COVID-19 Current Events Tracker (CET)
ASPA COVID-19 Public Education Campaign Market Research
ASPA CET Weekly Survey W63 FINAL
COVID-19 Current Events Tracker (CET)
OMB: 0990-0476
ASPA
COVID-19 PUBLIC EDUCATION CAMPAIGN A
campaign to increase vaccine acceptance and reinforce basic
prevention measures


CET – Annotated Questionnaire (Wave 63)
Note: The questions below are the proposed questions for the 63rd wave of the Weekly Current Events Tracker (CET). Questions highlighted in yellow will be asked every week; questions highlighted in blue will be rotated into the survey on a monthly basis; and questions highlighted in green are meant to be asked in this wave only or are being asked again to update data on a variable of interest. We will be fielding questions about reasons for waiting to get a booster, parents’ vaccine concerns, the CDC COVID-19 Community Levels tracker, COVID treatment for children under 12, and the Novavax vaccine. |
For the next section we would like to talk about current events.
// Page Break //
//BASE: All respondents//
Item #: Q1
Question Type: Single punch
// Soft Prompt: “We would like your response to this question.” //
beh1_cet_r: Have you received a COVID-19 vaccine?
Variable Label: beh1_cet_r: Vaccination behavior
Value |
Value Label |
0 |
No, I have not received a COVID-19 vaccine |
1 |
Yes, but I have only received one shot out of the two required shots |
2 |
Yes, I have received all of the required shots |
-99 |
Refused |
// Page Break //
//BASE: beh1_cet_r=1 or 2//
Item #: Q2
Question Type: Single punch
// Soft Prompt: “We would like your response to this question.” //
vaccine_id: Which COVID-19 vaccine did you receive?
Variable Label: vaccine_id: Vaccine ID
Value |
Value Label |
2 |
Johnson & Johnson/Janssen |
3 |
Moderna |
4 |
Pfizer-BioNTech |
5 |
Other |
99 |
I do not remember |
-99 |
Refused |
-100 |
Valid skip |
// Page Break //
//BASE: beh1_cet_r=2//
Item #: Q3
Question Type: Dropdown menu
// Soft Prompt: “We would like your response to this question.” //
fully_vacc_month: In which month were you considered fully vaccinated (i.e., two weeks after your final COVID-19 vaccine dose)? Final vaccine dose refers to either the second dose of the Pfizer or Moderna vaccine, or the single dose of the Johnson & Johnson vaccine. Please do not consider booster shots for this question. If you do not remember the specific month, give your best guess.
Variable Label: fully_vacc_month: Month of vaccination
Value |
Value Label |
1 |
December, 2020 |
2 |
January, 2021 |
3 |
February, 2021 |
4 |
March, 2021 |
5 |
April, 2021 |
6 |
May, 2021 |
7 |
June, 2021 |
8 |
July, 2021 |
9 |
August, 2021 |
10 |
September, 2021 |
11 |
October, 2021 |
12 |
November, 2021 |
13 |
December, 2021 |
14 |
January, 2022 |
15 |
February, 2022 |
16 |
March, 2022 |
17 |
April, 2022 |
18 |
May, 2022 |
-99 |
// Page Break //
//BASE: beh1_cet_r=2//
Item #: Q4
Question Type: Multi punch
// Soft Prompt: “We would like your response to this question.” //
vax_effects: What side effects, if any, did you experience after receiving your COVID vaccine?
//PROGRAMMING NOTE: randomize variables in grid//
Value |
Value Label |
1 |
I did not experience any side effects [EXCLUSIVE] [ANCHOR] |
2 |
Pain, redness, or swelling at the injection site |
3 |
Tiredness or fatigue |
4 |
Headache |
5 |
Muscle pain |
6 |
Fever |
7 |
Chills |
8 |
Nausea |
9 |
Allergic reaction |
10 |
Other – please describe [TEXT ENTRY] [ANCHOR] |
-99 |
Refused |
// Page Break //
//BASE: beh1_cet_r=2//
Item #: Q5
Question Type: Single punch
// Soft Prompt: “We would like your response to this question.” //
booster_uptake4: U.S. health officials and medical experts now recommend COVID-19 vaccine booster shots. Have you received a COVID-19 vaccine booster shot?
