulting from exposure to environmental contaminants. The Tracking Network provides data from existing health, exposure, and hazard surveillance systems and supports ongoing efforts within the public health and environmental sectors to improve data collection, accessibility, and dissemination as well as analytic and response capacity. Data that were previously collected for different purposes and stored in separate systems are now available in a nationally standardized format allowing programs to begin bridging the gap between health and the environment.
The changes to the ICR since the 2020 change request are in Section A.15.
A.2. Purpose and Use of the Information Collection
Tracking Network Data Collection and Dissemination
The purpose of this information collection is to support both general purpose statistics and research. Data on health, exposures, environmental hazards, and populations are obtained from existing data sources and integrated into the Tracking Network to address the critical gap in “knowledge that hinders our national efforts to reduce or eliminate diseases that might be prevented by better managing environmental factors” identified by the Pew Environmental Health Commission. Having integrated data in one network permits public health authorities at the national, state, and local level to (1) describe temporal and spatial trends in disease and potential environmental exposures, (2) identify populations most affected, (3) generate hypotheses about associations between health and environmental exposures, and (4) inform environmental public health policies and interventions aimed at reducing or eliminating diseases associated with environmental factors in state and local jurisdictions. Further, the availability of these types of data in a standardized network supports further agency research investigating the possible associations between the environment and adverse health effects and enables a better understanding of known associations among healthcare practitioners and the public. Our data are unique in that they undergo a very careful QA/QC process at the state/local levels and at CDC, as shared on the previous page. One key feature of the Tracking Program is the development of Nationally Consistent Data and Measures (NCDMs). The purpose of NCDMs is to ensure compatibility and comparability of data and measures useful for understanding the impact of our environment on health. There is a specific process for creating NCDMs that all recipients follow; a similar process is followed by our Tracking Program for national level data (Attachment 9). This information is shared on our Tracking Network: https://www.cdc.gov/nceh/tracking/pdfs/ncdm_requirements_april2017.pdf.
In collaboration with SLHD and federal partners, the Tracking Program identifies priority environmental health issues. When data are available nationally or publicly (for example, through another federal program or a public website), the Tracking Program obtains data from those national or public sources, placing no burden on recipients or unfunded SLHD. When data are not available nationally or publicly, the Tracking Program relies on recipient SLHDs to obtain these data from the original data stewards and submit them to the National Tracking Network. Unsolicited and unfunded SLDH also voluntarily contribute data to the Network. Tracking Section data management processes are detailed in Attachment 10.
Data from recipients or other SLHD are submitted annually following standardized procedures. Data submitted annually by recipients and other SLHD to the Tracking Program include 7 datasets and the metadata form, specifically (1) birth defects prevalence, (2) childhood blood lead levels, (3) drinking water monitoring, (4) emergency department visits, (5) hospitalizations, (6) radon testing, (7) biomonitoring, and (8) metadata. Each dataset contains aggregated data at the county or sub-county level and either day, month, or year as the temporal resolution. The data collection forms are Attachments 4A-4H.
A metadata record, based on standards created by the Federal Geographic Data Committee, is also submitted with each dataset using the Tracking Program’s metadata creation tool. Metadata describes the original source and collection procedures for the data being submitted. SLHD provide one metadata record per dataset per year; SLHD currently submit up to 6 datasets. National data providers also provide metadata or the equivalent documentation. Metadata records are used by the Tracking Program to capture and understand any differences or nuances for a dataset between awardees. The metadata record is also disseminated via the Tracking Network so other users of the data can understand the data as well. A blank metadata template form can be found in Attachment 4H.
In the past 3 years under Program Announcement CDC-RFA-EH17-1702 (Attachment 6), Tracking data were:
Collected and updated from funded and unfunded SLHD partners
Used to calculate standardized measures for environmental health surveillance
Integrated into the Tracking Network and disseminated to the public via the Tracking Network’s National Public Portal at https://ephtracking.cdc.gov/DataExplorer/.
Queried 10,900,00 times via the Tracking Network’s National Public Portal
Used for analyses by CDC researchers, for example:
Shin M, Hawley C, Strosnider H. Common and Unique Barriers to the Exchange of Administrative Healthcare Data in Environmental Public Health Tracking Program. Int J Environ Res Public Health. 2021 Apr 20;18(8):4356.
Werner AK, Strosnider HM. Developing a surveillance system of sub-county data: Finding suitable population thresholds for geographic aggregations. Spat Spatiotemporal Epidemiol. 2020 Jun;33:100339. doi: 10.1016/j.sste.2020.100339.
Boulet SL, Zhou Y, Shriber J, Kissin DM, Strosnider H, Shin M. Ambient air pollution and in vitro fertilization treatment outcomes. Hum Reprod. 2019 Oct 2;34(10):2036-2043.
Monti MM, David F, Shin M, Vaidyanathan A. Community drinking water data on the National Environmental Public Health Tracking Network: a surveillance summary of data from 2000 to 2010. Environ Monit Assess. 2019. 191(9):557.
Shin M, Werner AK, Strosnider H, Hines LB, Balluz L, Yip FY. Public Perceptions of Environmental Public Health Risks in the United States. Int J Environ Res Public Health. 2019 Mar 22;16(6):1045.
