83-C Premarket Tobacco Product Applications and Recordkeeping Requirements, Form FDA 4057b
0910-0879 OMB Change Request Memo 6.2023.docx
Premarket Tobacco Product Applications and Recordkeeping Requirements
83-C Premarket Tobacco Product Applications and Recordkeeping Requirements, Form FDA 4057b
OMB: 0910-0879
U.S. Food and Drug administration
Center for Tobacco Products
Premarket Tobacco Product Applications and Recordkeeping Requirements
(OMB Control Number 0910-0879)
Justification for No Material/Nonsubstantive Change (83-C)
Date: June 2023
The
Food and Drug Administration (FDA)/Center for Tobacco Products (CTP)
is submitting this non substantive change request for updates to FDA
Form 4057b to assist industry users to complete the form
correctly. This enhanced spreadsheet was developed to address
the most common PMS error messages received on industry provided
spreadsheets after 8/1/2022. The most common error messages were
identified as:
TP number is included but is not a TP number (usually a product ID or SKU)
Unhiding columns and entering data that is not allowed for the cat/subcat combination
Dependency rules between columns not followed (example: characterizing flavor not equal to ‘flavored’ with entries in ‘Flavored, if Flavored’ column)
Use of unsupported characters (The dash (—), also called the em dash, is the long horizontal bar, much longer than a hyphen, è, ñ) These mostly appear in the product name and flavor columns (examples: crème coffee, pina colada).
We do not expect any changes in burden per these form updates. Additionally, included in this change request, for efficiency we are reallocating burden hours for mandatory CTP Adverse Experience (AE) reporting that are currently approved under this OMB control number to OMB control number 0910-0291 “Adverse Experience Reporting”. The result of these changes is a net decrease of 24 hours to the overall burden for this collection (0910-0879).
Tracked Changes Document |
Modification |
|
|
|
|
Detailed Form Edits:
FDA 4057b v3.0 Form Change Log |
|
Form Section |
Description of Change |
Instruction Tab |
Adding a new “Instructions Tabs” to the spreadsheet containing field attributes and associated instructions. |
|
Renaming Product Category “Cigarette” to “Cigarettes” |
Renaming Product Category “Roll-Your-Own” to “Roll-Your-Own Tobacco Products” |
|
Renaming Product Category “Smokeless” to “Smokeless Tobacco Products” |
|
Renaming Product Category “ENDS(VAPES)” to “Electronic Nicotine Delivery System (ENDS) (Vapes) |
|
Renaming Product Category “Cigar” to “Cigars” |
|
Renaming Product Category “Pipe” to “Pipe Tobacco Products” |
|
Renaming Product Category “Waterpipe” to “Waterpipe Tobacco Products” |
|
Renaming Product Category “Heated Tobacco Product” to “Heated Tobacco Products (HTP)” |
|
Renaming one Product Subcategory option under “Roll-Your-Own Tobacco Products” From “Roll Your Own” to “Roll-Your-Own” |
|
“Product Category, If other” and “Product Subcategory, if Other” fields must be hidden and will only be unhidden when the option “Other” is selected in the “Product Category” and/or “Product Subcategory” fields. |
|
|
Replacing the text under the “Instructions” section with the following:
1. Applicant name must be entered as required by 21 CFR § 1114.7(c)(3). 2. Product Category must be entered as required by 21 CFR § 1114.7(c)(3). To ensure ingestion use the drop-down menu. 3. Product Subcategory must be entered as required by 21 CFR § 1114.7(c)(3). To ensure ingestion use the drop-down menu. 4. To populate your unique identification of your products, once you have completed entry of applicant name, product category, product subcategory, and application type, click on the “Enter Unique Product Properties” button to enter each tobacco product. This will allow for a new tab called “Product” to generate. 5. Complete product tab for all tobacco products you are submitting. Refer to the “Instructions Tab” for information to correctly populate each product property for ingestion. Enter applicable product information as specified in Table 1 to 21 CFR § 1114.7(c)(3)(iii) under the Product Tab. 6. If you click on the "reset" button you will lose your product tab and all product data entered. 7. Once you have completed data entry for both your introduction and product tabs, SAVE AS .XLS or .XLSX and name appropriately. For file naming convention, reference the Electronic Submission File Formats and Specifications which can be found on the FDA website. 8. Your spreadsheet must be XLS or XLSX to attach to eSubmitter file for submission, otherwise it will not be processed. 9. Create a separate spreadsheet for each product Category/Subcategory. Do not exceed 5000 rows of products as the spreadsheet will not validate. If total number of products for each product Category/Subcategory exceeds 5000 products, then create a new spreadsheet for the additional products. |