Variable Label: booster_uptake4: Booster uptake – April 2022 guidance
Value |
Value Label |
0 |
No |
1 |
Yes, I have received 1 booster shot |
2 |
Yes, I have received 2 booster shots |
-99 |
Refused |
-100 |
Valid skip |
// Page Break //
//BASE: (booster_uptake4=0 & vaccine_id=2 & fully_vacc_month=1-16) OR (booster_uptake4=0 & vaccine_id=3-4 & fully_vacc_month=1-13) //
Item #: Q6
Question Type: Single punch
// Soft Prompt: “We would like your response to this question.” //
booster_elig_uptake3: You are currently eligible to receive a COVID-19 vaccine booster shot. What is the likelihood that you will get one?
Variable Label: booster_elig_uptake4: Booster uptake likelihood – eligible adults
Value |
Value Label |
1 |
Very unlikely |
2 |
Somewhat unlikely |
3 |
Neither likely nor unlikely |
4 |
Somewhat likely |
5 |
Very likely |
-99 |
Refused |
-100 |
Valid skip |
// Page Break //
//BASE: (booster_uptake4=0 & vaccine_id=2 & fully_vacc_month=17-18) OR (booster_uptake4=0 & vaccine_id=3-4 & fully_vacc_month=14-18)
Item #: Q7
Question Type: Single punch
// Soft Prompt: “We would like your response to this question.” //
booster_likely_v2: What is the likelihood that you will get a COVID-19 vaccine booster shot when eligible?
Variable Label: booster_likely_v2: Booster uptake likelihood – not yet eligible
Value |
Value Label |
1 |
Very unlikely |
2 |
Somewhat unlikely |
3 |
Neither likely nor unlikely |
4 |
Somewhat likely |
5 |
Very likely |
-99 |
Refused |
-100 |
Valid skip |
// Page Break //
//BASE: (booster_uptake4=0 OR -99)//
Item #: Q8
Question Type: Single punch
// Soft Prompt: “We would like your response to this question.” //
booster_3a. How soon will you get a booster?
Variable Label: booster_3a: Wait to get a booster
Value |
Value Label |
1 |
I will get a booster as soon as I can |
2 |
I will wait to get a booster for one or more reasons |
3 |
I will never get a booster |
-99 |
Refused |
-100 |
Valid Skip |
// Page Break //
//BASE: booster_3a=2//
Item #: Q9
Question Type: Open-ended
// Soft Prompt: “We would like your response to this question.” //
asap_boost_oe. What are the main reasons that you have not yet gotten a COVID vaccine booster?
________________________________________________________________________________________________________________________________________________________
// Page Break //
//BASE: booster_3a=1//
Item #: Q10
Question Type: Open-ended
// Soft Prompt: “We would like your response to this question.” //
wait_boost_oe. What are the main reasons that you plan to wait to get a COVID vaccine booster?
________________________________________________________________________________________________________________________________________________________
// Page Break //
//BASE: beh1_cet_r=0 OR -99//
Item #: Q11
Question Type: Single punch
// Soft Prompt: “We would like your response to this question.” //
beh2a_cet: What is the likelihood that you will get a COVID-19 vaccine?
Variable Label: beh2a: Intention to get vaccinated
Value |
Value Label |
1 |
Very unlikely |
2 |
Somewhat unlikely |
3 |
Neither likely nor unlikely |
4 |
Somewhat likely |
5 |
Very likely |
-99 |
Refused |
-100 |
Valid Skip |
// Page Break //
//BASE: beh1_cet_r=0 OR -99//
Item #: Q12
Question Type: Single punch
// Soft Prompt: “We would like your response to this question.” //
beh3a_cet_r: How soon will you get vaccinated?
Variable Label: beh3a_cet_r: Wait to get vaccinated
Value |
Value Label |
1 |
I will get a vaccine as soon as I can |
2 |
I will wait to get a vaccine for one or more reasons |
3 |
I will never get a COVID-19 vaccine |
-99 |
Refused |
-100 |
// Page Break //
//BASE: All respondents//
Item #: Q13
Question Type: Multi punch
// Soft Prompt: “We would like your response to this question.” //
parent: Are you the parent of a child or children in the following age groups?