Program Data
In addition to standard reporting required by CDC’s Office of Grants Services (OGS), CDC’s Tracking Program also collects information from recipients for the purposes of program evaluation and monitoring. Data collection forms are provided to assist recipients in gathering the necessary information (Attachments 5A-5I). This information includes Attachment 5A: Workplan Template, Attachment 5B: Work Plan REDCap Form, Attachment 5C - Program Accomplishments-Public Health Actions, Attachment 5D - Program Accomplishments-Public Health Actions - REDCap Form, Attachment 5E - Performance Measures Report, Attachment 5F - Performance Measures REDCap Form, Attachment 5G - PHA Impact Follow Up - REDCap form, Attachment 5H - Communication Plan Template, Attachment 5I - Web Stats Template. Each of these forms are collected by CDC via an electronic data capture system (EDCS) annually. The program accomplishment/public health action report is submitted as available but at least twice a year via an EDCS to the Tracking Program. In the past three years, the program data were collected from all 26 funded SLDH. Collectively, the 26 funded SLHD submitted almost 600 PHAs to CDC for review.
These data were used to identify funded SLHD in need of additional technical assistance, identify common challenges and successes, improve communication between funded SLHD and CDC, and to monitor funded SLHD compliance with funding requirements. Specifically, each report was used in the following ways.
Work Plan Template
Ongoing monitoring of the award to evaluate its effectiveness, and for continuous program improvement
Outline projects and related activities, with timelines and expected targets for each
Program Accomplishments and Public Health Actions Report
Provide CDC leadership with program performance data
Collect examples of how Tracking surveillance data have been used
Provide recipient successes that can be shared as success stories
Evaluate overall program impact
Eatman S, Strosnider HM. CDC's National Environmental Public Health Tracking Program in Action: Case Studies From State and Local Health Departments. J Public Health Manag Pract. 2017 Sep/Oct;23 Suppl 5 Supplement, Environmental Public Health Tracking:S9-S17.
Public Health Action Impact Follow-Up Report
Identify and collect further evidence of impact beyond initial reporting of Public Health Actions
Provide narrative continuity for Public Health Actions as they realize impact and collect evidence
Inform Tracking evaluation reports of the additional evidence of impact collected
Performance Measures Report
Tracks 22 measures of program progress that address Surveillance, Outreach/Communications, Information Technology, Partnerships, and Program Capacity aspects of recipient work.
Provides a format of data collection useful for capturing performance measures recipients report annually.
Describes the data gaps and limitations recipients addressed, identifies data that measures the performance of grant recipients’ activities, and informs program of activities that occurred after tracking data identified a disproportionately affected population
Establishes volume and type of communication activities completed by grant recipients and informs program of the partnerships and mentorships established by grant recipients
Captures new tools, processes, and data pipeline enhancements grant recipients completed; how many real-time/near real-time granular datasets grant recipients maintain; and describes the volume and description of routine analyses that result in impact
Communication Plan
Determine program outcomes and create communication objectives
Identify communications activities including the target audience, timeline, evaluation measures, and targets
Identify innovative audiences and partnerships
Inform PMO workgroup activities (opportunities for trainings and presentations)