Introduction Tab |
|
|
|
|
Under the “Please Note” section removing verbiage that states “Using "Additional Property" to differentiate the products if all the other unique product properties are exactly same.” |
Removing “(Continue through the errors and save the file as "Tobacco_Product_list.xls" or "Tobacco_Product_list.xlsx". If there are multiple product files, save the files as "Tobacco_Product_list_n.xls" or "Tobacco_Product_list_n.xlsx"(where n=1,2,3,etc.) from “Please Note” section and replacing the text with “For file naming convention, reference the Electronic Submission File Formats and Specifications which can be found on the FDA website.” |
|
Removing text “All Application Types (Internal Use Only)” from cell D1/Introduction Header. Header will display only “FDA Form 4057b” |
|
Changing form version number from 2.1 to 3.0 |
|
|
The Product tab will no longer be visible until the user clicks on “Enter Unique Product Properties” on the Introduction tab |
Product tab |
Locking the header row so that industry cannot ‘unhide’ columns or change the header text once “Enter Unique Product Properties” has been selected. |
Adding validation to TP number column where the entry must start with ‘TP’, all characters must be alphanumerical (no special characters) and total length of characters must be 8-11. |
|
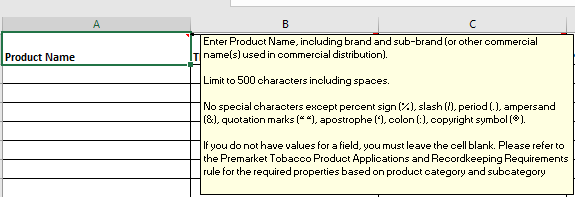
Adding tooltips when the user hovers over the first empty row- see example below:
|
|
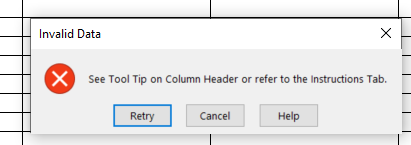
Adding error messages if an entry does not comply with the validations in the spreadsheet and if dependency rules are not adhered to. For example, if Characterizing flavor = Flavored, then a text value must be entered into the “Characterizing Flavor, if Flavor” field as it cannot be null. See example below of error message display:
|
|
Adding validation to the following numeric columns where the max length of digits is 7, no special characters, no alpha characters, no ranges, and no negative values:
Product Quantity Numeric Value Product Quantity Mass Numeric Value Portion Count Numeric Value Length Numeric Value Width Numeric Value Diameter Numeric Value Portion Thickness Numeric Value E-liquid Volume Nicotine Concentration PG Numeric Value VG Numeric Value Wattage Numeric Value Battery Capacity Numeric Value Height Numeric Value |
|
Adding validation to the following numeric column where field cannot permit number greater than 100 (maximum of 3 digits) since it is a percentage - no special characters, no alpha characters, no ranges, no negative values:
Filter Ventilation
Adding validation to the following numeric column where field cannot permit number greater than 99 (maximum of 2 digits), no special characters, no alpha characters, no ranges, no negative values:
Number of hoses |
|
Limiting the number of allowable characters (including spaces and permitted special characters) for the following columns to 500 or less:
Product Name column. Characterizing Flavor, if flavored |
|
Limiting the number of allowable characters (including spaces and permitted special characters) for the following column to 5000 or less:
Additional Properties |
|
Restricting the use of unsupported characters such as longer em dash (—), è, ñ in the ‘product name’ and ‘characterizing flavor, if flavored’ columns
Only the following special characters are permitted " % / . & " " ' : © (percent, slash, period, quotes, apostrophe, colon, copyright symbol). |
|
Increasing the column size for Units (Length) to accommodate the word “Centimeter” |
|
Adding “Other” as an option in the drop-down list for Length Description. |
|
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | dgittleson |
| File Modified | 0000-00-00 |
| File Created | 2023-09-09 |
© 2026 OMB.report | Privacy Policy