Variable Label: parent: Parent of children in following age groups
Value |
Value Label |
1 |
Younger than 6 months old |
2 |
6 months to <2 years old |
3 |
2 to 4 years old |
4 |
5 to 11 years old |
5 |
12 to 15 years old |
6 |
16 to 17 years old |
99 |
None of the above, I do not have children in those age groups [EXCLUSIVE] |
-99 |
Refused |
// Page Break //
//BASE: Parent=4-6//
Item #: Q14
Question Type: Single punch grid
// Soft Prompt: “We would like your response to this question.” //
child_vaxxed_2: Has your child(ren) in the following age group(s) received a COVID-19 vaccine?
Note: If you have more than one child in the same age group, please answer for at least one of them.
Variable Name |
Variable Text |
Variable Label |
child_vaxxed_2_4 |
5 to 11 years old [ONLY SHOW IF parent=4] |
child_vaxxed_2_4: 5 to 11 years old |
child_vaxxed_2_5 |
12 to 15 years old [ONLY SHOW IF parent=5] |
child_vaxxed_2_5: 12 to 15 years old |
child_vaxxed_2_6 |
16 to 17 years old [ONLY SHOW IF parent=6] |
child_vaxxed_2_6: 16 to 17 years old |
Value |
Value Label |
0 |
No, has not received a COVID-19 vaccine |
1 |
Yes, but has only received one shot out of the two required shots |
2 |
Yes, has received all of the required shots |
-99 |
Refused |
-100 |
Valid skip |
// Page Break //
//BASE: child_vaxxed_2_5=2 AND/OR child_vaxxed_2_6=2//
Item #: Q15
Question Type: Single punch grid
// Soft Prompt: “We would like your response to this question.” //
child_boosted: Has your child(ren) in the following age group(s) received a COVID-19 vaccine booster shot?
Note: If you have more than one child in the same age group, please answer for at least one of them.
Variable Name |
Variable Text |
Variable Label |
child_boosted_5 |
12 to 15 years old [ONLY SHOW IF child_vaxxed_2_5=2] |
child_boosted_5: 12 to 15 years old |
child_boosted_6 |
16 to 17 years old [ONLY SHOW IF child_vaxxed_2_6=2] |
child_boosted_6: 16 to 17 years old |
Value |
Value Label |
0 |
No, has not received a COVID-19 vaccine booster shot |
1 |
Yes, has received a COVID-19 vaccine booster shot |
-99 |
Refused |
-100 |
Valid skip |
// Page Break //
//BASE: parent=1-6//
Item #: Q16
Question Type: Single punch grid
// Soft Prompt: “We would like your response to this question.” //
child_covid_concern: How concerned are you about your child(ren) in the following age groups getting COVID-19?
Note: If you have more than one child in the same age group, please answer for at least one of them.
Variable Label: child_covid_concern: Concern about child(ren)’s COVID-19 risk
//PROGRAMMING NOTE: PIPE 1-6 responses from parent//
Variable Name |
Variable Text |
Variable Label |
child_covid_concern_1 |
Younger than 6 months old |
child_covid_concern_1: Younger than 6 months old |
child_covid_concern_2 |
6 months to <2 years old |
child_covid_concern_2: 6 months to <2 years old |
child_covid_concern_3 |
2 to 4 years old |
child_covid_concern_3: 2 to 4 years old |
child_covid_concern_4 |
5 to 11 years old |
child_covid_concern_4: 5 to 11 years old |
child_covid_concern_5 |
12 to 15 years old |
child_covid_concern_5: 12 to 15 years old |
child_covid_concern_6 |
16 to 17 years old |
child_covid_concern_6: 16 to 17 years old |
Value |
Value Label |
1 |
Not concerned |
2 |
Slightly concerned |
3 |
Somewhat concerned |
4 |
Very concerned |
5 |
Child has already had COVID |
-99 |
Refused |
// Page Break //
//BASE: Parent=1-6//
Item #: Q17
Question Type: Single punch grid
// Soft Prompt: “We would like your response to this question.” //
vacc_child_parent: If a COVID-19 vaccine was authorized and available for children in the following age groups, how likely would you be to get your child(ren) vaccinated?