Provide a picture of the national reach and usage of the Tracking Network and share that picture with internal and external audiences.
Web Stats Template
Provide a picture of the national reach and usage of the Tracking Network, including funded SLHD components of the network.
Monitor and evaluate the use of funded SLHD’s public portal on the Tracking Network by logging measures such as the number of visitors, number of data queries, and the data most frequently queried.
Identify needs of the users of the Tracking Network and ensure that resources are focused on those data with the greatest utility.
Inform program activities and recommendations including what additional data should be implemented by all funded SLHD because of the frequent use of the data on individual funded SLHD public portals
Terms of clearance: Approved consistent with the understanding that CDC endeavors to
more prominently display the data sources, limitations, scope/scale, and recommendations for
interpretation of the tracking system (currently available on:
https://ephtracking.cdc.gov/showIndicatorPages, and will communicate these limitations in
any presentations or dissemination of the Tracking Network data. As the Tracking Network
primarily collects certain health information from only 33 funded state and local health
departments, and since there may exist variation across the jurisdictions' methods for
collecting the information –collected data are not nationally representative. This information is intended to gain insight into issues that are present at the state and local levels, and can be
employed to inform regional or multi-state public health actions for those 33 recipients.
Additionally, the Tracking Network also includes some national-level data that are relevant to
environmental health, which is collected in collaboration with other federal programs and from publicly available data sources.
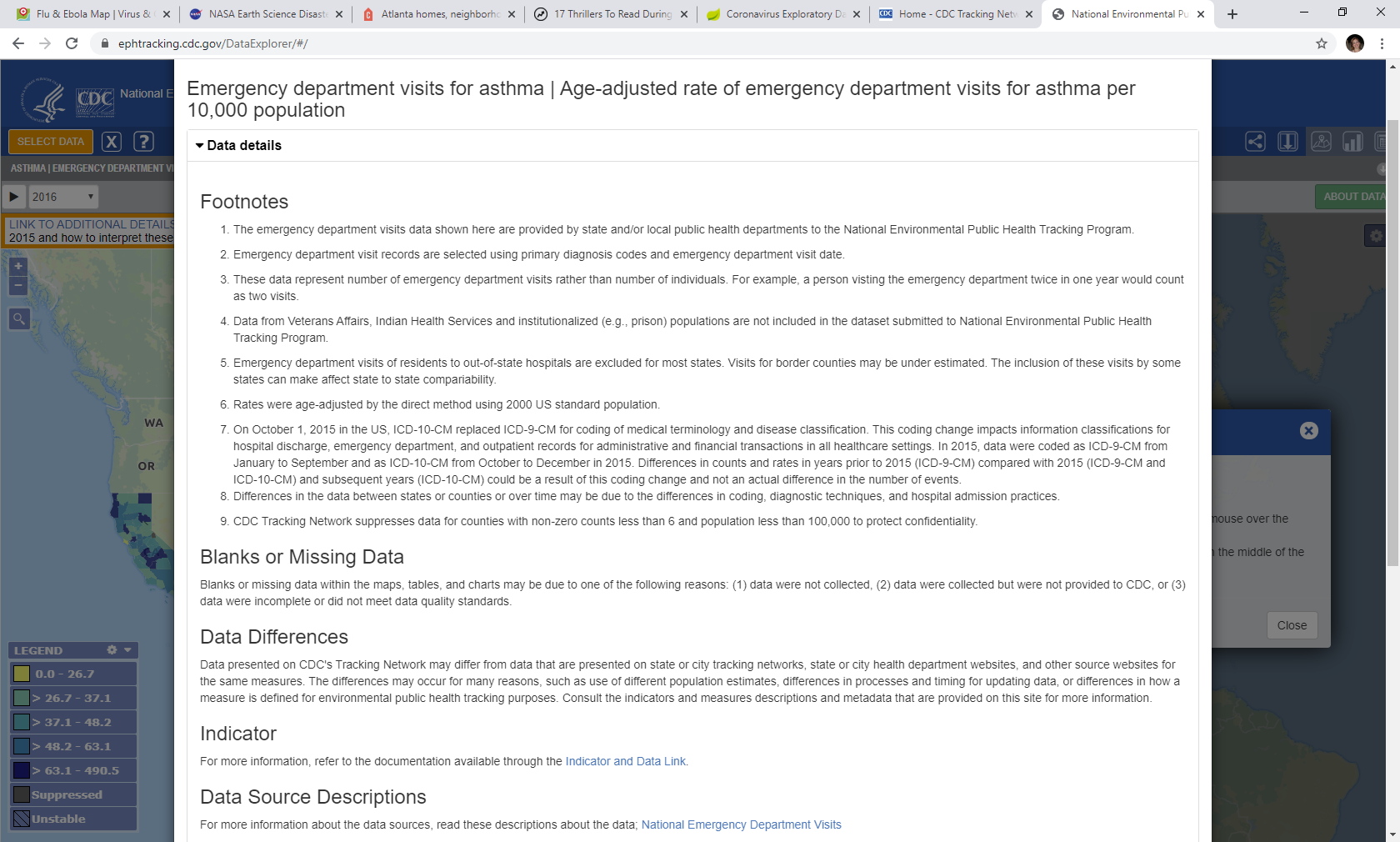
The Tracking Program continues to effectively communicate the limitations of the data to the users to address the terms of clearance. In addition to the indicator templates (https://ephtracking.cdc.gov/showIndicatorPages), the program provides footnotes for each measure and has implemented a toast message above the map to display highly important limitations. Further, these data are never aggregated or presented in a way to imply that they are nationally represented.
Figure A: Data Explorer Toast Message and About Data Button


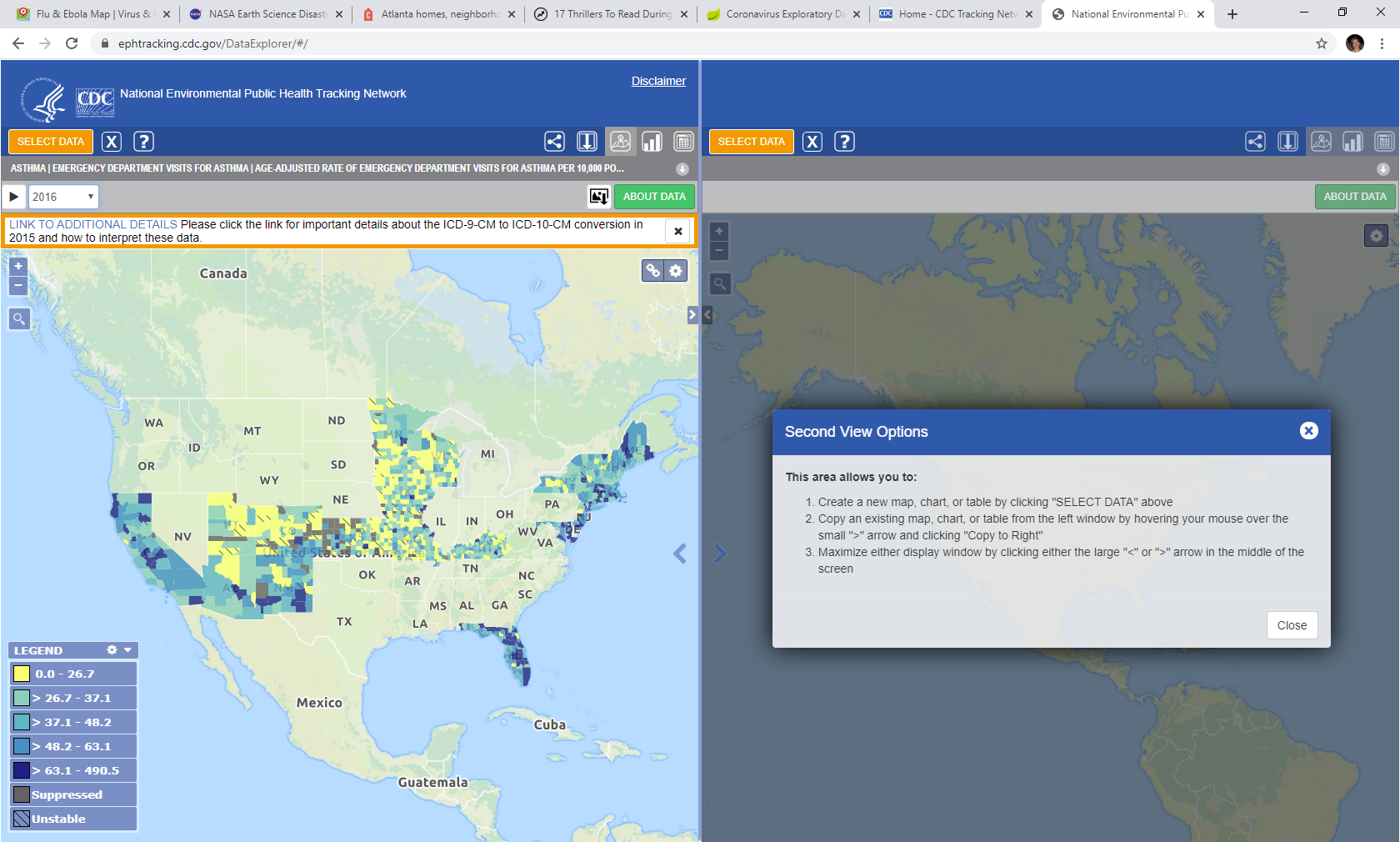
Figure B: About Data Text
A.3. Use of Improved Information Technology and Burden Reduction
The Tracking Network is a web-based information system that collects and disseminates standardized data by state or local jurisdiction at a national level. Special attention has been given to ensuring the system is easy to use and collects information that can later be queried and summarized to public health professionals and their stakeholders using the Tracking Network’s National Public Portal. The system was developed for recipient participation with the following objectives:
Shortening the time period for collecting information
Standardizing the information collection and dissemination processes
Identifying promising practices
Measuring system usage and user preferences
Sharing knowledge and experience
Reducing dependence on paper
The Tracking Network fosters consistency of information through its uniform collection process and well-defined information components. This collection process takes advantage of technology that minimizes the number of errors and redundancy. The process allows all data to be carefully reviewed and validated to ensure accuracy. Data are submitted electronically using an establish XML protocol through a CDC’s secure file transfer (Attachments 4A-4H).
Program data are submitted to CDC via email and EDCS using data collection forms (Attachments 5A-5I).
In 1702, sorting through and organizing the data by the program before using it for analysis and evaluation posed challenges. Using an EDCS helps significantly reduce this burden by optimizing the data capture method to eliminate the need for personnel to complete manual data cleaning and organization before using data for analysis and evaluation upon submission. An EDCS automatically codes data with searchable variables, showcases data through expedient report generations, and can export data into a data lake so the program can create dashboards that automate routine analysis and present data for further analysis and evaluation. The result is our Program Services, and Evaluation team can invest their time focusing on richer analysis and evaluation.
Additionally, an EDCS provides improvements for connecting recipient projects and their activities. Using an excel template offers an additional challenge for connecting activities with respective projects. Recipients often merge cells to convey the connection between the project and its activities resulting in inconsistent data delivery formatting. This significantly increases the burden of sorting and organizing the data before it can be reviewed for analysis and evaluation. Using an EDCS eliminates this problem.
Improving and consolidating data collection field types also contributes to reducing the time it takes to complete forms by recipients. EDCS allows for easier multi-select and file upload methods than Excel spreadsheet support. Using an EDCS allows for consolidating uploaded files with recipient records and streamlining the capture of different types of field collections.
Using an EDCS allows recipients to work on their deliverables using an internal system and provide a single exported file for delivering their data in one step. This gives the recipient more control over the development of their deliverable data and significantly easy submission to the program.
Program data are submitted to CDC via a direct EDCS. Using an EDCS enables the creation of a database of recipient data which allows all data captured to be streamlined and ready to use for analysis, and evaluation, upon submission. By populating this database with consistent and accurate data, program staff will be able to develop reports outlining recipient success and technical assistance needs. Implementing data collection using an EDCS will decrease recipient burden hours and improve recipient data reporting streams on a quarterly (PA/PHA) and yearly (APR, PM) basis. Using an EDCS, recipients gain improvements to collaborate within both their network and with CDC programmatic staff. The added functionality will also decrease burden hours and allow CDC program staff to provide real-time oversight and guidance.
A.4. Efforts to Identify Duplication and Use of Similar Information
The collection of this information is part of a federal reporting requirement for funds received by recipients. The Tracking Program’s efforts to identify duplication included attendance at national meetings and consultations with SLHD, other federal agencies, and academia.
As previously described in Part A.1, in 2000, the Pew Environmental Health Commission documented a critical gap in “knowledge that hinders our national efforts to reduce or eliminate diseases that might be prevented by better managing environmental factors” due largely to the fact that existing environmental health systems were inadequate and fragmented. To address the gap, Congress appropriate funds to CDC to develop the Tracking Network. The standardized data received by the Tracking Network from SLHD are not duplicated elsewhere.
To avoid duplication, the Tracking Program does not collect from SLHD any data which are already submitted to the federal government as needed by the Tracking Program. For example, the Tracking Program receives data on cancer, vital statistics, and air pollution from federal partners. Further, the Tracking Program does not request duplicate childhood blood lead levels from recipients that already report to CDC’s Lead Poisoning Program (under the Blood Lead Surveillance System (BLSS) - OMB Control No. 0920-1175, expiration date 07/31/2023).
A.5. Impact on Small Businesses or Other Small Entities
This data collection will not involve small businesses.
A.6. Consequences of Collecting the Information Less Frequently
Tracking Network Data
Each dataset is collected annually during either the fall or the spring data call in fulfillment of requirements outlined in Notice of Funding Announcement CDC-RFA-EH22-2202 (Attachment 3). Metadata are collected 2 times a year because metadata are required for each of the 7 datasets collected once a year (during either the fall or the spring data call). Less frequent data submissions will negatively impact the timeliness and utility of the data on the Tracking Network. Annual collection of data allows the Tracking Network to stay current and provide the most recently available data.
Program Data
Program data are collected at varying intervals throughout the year, from once a year to quarterly. Less frequent collection of these performance measures would negatively impact the program’s ability to make necessary adjustments to ensure program success; demonstrate utility of data; to document program impact on environmental-related disease burden; and to be accountable to CDC leadership and appropriators. Other reports are collected less frequently and are consistent with guidance from other offices at CDC.