Note: COVID-19 vaccines have now been authorized and are available for use in children as young as 5 years old. If you have more than one child in the same age group, please answer for at least one of them.
Variable Label: vacc_child_parent: Parent likelihood to get child(ren) vaccinated
//PROGRAMMING NOTE: PIPE 1-6 responses from parent.//
Variable Name |
Variable Text |
Variable Label |
vacc_child_parent_6m |
Younger than 6 months old |
vacc_child_parent_6m: Younger than 6-months-old |
vacc_child_parent_6mto2 |
6 months to <2 years old |
vacc_child_parent_6mto2: 6 months- to 2-years-old |
vacc_child_parent_2to4 |
2 to 4 years old |
vacc_child_parent_2to4: 2- to 4-years-old |
vacc_child_parent_5to11 |
5 to 11 years old [ONLY SHOW IF child_vaxxed_2_4=0 or 99] |
vacc_child_parent_5to11: 5- to 11-years-old |
vacc_child_parent_12to15 |
12 to 15 years old [ONLY SHOW IF child_vaxxed_2_5=0 or 99] |
vacc_child_parent_12to15: 12- to 15-years-old |
vacc_child_parent_16to17 |
16 to 17 years old [ONLY SHOW IF child_vaxxed_2_6=0 or 99] |
vacc_child_parent_16to17: 16- to 17-years-old |
Value |
Value Label |
1 |
Very unlikely |
2 |
Somewhat unlikely |
3 |
Neither likely nor unlikely |
4 |
Somewhat likely |
5 |
Very likely |
-99 |
Refused |
-100 |
Valid Skip |
// Page Break //
//BASE: Parent=1-6//
Item #: Q18
Question Type: Single punch grid
// Soft Prompt: “We would like your response to this question.” //
child_vaccine_concern: How concerned are you about your child(ren) in the following age groups having any side effects from a COVID-19 vaccine?
Note: If you have more than one child in the same age group, please answer for at least one of them.
Variable Label: child_vaccine_concern: Concern about child(ren)’s vaccine risk
//PROGRAMMING NOTE: PIPE 1-6 responses from parent//
Variable Name |
Variable Text |
Variable Label |
child_vaccine_concern_1 |
Younger than 6 months old |
child_vaccine_concern_1: Younger than 6 months old |
child_vaccine_concern_2 |
6 months to <2 years old |
child_vaccine_concern_2: 6 months to <2 years old |
child_vaccine_concern_3 |
2 to 4 years old |
child_vaccine_concern_3: 2 to 4 years old |
child_vaccine_concern_4 |
5 to 11 years old [ONLY SHOW IF child_vaxxed_2_4=0 or 99] |
child_vaccine_concern_4: 5 to 11 years old |
child_vaccine_concern_5 |
12 to 15 years old [ONLY SHOW IF child_vaxxed_2_5=0 or 99] |
child_vaccine_concern_5: 12 to 15 years old |
child_vaccine_concern_6 |
16 to 17 years old [ONLY SHOW IF child_vaxxed_2_6=0 or 99] |
child_vaccine_concern_6: 16 to 17 years old |
Value |
Value Label |
1 |
Not at all concerned |
2 |
Slightly concerned |
3 |
Somewhat concerned |
4 |
Very concerned |
-99 |
Refused |
-100 |
Valid skip |
// Page Break //
//BASE: All respondents//
Item#: Q19-Q20
Question Type: Single punch grid
// Soft Prompt: “We would like your response to this question.” //
cdc_commlevels2: Earlier this year, the Centers for Disease Control and Prevention (CDC) dropped its recommendation for universal mask-wearing in areas of low or medium risk of COVID. They also unveiled a new method for calculating risk in each U.S. county, called COVID-19 Community Levels, which focuses on the impact of severe COVID on local hospitals and helps people decide what precautions to take against COVID—including when to wear a mask. The below map depicts COVID-19 Community Levels across the United States as of mid May.