There are no legal obstacles to reduce the burden.
A.7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
There are no special circumstances. This request fully complies with the regulation 5 CFR 1320.5. Metadata are collected for each dataset. Datasets are collected annually during either the fall or spring data call, and each dataset is required to have metadata submitted as part of the data call process.
A.8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
In accordance with 5 CFR 1320.8(d), a 60-day notice for public comment and recommendations was published in the Federal Register on 12/20/2022, Vol. 84, No. 243, pp. 77840. One comment was received, the majority of which was non-substantive and the majority of which was not relevant to Tracking Section work (Attachment 2a). A commenter noted the estimated annualized burden hours for birth defects data collection and questioned why it is only 30 respondents. CDC addressed this by updating the text in the section on annualized burden hours (Section A.12).
The Tracking Program consults annually with its state and local external partners (Table 1). These consultants are experts in environmental public health surveillance and provide strategic input for the program. These meetings last two days and provided a forum for open discussions with the program.
Table 1. 2022 CDC External Consultations
Name |
Title |
Affiliation |
Phone |
|
OUTSIDE CONSULTANTS |
||||
Matthew Roach, MPH |
Principal Investigator |
Arizona Department of Health Services |
602-542-1025 |
|
Paul B. English, PhD, MPH
|
Branch Scientific Advisor |
California Department of Health |
510-620-3684 |
|
Kristy Richardson, PhD |
Principal Investigator |
Colorado Department of Public Health and Environment |
303-692-2606 |
|
Jim Vannoy
|
Principal Investigator |
Connecticut Department of Public Health |
860) 509-7963 |
|
Tabetha Offutt-Powell, PhD |
Principal Investigator |
Executive Office Of The Governor Of Delaware |
903-399-2761 |
|
Chris DuClos, MS |
Principal Investigator
|
Florida Department of Health |
850-245-4264 |
|
Jason Ravenscroft |
Principal Investigator |
Health and Hospital Corporation of Marion County |
317-221-3358 |
|
Neil Muscatiello
|
Principal Investigator |
Health Research, Inc. (New York State) |
(518) 431-1242 |
|
Ken Sharp, MPA, RS |
Principal Investigator |
Iowa Department of Public Health |
515-281-5099 |
|
Farah S. Ahmed, PhD., MPH
|
Environmental Health Officer
|
Kansas Department of Health & Environment |
785-296-6426 |
|
Kathleen Winter, PhD |
Principal Investigator |
Kentucky Cabinet for Health and Human Services |
502-564-3418 |
|
Kate Friedman, MNS |
Principal Investigator |
Louisiana Department of Health & Hospitals |
225-342-7135 |
|
Andrew E. Smith, S.M., ScD |
Principal Investigator |
Maine Center for Disease Control and Prevention |
207-287-5189 |
|
Clifford S. Mitchell, MS, MD, MPH |
Director, Environmental
Health Coordination |
Maryland Department of Health and Mental Hygiene |
410-767-7438 |
|
Melanie Jetter |
Principal Investigator |
Massachusetts Department of Public Health |
(617) 624-5757 |
|
Thomas Largo, MPH |
Principal Investigator
|
Michigan Department of Community Health |
800-648-6942 |
|
Jessie Shmool, MPH |
Principal Investigator
|
Minnesota Department of Health |
651-201-5000 |
|
Principal Investigator |
Missouri Department of Health & Senior Services |
573-751-6102 |
||
Principal Investigator |
New Hampshire Department of Health & Human Services |
603-271-4988 |
|
|
Barbara Goun, Ph.D., MPH |
Principal Investigator
|
New Jersey Department Health and Senior Services |
609-826-4932 |
|
Heidi Krapfl, MPH |
Bureau Chief |
New Mexico Department of Health |
505- 476-3577 |
|
Neil Muscatiello, MPH |
Director |
New York State Department of Health |
518- 402-7950 |
|
Kim Gaetz, PhD |
Principal Investigator |
North Carolina Department Of Health & Human Services |
919-707-5902 |
|
Curtis Cude |
Principal Investigator |
Oregon Public Health Division |
971- 673-0975 |
|
Anil Nair, PhD |
Principal Investigator |
Pennsylvannia Department of Health |
717-787-3350 |
|
Peter DiPippo |
Principal Investigator |
Rhode Island Department of Health |
401-222-5960 |
|
Lara Raymon, MSPH
|
Principal Investigator |
South Carolina Department of Health and Environmental Control |
(803) 806-9440 |
|
David Boroski
|
Principal Investigator |
Tennessee Department of Health |
615.253.2257 |
|
Surveillance Section Manager |
Utah Department of Health |
801- 538-6191 |
||
Meagan Robinson, DrPH
|
Principal Investigator |
Virginia Department of Health |
804-864-7745 |
|
David Grass, PhD
|
Principal Investigator |
Vermont Department of Health |
(802) 951-4064 |
|
Jennifer Sabel, Ph.D.
|
Principal Investigator |
Washington State Department of Health |
360- 236-3177 |
|
Carrie Tomasallo, MPH, Ph.D. |
Principal Investigator
|
Wisconsin Division of Public Health |
608-267-4465 |
|
In addition to data shared by SLHD, the Tracking Program also works closely with other federal partners to obtain data at the national level. For example, we work with EPA to provide data for all 50 states on specific air pollutants. We also collaborate with other CDC centers to obtain national-level data on specific health effects such as reproductive and birth outcomes, heart disease, and childhood lead poisoning.
A.9. Explanation of Any Payment or Gift to Respondents
The Agency will not provide payment or other forms of remuneration to respondents of its various forms of collecting feedback. Recipients’ activities are funded through the cooperative agreement.
A.10. Protection of the Privacy and Confidentiality of Information Provided by Respondents
The CDC Chief Privacy Officer has determined that the Privacy Act does not apply (Attachment 7). For CDC, the data collection (e.g., aggregate counts of birth defects prevalence, childhood blood lead levels, drinking water monitoring, emergency department visits, hospitalizations, radon testing, biomonitoring) does not involve collection of information in identifiable form (IIF). Information collected is from a standardized form of existing data de-identified by the SLHDs. All data are kept private to the extent permitted by law. No Privacy Act System of Records Notice is required to maintain the data at CDC.
To maintain confidentiality and IT security, these data are transported through the Tracking Network’s secure file transfer gateway and maintained in in Tracking Network’s secure data repository with restricted access. A security plan establishing controlled access to the information and following CDC guidelines has been developed. SLHD are required to use CDC’s Security Access Management Services (SAMS) to securely submit data to the program. Before data are disseminated to the public via the Tracking Network’s National Public Portal, data are aggregated to reduce information with low case counts and population and to increase stability of rates. Remaining small numbers are suppressed and if needed additional suppression is applied to prevent back calculation of potentially sensitive information.
A.11. Institutional Review Board (IRB) and Justification for Sensitive Questions
The NCEH/ATSDR Human Subjects Contact has reviewed this information collection and determined that these CDC collections are non-research under Notice of Funding Opportunity CDC-RFA-EH22-2202 (Attachment 3). A copy of the NCEH/ATSDR research determination can be found in Attachment 8.
The requirements for IRB review and informed consent are the responsibility of the agencies or organizations that collect and own the primary data (i.e., the sources of the secondary datasets in the Tracking Network).
The CDC does not obtain sensitive information.
A.12. Estimates of Annualized Burden Hours and Costs
For this IC, respondents are defined as SLHD. Thirty-three SLHD will provide both Tracking Network data and program data to the Tracking Program as part of their cooperative agreement. In some cases, one or more of the funded SLHD does not respond to one or more forms because data are not available. For example, there are states that do not have a birth defects registry, so not all SLHD would be submitting birth defects data. This means the number of respondents for that dataset would be less than the number of total funded recipients. Additionally, a few unfunded SLHD have responded, unsolicited, because of their interest in having their data in the Tracking Network. The total number of respondents in the table is 37. This number reflects the current 33 SLHD respondents plus four (4) to allow for future funding of new SLHD or to collect voluntary responses from unfunded SLHD.
Table 2 displays the annualized report burden computations. The total burden hours requested are 14,384. This estimate includes the time it takes to extract the data from the original data source(s), standardize and format the data to match the corresponding Tracking Network data form, and submit the data to the Tracking Network. In some cases, the data at the source are centralized and easily extracted. In other cases, like for radon data, the data are not. In those cases, the number of hours for extracting and standardizing the data is much greater. But part of the mission of the Tracking Program is to improve data management and accessibility. Data which are not centralized or easily standardized will be over time as recipients work to improve how the data are maintained and build processes for standardizing, formatting, and updating the data. This will reduce the number of hours needed to extract, standardize, format, and submit the data to the Tracking Network.
Table 2: Estimated Annualized Burden Hours
Type of Respondent |
Form Name |
No. of Respondents |
No. of Responses per Respondent |
Avg. Burden per Response (in hrs.) |
Total Burden (in hrs.) |
State and local health department |
Birth defects prevalence |
30 |
1 |
40 |
1200 |
Childhood blood lead levels |
37 |
1 |
40 |
1480 |
|
Drinking water monitoring |
37 |
1 |
50 |
1850 |
|
Emergency department visits |
37 |
1 |
40 |
1480 |
|
Hospitalizations |
37 |
1 |
40 |
1480 |
|
Radon testing |
25 |
1 |
50 |
1250 |
|
Biomonitoring |
25 |
1 |
40 |
1000 |
|
Metadata records |
37 |
2 |
20 |
1480 |
|
Work Plan Template |
37 |
1 |
21 |
777 |
|
Program Accomplishments and Public Health Actions Report |
37 |
2 |
20 |
1480 |
|
Performance Measures Report |
37 |
1 |
20 |
740 |
|
PHA Impact Follow Up Form |
37 |
2 |
0.25 |
18.5 |
|
Communications plan |
37 |
1 |
2 |
74 |
|
|
Web Stats Template |
37 |
2 |
1 |
74 |
Total |
|||||
Table 3 describes the annualized cost burden to the SLHD. The hourly wage rates are based on average rates from Occupational Employment and Wages, May 2022. https://www.bls.gov/oes/current/oes_stru.htm
19-0000 Life, Physical, and Social Science Occupations (Major Group), State Government, excluding schools and hospitals, median hourly rate of $40.21.
13-1111 Management Analysts, State Government, excluding schools and hospitals, median hourly rate of $50.32.