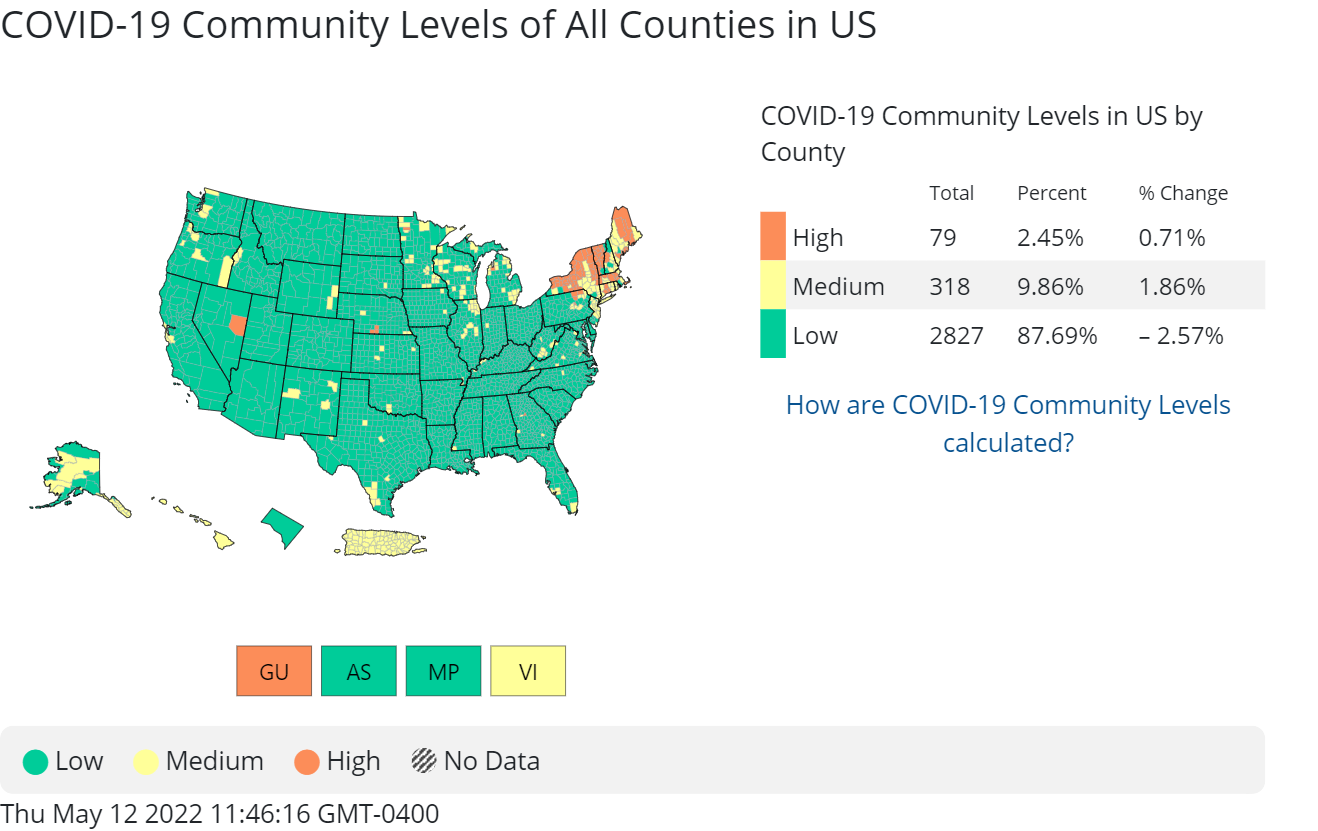
How much do you agree or disagree with the following statements about the CDC’s current masking guidance?