Table 3: Estimated Annualized Costs to Respondents
Type of Respondent |
Form Name |
Total Burden (in hrs.) |
Hourly Wage Rate |
Total Respondent Costs |
State and local health department |
Birth defects prevalence |
1200 |
$ 40.21 |
$ 48,252 |
Childhood blood lead levels |
1480 |
$ 40.21 |
$ 59,511 |
|
Drinking water monitoring |
1850 |
$ 40.21 |
$ 74,389 |
|
Emergency department visits |
1480 |
$ 40.21 |
$ 59,511 |
|
Hospitalizations |
1480 |
$ 40.21 |
$ 59,511 |
|
Radon testing |
1250 |
$ 40.21 |
$ 50,263 |
|
Biomonitoring |
1000 |
$ 40.21 |
$ 40,210 |
|
Metadata records |
1480 |
$ 40.21 |
$ 59,511 |
|
Work Plan Template |
777 |
$ 50.32 |
$ 39,099 |
|
Program Accomplishments and Public Health Action Report |
1480 |
$ 50.32 |
$ 74,474 |
|
Performance Measures Report |
740 |
$ 50.32 |
$ 37,237 |
|
PHA Impact Follow Up Form |
18.5 |
$ 50.32 |
$ 931 |
|
Communications plan |
74 |
$ 50.32 |
$ 3,724 |
|
Web Stats Template |
74 |
$ 50.32 |
$ 3,724 |
|
Total |
$ 610,347 |
|||
A.13. Estimates of Other Total Annual Cost Burden to Respondents and Record Keepers
The Tracking Program will begin using an EDCS to collect information from the CDC-RFA-EH22-2202 recipients. The EDCS provides an innovative and collaborative approach to address data quality and reduce burden hours and costs. There will be no direct costs to the recipients. The EDCS will be designed to use existing hardware within funded sites, including access to the internet. REDCap is an example of an EDCS. REDCap is an easy-to-use, free software tool useful for programmatic deliverable management and data capture. Included in this package are 5 forms utilizing CDC’s REDCap platform to capture programmatic data from the CDC-RFA-EH22-2202 recipients.
The data submission system was designed to use existing hardware within funded sites, and all respondents currently have access to the internet to use the information system. There will be no direct costs to the respondents or record keepers.
A.14. Annualized Cost to the Federal Government
The total estimated annualized cost to the federal government is $21,571,142. Table 4 contains a detailed breakdown of the costs per year.
Personnel: $670,699 per year salary and benefits.
Cooperative agreement awards: $19,960,000.
Contract: $896,443 per year. The contract supports four on-site IT or Systems Analysts and several part-time staff that develop and maintain the web-based data query system and its data tables.
Travel: $25,000 per year. To promote the use of the Tracking Network, staff will conduct site visits and present data at several regional and national conferences, including the annual meeting of the American Public Health Association, Council of State and Territorial Epidemiologists, and the National Environmental Health Association. Attendance for one person at each of these three conferences is approximately $1,700 per conference.
Software: $11,000 - Additional software is utilized to support the program’s activities.
Hardware or storage: $8,000.
Table 4: Estimated Annualized Cost to the Federal Government
|
||||
Personnel |
Average Annual Hours |
Average Hourly Rate |
|
Average Annual Cost |
5 Public Health Advisors (GS 9-13) |
6,240 |
$42.03 |
|
$262,267 |
6 Epidemiologists (GS 13-14) |
1,248 |
$52.05 |
|
$64,958 |
4 Informatics Professionals (GS 12-14) |
3,280 |
$48.26 |
|
$158,293 |
3 Health Communication Specialists (GS 12-14) |
630 |
$48.26 |
|
$30,404 |
Total Personnel |
|
|
|
$515,922 |
Total Benefits (30%) |
|
|
|
$154,777 |
Total Salary and Benefits |
|
|
|
$670,699 |
Cooperative Agreements |
|
|
|
$19,960,000 |
Contracts |
|
|
|
$896,443 |
Travel |
|
|
|
$25,000 |
Software/Hardware |
|
|
|
$19,000 |
|
|
|
|
|
Total Annualized Costs |
|
|
|
$21,571,142 |
A.15. Explanation for Program Changes or Adjustments
For Tracking Data, changes are requested for the following instruments:
Attachment 4A. Birth defect: No change
Attachment 4B. Childhood blood lead levels
In addition to ‘blood lead levels by birth cohort’ and ‘annual blood lead levels,’ we added five data elements to collect State Blood Lead Testing Practices to evaluate screening practices.
Revised value set for the data element ‘BLLCategory.’-CDC recently updated its blood lead reference value (BLRV) from 5 µg/dL to 3.5 µg/dL in response to the Lead Exposure Prevention and Advisory Committee (LEPAC) recommendation made on May 14, 2021.
Attachment 4C. Drinking water
Changed data set name from ‘community drinking water’ to ‘drinking water’ to make consistent terminology (e.g., community drinking water system).
After a few submissions and review of the data, we added three important analyte codes (1030=Lead; 2805=PFOS; 2806=PFOA) and removed three data elements.
Attachment 4D. Emergency department visits: No changes
Attachment 4E. Hospitalizations: No changes
Attachment 4F. Radon testing
To standardize the consistent data element name across contents, we changed the name of a data element from ‘AddressID’ to ‘Addressidentifier.’ This is a unique ID that can be linked to the address but maintain confidentiality. We removed a data element ‘StreetAddress.’
After a few submissions and review of the data, we have identified the key data element ‘AddressPostalCode’ and added an additional value set (K= Skirted in pier, O = Other) for a data element ‘FoundationTypeCode.’
Attachment 4G. Biomonitoring
This is a new data collection. We used data from the National Health and Nutrition Examination Survey (NHANES) Program, which is the National Report on Human Exposure to Environmental Chemicals. The measures cannot be used to examine exposure levels by locality, state, or region. To collect state or sub-state level biomonitoring data, we added new 17 data elements.
Attachment 4H. Meta data: No Changes
For Program Data, minor changes are requested for the following instruments:
(Attachment 5A) Work Plan Template – streamlined Excel template for more efficient reporting of program projects and activities. Removed 1 field
(Attachment 5B) Work Plan – REDCap Form - streamlined electronic form for more efficient reporting of program projects and activities. Non collection form which is included to visualize how the data will look in REDCap. This form will not replace Attachment 5A. The RedCap form will be replaced once we utilize RedCap system.
(Attachment 5C) - Program Accomplishments-Public Health Actions – Added 2 new fields to collect more information about the accomplishments and actions reported. The new fields allow us to collect information about the action type, partners responsible for the action, and the action’s anticipated long-term outcomes. The 2 new fields are: Is this action/decision expected to realize longer-term outcomes? Dropdown – Yes, No, Not Sure; and Expected timeframe for outcomes? Dropdown – 6 months, 1 year, 1-5 years, 5+ years.