//PROGRAMMING NOTE: RANDOMIZE options //
Variable Name |
Variable Text |
Variable Label |
cdc_commlevels2_1 |
I have heard about the CDC’s COVID-19 Community Levels tool. |
cdc_commlevels2_1: Have heard of Community Levels |
cdc_commlevels2_2 |
I understand the CDC’s current masking guidance. |
cdc_commlevels2_2: Understand new masking guidance |
cdc_commlevels2_3 |
I have visited the CDC’s website to find out more about masking guidance for my area. |
cdc_commlevels2_3: Visited CDC website |
cdc_commlevels2_4 |
I know how to find the COVID-19 Community Level for my area. |
cdc_commlevels2_4: Know how to find Community Level |
cdc_commlevels2_5 |
I know how to determine whether masking is recommended in my area based on the CDC’s COVID-19 Community Levels. |
cdc_commlevels2_5: Know how to determine masking guidance |
cdc_commlevels2_6 |
I plan to continue masking indoors in public, regardless of the CDC’s guidance for my community. |
cdc_commlevels2_6: Plan to continue masking |
cdc_commlevels2_7 |
I do not plan on masking indoors in public, even if the COVID-19 Community Level is high and wearing a mask is recommended. |
cdc_commlevels2_7: Do not plan to continue masking |
cdc_commlevels2_8 |
I plan to use the CDC guidance for my community when deciding whether to wear a mask indoors in public. |
cdc_commlevels2_8: Plan to use CDC guidance |
cdc_commlevels2_9 |
I have used the COVID-19 Community Levels tool to figure out what precautions (such as mask-wearing) I should take against COVID. |
cdc_commlevels2_9: Have used Community Level Tracker |
cdc_commlevels2_10 |
I regularly check the COVID-19 Community Levels tool to find out about currently recommended precautions. |
cdc_commlevels2_10: Regularly used Community Level tracker |
Value |
Value Label |
1 |
Strongly disagree |
2 |
Somewhat disagree |
3 |
Neither agree nor disagree |
4 |
Somewhat agree |
5 |
Strongly agree |
-99 |
Refused |
// Page Break //
/BASE: All respondents//
Item
#: Q21
Question
Type: Single
punch grid
// Soft Prompt: “We would like your response to this question.”//
novavax_aware: Novavax has submitted a request for Emergency Use Authorization of their COVID vaccine to the Food and Drug Administration (FDA). How familiar are you with the Novavax COVID vaccine?
Variable Label: novavax_aware: Familiarity with Novavax
Value |
Value Label |
1 |
Not at all familiar |
2 |
Slightly familiar |
3 |
Moderately familiar |
4 |
Very familiar |
-99 |
Refused |
// Page Break //
/BASE: All respondents//
Item
#: Q22
Question
Type: Single
punch grid
// Soft Prompt: “We would like your response to this question.”//
novavax_perc: The Novavax COVID vaccine is not an mRNA vaccine—it contains the spike protein that is on the surface of the virus that causes COVID, but formulated in a way which cannot cause disease. When the vaccine is injected, it causes the immune system to produce antibodies and immune responses so that the body will be able to recognize and attack the coronavirus when it enters the body.
Thinking about the Novavax COVID vaccine, how much do you agree or disagree with the following questions?
//PROGRAMMING NOTE: RANDOMIZE options //
Variable Name |
Variable Text |
Variable Label |
novavax_perc1 |
I think the Novavax COVID vaccine is safer than the other COVID vaccines that are available (Pfizer, Moderna, or Johnson & Johnson). |
novavax_perc1: Novavax is safer |
novavax_perc2 |
I am worried about the potential side effects of the Novavax COVID vaccine. |
novavax_perc2: Worried about side effects |
novavax_perc3 |
I think more research should be done on the Novavax COVID vaccine before it is authorized by the FDA. |
novavax_perc3: More research needed |
novavax_perc4 |
I am more likely to get the Novavax COVID vaccine than I am to get one of the other COVID vaccines that are available. [ONLY SHOW IF beh1_cet_r=0 OR -99] |
novavax_perc4: More likely to get Novavax |
novavax_perc5 |
I plan to get the Novavax COVID vaccine. [ONLY SHOW IF beh1_cet_r=0 OR -99] |
novavax_perc5: Plan to get Novavax |
Value |
Value Label |
1 |
Strongly disagree |
2 |
Somewhat disagree |
3 |
Neither agree nor disagree |
4 |
Somewhat agree |
5 |
Strongly agree |
-99 |
Refused |
// Page Break //
/BASE: All respondents//
Item
#: Q23
Question
Type: Single
punch
// Soft Prompt: “We would like your response to this question.”//
treat_kids_know: True or false: The FDA recently expanded its approval for another COVID-19 treatment, Veklury (also known as remdesivir), making it the first treatment approved for children under 12.
Variable Label: treat_kids_know: Awareness of Veklury approval for kids under 12
Value |
Value Label |
1 |
True |
2 |
False |
99 |
I don’t know |
-99 |
Refused |
// Page Break //
//BASE: All respondents//
Item
#: Q24
Question
Type: Single
punch
// Soft Prompt: “We would like your response to this question.”//
treat_kids_perc: This is true. Veklury (also known as remdesivir) is an antiviral treatment that will be available for children 28 days and older who weigh at least 7 pounds, have tested positive for COVID, and are hospitalized or have mild to moderate COVID and are at high risk for severe COVID.
How much do you agree or disagree with the following statements?
//PROGRAMMING NOTE: randomize variables in grid//
Variable Name |
Variable Text |
Variable Label |
treat_kids_perc1 |
The availability of an antiviral treatment for children makes me more reluctant to get my child(ren) a COVID vaccine. [ONLY SHOW IF parent=4-6 & child_vaxxed_2_4=0, child_vaxxed_2_5=0, or child_vaxxed_2_6=0] |
treat_kids_perc1: Less likely to vaccinate eligible child |
treat_kids_perc2 |
The availability of an antiviral treatment for children makes me more reluctant to get my child(ren) under 5 a COVID vaccine, once one is available for them. [ONLY SHOW IF parent=1-3] |
treat_kids_perc2: Less likely to vaccinate child under 5 when eligible |
treat_kids_perc3 |
I would be willing to have my child(ren) treated with an antiviral treatment if they had COVID and were eligible for the treatment. [ONLY SHOW IF parent=1-6] |
treat_kids_perc3: Willing to give children antiviral treatment |
treat_kids_perc4 |
I am worried that the COVID antiviral treatment for children could be unsafe. |
treat_kids_perc4: Worried about safety |
treat_kids_perc5 |
I am worried about the potential side effects of the COVID antiviral treatment for children. |
treat_kids_perc5: Potential side effects |
treat_kids_perc6 |
The availability of a COVID antiviral treatment for children is an important step forward in the fight against COVID. |
treat_kids_perc6: Important step forward |
Value |
Value Label |
1 |
Strongly disagree |
2 |
Somewhat disagree |
3 |
Neither agree nor disagree |
4 |
Somewhat agree |
5 |
Strongly agree |
99 |
I don’t know |
-99 |
Refused |
// Page Break //
//BASE: All respondents//
Item
#: Q25
Question
Type: Single
punch grid
// Soft Prompt: “We would like your response to this question.”//
ptn_w63: We are interested in your opinion of a few messages about COVID-19 vaccines or boosters.
For each of the below messages, please indicate how much you agree or disagree with the following statement:
“I would share the information in the message with a friend or family member who wants to know more about COVID-19 vaccines or boosters.”
//PROGRAMMING NOTE: randomize variables in grid//
Variable Name |
Variable Text |
Variable Label |
ptn_w63_1 |
At the peak of Omicron, nearly one third of older people who died of COVID were vaccinated but had not been boosted. |
ptn_w63_1: One third unboosted |
ptn_w63_2 |
A COVID booster just might save your life, especially if you are over 50. |
ptn_w63_2: Over 50 |
ptn_w63_3 |
Regardless of your age, race, sex, or heart health, if you get COVID your risk for heart disease is much higher than for people who have not had COVID. |
ptn_w63_3: Heart disease risk is higher |
ptn_w63_4 |
With COVID cases on the rise again, it’s important for everyone to be up to date on their vaccines and boosters. |
ptn_w63_4: Cases on the rise |
ptn_w63_5 |
Some people have no side effects from COVID vaccines and boosters, and most who have side effects report that they are mild and only last a day or two. |
ptn_w63_5: Mild side effects |
Value |
Value Label |
1 |
Strongly disagree |
2 |
Disagree |
3 |
Neither agree nor disagree |
4 |
Agree |
5 |
Strongly agree |
-99 |
Refused |
For
internal communications only

| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Modified | 0000-00-00 |
| File Created | 0000-00-00 |
© 2026 OMB.report | Privacy Policy