(Attachment 5D) - Program Accomplishments-Public Health Actions - REDCap Form - streamlined electronic form for more efficient reporting of program accomplishments and public health actions. Non collection form which is included to visualize how the data will look in REDCap. This form will not replace Attachment 5C. The RedCap form will be replaced once we utilize RedCap system.
(Attachment 5E) - Performance Measures Report - Removed 2 performance measures. Optimized the fields for collecting data points resulting in a reduction of 100 fields recipients previously encountered. Additionally, 2 performance measures recipients complete have been restructured to capture data that can provide content that serves both performance measures.
(Attachment 5F) - Performance Measures REDCap Form - streamlined electronic form for more efficient reporting of program surveillance, information technology, communications, partnerships, and program capacity performance measures. Non collection form which is included to visualize how the data will look in REDCap. This form will not replace Attachment 5E. The RedCap form will be replaced once we utilize RedCap system.
(Attachment 5G) - PHA Impact Follow Up - REDCap form – A new electronic form to efficiently capture reporting of longer-term impact of previously reported public health actions.
(Attachment 5H) - Communication Plan Template - streamlined template for more efficient reporting of communications and partnership activities. The new template allows for more consistent reporting between funded SLHD. Deleted the Partnership Plan Template and Guide. Combined the questions originally in the Partnership plan into the Communication Plan Template.
(Attachment 5I) – Web Stats Template – created an Excel reporting template with one cell for each question. In comparison to the previous version of the template, the new version request state or county name be included but reduced the total required web statistics variables being collected from 53 to 37. Additionally, a new auto populated field has been added to the template that automatically looks up a jurisdiction's FIPS code based on the data entered into the state or county name field. This reporting template supports better data collection, reduces reporting burden, and supports easier analysis.
Additionally, for the program data, we request to increase the number of respondents from 26 to 37 to incorporate the 7 new recipients funded under CDC-RFA-EH22-2202, potential future funding of new SLHD, and to collect voluntary responses from unfunded SLHD.
Based on the above changes, we are requesting to increase the annualized number of responses from 599 to 635 (due to an increased number of recipients) but decrease the annualized time burden from 21,860 to 14,384 hours.
A.16. Plans for Tabulation and Publication and Project Time Schedule
Tracking Network Data
Data from recipients or other SLHD are submitted once a year in a standardized XML format to CDC using a secure web-based file transfer system during either a fall or spring data call. Recipients receive a notification letter 60 days prior to the data call which describes the data requested and which data forms to complete. Corresponding metadata are submitted for each of the 7 datasets for a total of 6 metadata submissions per year. On average, the time from data submission to measure dissemination is 4 to 6 months.
Table 4a. Project Time Schedule – Tracking Network Data
Activity |
Time Schedule after PRA Clearance |
Data call letter sent to respondents (once in the fall and once in the spring) |
Day 0 |
Data information/Data collection |
Day 1 – Day 60 |
Data and metadata submission and validation |
Day 61 - 81 |
Measure generation |
Day 82 - 127 |
Data integration into Tracking Portal |
Day 128 – Day 173 |
Measure Dissemination |
Day 174 |
Scientific Analyses and Reports |
Ongoing activity following data validation |
Data obtained by the Tracking Program are integrated into the Tracking Network and disseminated to the public via the Tracking Network’s National Public Portal at http://ephtracking.cdc.gov. Tracking Program staff also analyze the data to advance the science of environmental public health tracking. For example, staff conduct analyses to:
Assess temporal and spatial trends in health, exposure, and environmental hazards
In addition to conducting QA/QC procedures and preparing data for the National Public Portal, Tracking Program staff analyze the data we receive from SLHD and national partners. The type of analysis varies depending on the research question and the available data. We frequently conduct descriptive analyses for surveillance purposes and analysis the data to identify temporal or spatial trends.
Monitor known or suspected associations between health and environment
Generate hypotheses about the association between health and environment
Develop and test new methods and tools for surveillance
Facilitate and conduct surveillance summaries and descriptive analyses
Results are published in peer review literature or as white papers and used to inform the practice of environmental public health tracking at the federal, state, and local level.
Program Data
Table 4b. Project Time Schedule – Tracking Network Data
Activity |
Time Schedule after PRA Clearance |
Tracking Program Work plan submitted and reviewed |
Quarter 3 (with continuation application) or 90 days after the cooperative agreement ends |
PA/PHA report submitted and reviewed |
Once a quarter, at least twice a year |
Performance measures submitted and reviewed |
Quarter 3 |
Communications plan and partnership plan submitted and reviewed |
Quarter 3 (with continuation application) |
Web Stats Template submitted and reviewed |
Quarter 1 |
Analyses and Reports |
Ongoing activity upon receipt of updated information |
The program does not use complex statistical methods for analyzing program data. Collected program data are reported in internal documents and shared with funded SLHD. Results are presented during webinars and the implications of the findings are discussed and questions answered. Aggregated information may also be included in reports to CDC leadership, Congress, and other stakeholders.
A.17. Reason(s) Display of OMB Expiration Date is Inappropriate
The Tracking program will display the expiration date for OMB approval of the information system data collection on each information collection form listed in the burden table in the required format.
A.18. Exceptions to Certification for Paperwork Reduction Act Submissions
There are no exceptions to the certification. These activities comply with the requirements in 5 CFR 1320.9.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Richardson, Tony (CDC/OD/OADS) |
| File Modified | 0000-00-00 |
| File Created | 2023-07-29 |
© 2026 OMB.report | Privacy Policy